Multiwavelength Photoacoustic Doppler Flowmetry of Living Microalgae Cells
Abstract
1. Introduction
2. Methods
2.1. Sample Preparation
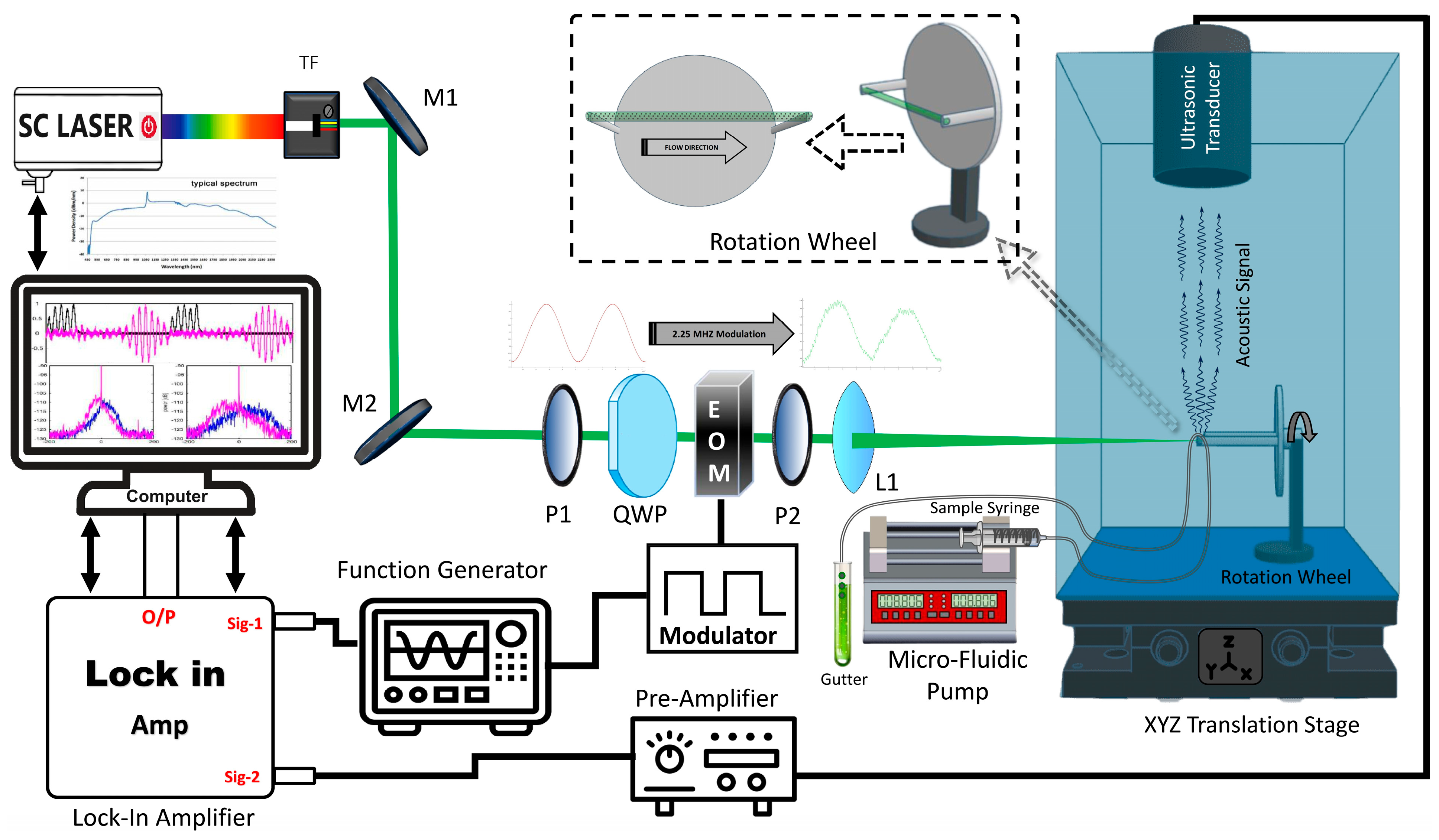
2.2. Experimental Setup
2.3. Signal Processing
3. Results
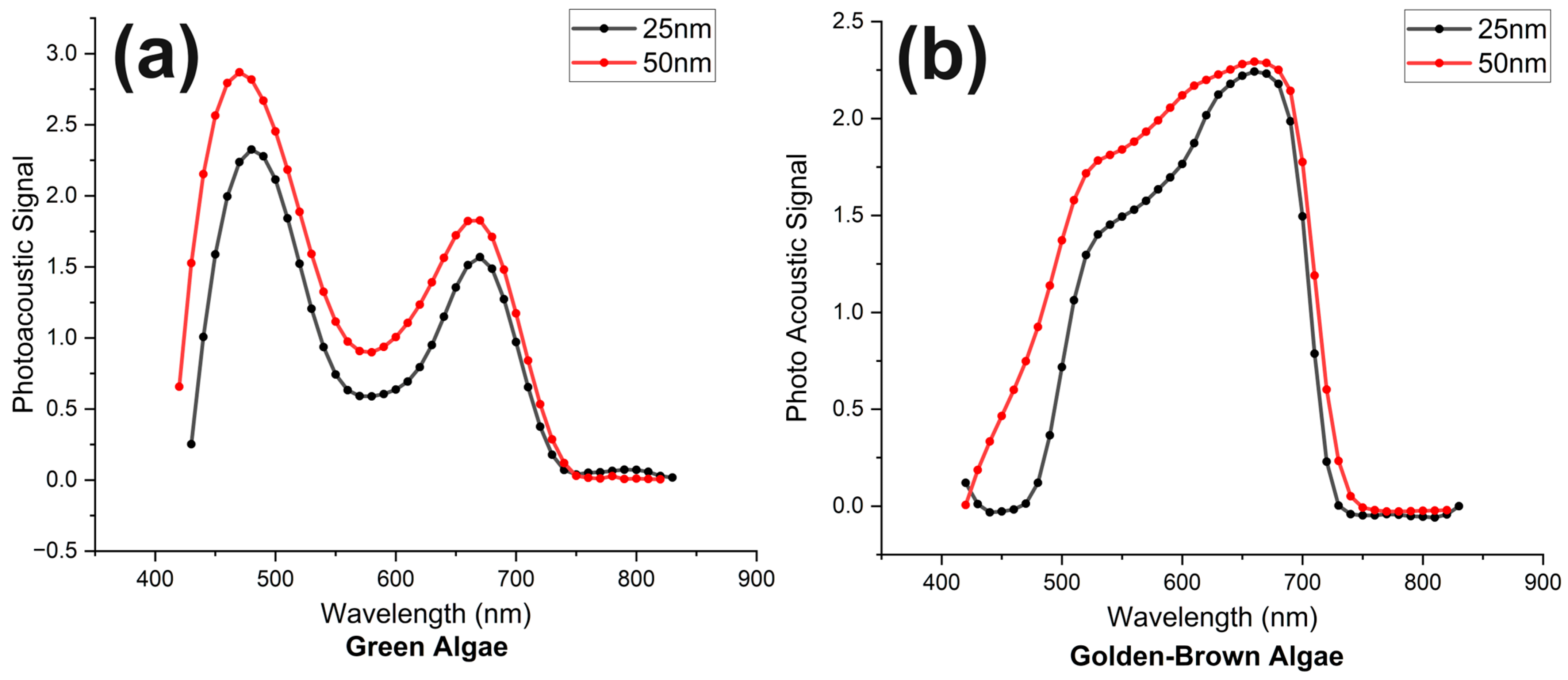
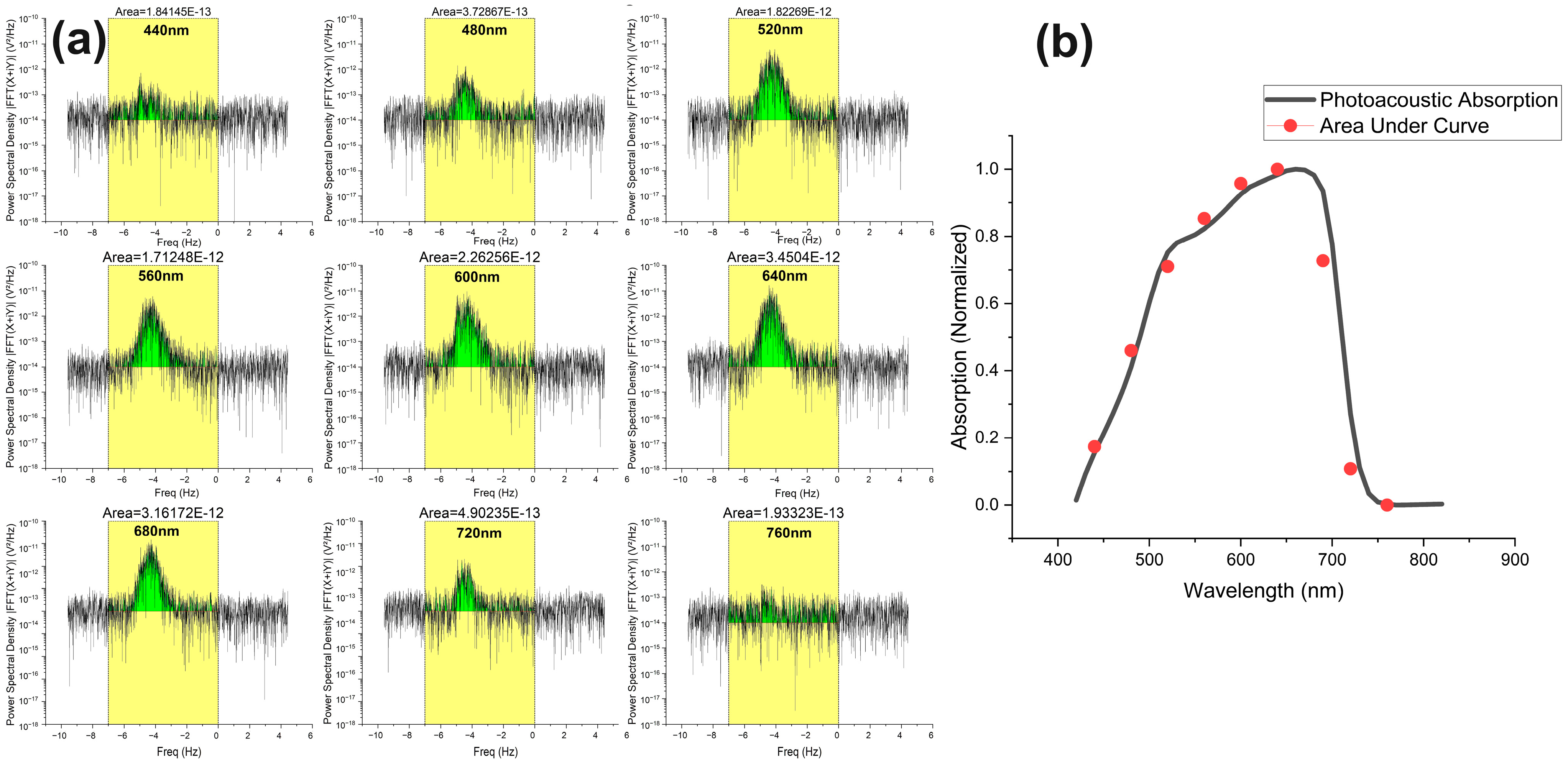
3.1. Absorption Spectra of Algae Samples in the Stationary State
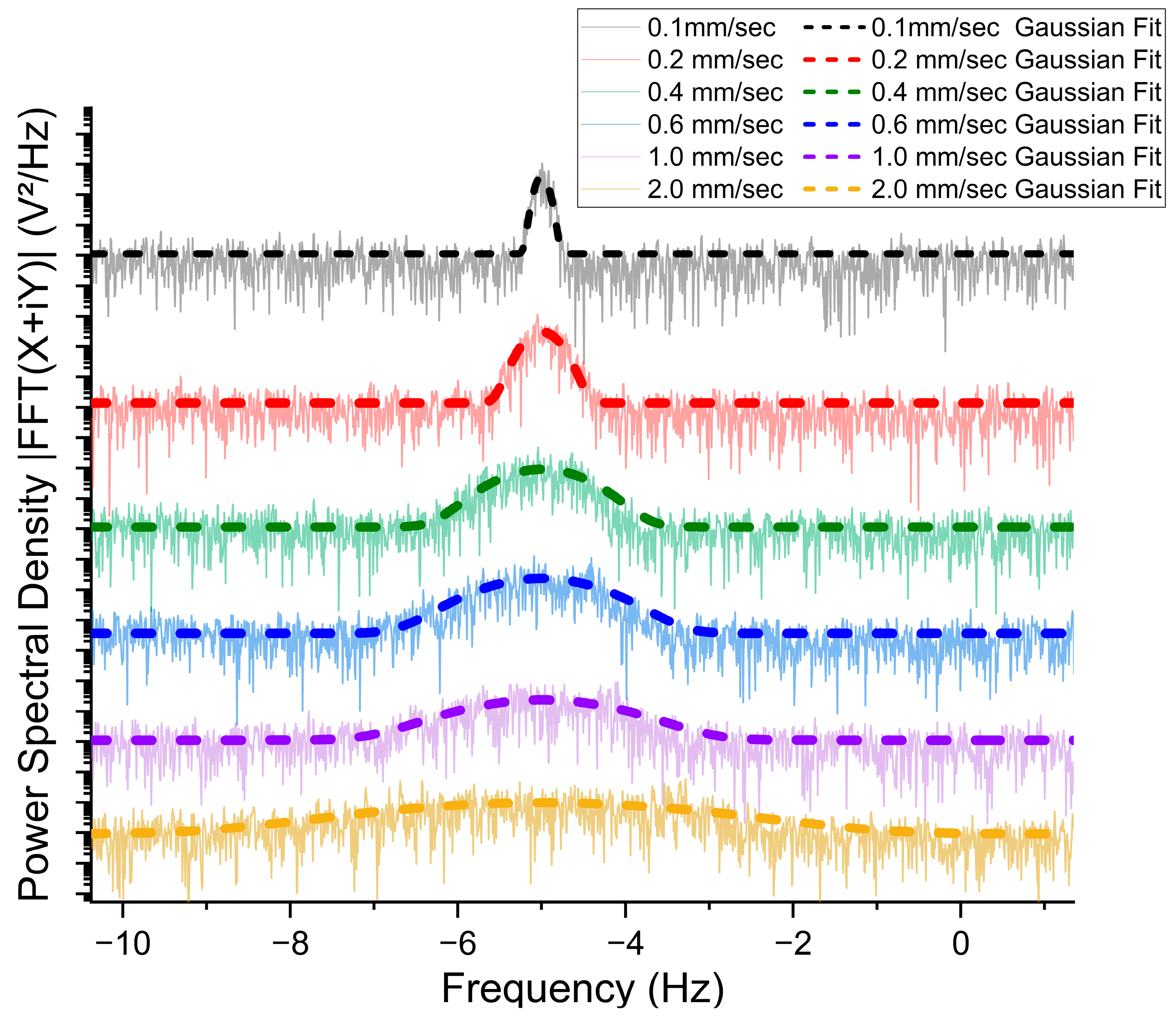
3.2. Flow Speed Measurement at Horizontal Flow Direction
3.3. Flow Direction Measurement
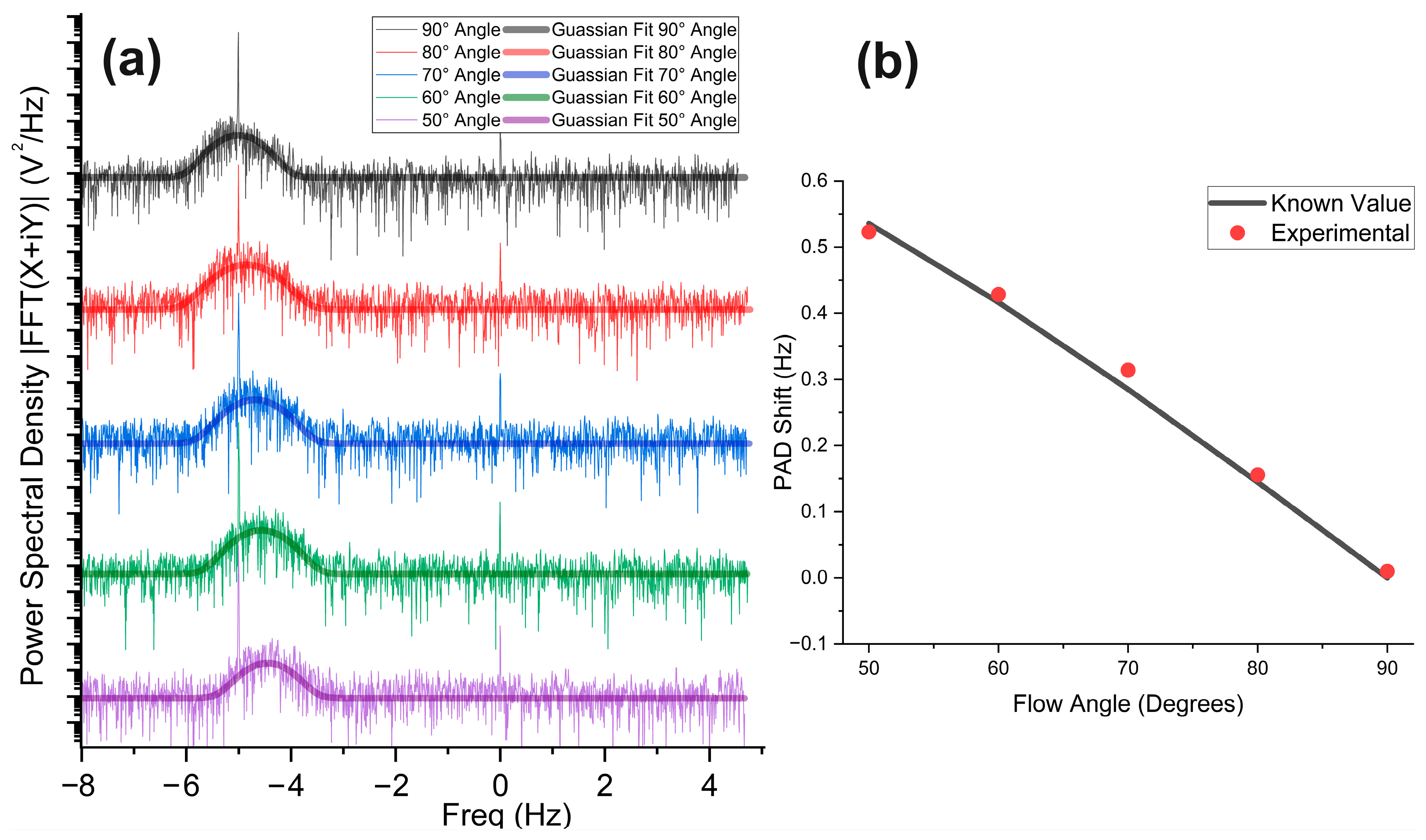
3.4. Flow Angle Measurement
3.5. Photoacoustic Doppler Shift Absorption Spectrum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, D.; Zhu, L.; Tong, S.; Shen, Y.; Gao, F.; Gao, F. Photoacoustic imaging plus X: A review. J. Biomed. Opt. 2003, 29, S11513. [Google Scholar] [CrossRef] [PubMed]
- Rolinger, L.; Rüdt, M.; Hubbuch, J. A Critical Review of Recent Trends, and a Future Perspective of Optical Spectroscopy as PAT in Biopharmaceutical Downstream Processing. Anal. Bioanal. Chem. 2020, 412, 2047–2064. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liao, R.; Xiong, Z.; Wang, Z.; Li, J.; Zhou, Q.; Tao, Y.; Ma, H. Simultaneously Acquiring Optical and Acoustic Properties of Individual Microalgae Cells Suspended in Water. Biosensors 2022, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Stylogiannis, A.; Kousias, N.; Kontses, A.; Ntziachristos, L.; Ntziachristos, V. A Low-Cost Optoacoustic Sensor for Environmental Monitoring. Sensors 2021, 21, 1379. [Google Scholar] [CrossRef] [PubMed]
- Galanzha, E.I.; Zharov, V.P. Photoacoustic Flow Cytometry. Methods 2012, 57, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.H.; Samson, A.-P.; Kocsis, T.; Viator, J.A. Photoacoustic Flow Cytometry Using Functionalized Microspheres for Selective Detection of Bacteria. Micromachines 2023, 14, 573. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Carey, K.A.; Nedosekin, D.A.; Menyaev, Y.A.; Sarimollaoglu, M.; Galanzha, E.I.; Stumhofer, J.S.; Zharov, V.P. In Vivo Photoacoustic Flow Cytometry for Early Malaria Diagnosis. Cytom. Part A 2016, 89, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Nedosekin, D.A.; Fahmi, T.; Nima, Z.A.; Nolan, J.; Cai, C.; Sarimollaoglu, M.; Dervishi, E.; Basnakian, A.; Biris, A.S.; Zharov, V.P. Photoacoustic Flow Cytometry for Nanomaterial Research. Photoacoustics 2017, 6, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Juratli, M.A.; Menyaev, Y.A.; Sarimollaoglu, M.; Siegel, E.R.; Nedosekin, D.A.; Suen, J.Y.; Melerzanov, A.V.; Juratli, T.A.; Galanzha, E.I.; Zharov, V.P. Real-Time Label-Free Embolus Detection Using In Vivo Photoacoustic Flow Cytometry. PLoS ONE 2016, 11, e0156269. [Google Scholar] [CrossRef] [PubMed]
- Brunker, J.; Beard, P. Velocity Measurements in Whole Blood Using Acoustic Resolution Photoacoustic Doppler. Biomed. Opt. Express 2016, 7, 2789–2806. [Google Scholar] [CrossRef] [PubMed]
- Brunker, J.; Beard, P. Acoustic Resolution Photoacoustic Doppler Flowmetry: Practical Considerations for Obtaining Accurate Measurements of Blood Flow. In Proceedings of the SPIE—The International Society for Optical Engineering, San Francisco, CA, USA, 3 March 2014; Oraevsky, A.A., Wang, L.V., Eds.; SPIE: Paris, France, 2014; p. 89431K. [Google Scholar]
- Čertnerová, D.; Galbraith, D.W. Best Practices in the Flow Cytometry of Microalgae. Cytom. Part A 2021, 99, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.; Seckbach, J. (Eds.) The Algae World; Springer: Dordrecht, The Netherlands, 2015; Volume 26, ISBN 978-94-017-7320-1. [Google Scholar]
- Ferreira de Oliveira, A.P.; Bragotto, A.P.A. Microalgae-Based Products: Food and Public Health. Future Foods 2022, 6, 100157. [Google Scholar] [CrossRef]
- Levine, I.A.; Gerk, C.L.; Gómez, S.M.; Nalley, J.O.; Nalley, M.M. The Algae Foundation® and Algae Technology Educational Consortium. J. World Aquac. Soc. 2021, 52, 1099–1117. [Google Scholar] [CrossRef]
- Hotos, G.N.; Bekiari, V. Absorption Spectra as Predictors of Algal Biomass and Pigment Content of the Cultured Microalgae Amphidinium carterae, Isochrysis galbana, Nephroselmis sp., and Anabaena sp. Int. J. Plant Biol. 2023, 14, 879–895. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, W.; Wen, Y.; Zhang, L.; Wu, Y.; Farid, M.S.; El-Seedi, H.R.; Capanoglu, E.; Zhao, C. Recent Advances of Natural Pigments from Algae. Food Prod. Process. Nutr. 2023, 5, 39. [Google Scholar] [CrossRef]
- Fork, D.C.; Amesz, J. Light-Induced Shifts in the Absorption Spectrum of Carotenoids in Red and Brown Algae. Photochem. Photobiol. 1967, 6, 913–918. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Zeng, L.-H.; Ren, Z.-H. Recent Application of Spectroscopy for the Detection of Microalgae Life Information: A Review. Appl. Spectrosc. Rev. 2020, 55, 26–59. [Google Scholar] [CrossRef]
- Lababpour, A.; Lee, C.-G. Simultaneous Measurement of Chlorophyll and Astaxanthin in Haematococcus Pluvialis Cells by First-Order Derivative Ultraviolet-Visible Spectrophotometry. J. Biosci. Bioeng. 2006, 101, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Sá, M.; Ferrer-Ledo, N.; Gao, F.; Bertinetto, C.G.; Jansen, J.; Crespo, J.G.; Wijffels, R.H.; Barbosa, M.; Galinha, C.F. Perspectives of Fluorescence Spectroscopy for Online Monitoring in Microalgae Industry. Microb. Biotechnol. 2022, 15, 1824–1838. [Google Scholar] [CrossRef] [PubMed]
- Tomar, V. Raman Spectroscopy of Algae: A Review. J. Nanomed. Nanotechnol. 2012, 3, 1000131. [Google Scholar] [CrossRef]
- Duygu, D. Fourier Transform Infrared (FTIR) Spectroscopy for Identification of Chlorella vulgaris Beijerinck 1890 and Scenedesmus obliquus (Turpin) Kützing 1833. Afr. J. Biotechnol. 2012, 11, 3817–3824. [Google Scholar] [CrossRef]
- Chauton, M. Nuclear Magnetic Resonance Spectroscopy of Marine Microalgae: Metabolic Profiling and Species Discrimination from High-Resolution Magic Angle Spinning NMR Analysis of Whole-Cell Samples; Fakultet for Naturvitenskap og Teknologi: Tromsø, Norway, 2005. [Google Scholar]
- Pinchasov-Grinblat, Y.; Dubinsky, Z. Photoacoustics—A Novel Tool for the Study of Aquatic Photosynthesis. In Photosynthesis; InTech: London, UK, 2013. [Google Scholar]
- Brunker, J.; Beard, P. Acoustic resolution photoacoustic Doppler velocimetry in blood-mimicking fluids. Sci. Rep. 2016, 6, 20902. [Google Scholar] [CrossRef]
- Fang, H.; Maslov, K.; Wang, L.V. Photoacoustic Doppler Flow Measurement in Optically Scattering Media. Appl. Phys. Lett. 2007, 91, 264103. [Google Scholar] [CrossRef]
- Fang, H.; Maslov, K.; Wang, L.V. Photoacoustic Doppler Effect from Flowing Small Light-Absorbing Particles. Phys. Rev. Lett. 2007, 99, 184501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yao, J.; Maslov, K.I.; Wang, L.V. Structured-Illumination Photoacoustic Doppler Flowmetry of Axial Flow in Homogeneous Scattering Media. Appl. Phys. Lett. 2013, 103, 094101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yao, J.; Maslov, K.I.; Wang, L.V. Photoacoustic Doppler Axial Flow Measurement of Homogenous Media Using Structured Illumination. In Proceedings of the SPIE—The International Society for Optical Engineering, San Francisco, CA, USA, 3 March 2014; Oraevsky, A.A., Wang, L.V., Eds.; SPIE: Paris, France, 2014; p. 89431U. [Google Scholar]
- Fang, H.; Wang, L.V. Photoacoustic Doppler Flowmetry. In Proceedings of the SPIE BiOS, San Francisco, CA, USA, 7 February 2008; Oraevsky, A.A., Wang, L.V., Eds.; SPIE: Paris, France, 2008; p. 68561M. [Google Scholar]
- Yao, J.; Wang, L.V. Transverse Flow Imaging Based on Photoacoustic Doppler Bandwidth Broadening. J. Biomed. Opt. 2010, 15, 021304. [Google Scholar] [CrossRef] [PubMed]
- Cvjetinovic, J.; Salimon, A.I.; Novoselova, M.V.; Sapozhnikov, P.V.; Shirshin, E.A.; Yashchenok, A.M.; Kalinina, O.Y.; Korsunsky, A.M.; Gorin, D.A. Photoacoustic and Fluorescence Lifetime Imaging of Diatoms. Photoacoustics 2020, 18, 100171. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zheng, X.; Liu, Z.; Dong, T.; Luo, Y.; Yan, W.; Wang, C.; Song, C. On-Chip Photoacoustics-Activated Cell Sorting (PA-ACS) for Label-Free and High-Throughput Detection and Screening of Microalgal Cells. Anal. Chem. 2024, 96, 1301–1309. [Google Scholar] [CrossRef]
- Kumar, S.; Baweja, P.; Sahoo, D. Diatoms: Yellow or Golden Brown Algae; Springer: Dordrecht, The Netherlands, 2015; pp. 235–258. [Google Scholar]
- Wong, J.F.; Hong, H.J.; Foo, S.C.; Yap, M.K.K.; Tan, J.W. A Review on Current and Future Advancements for Commercialized Microalgae Species. Food Sci. Hum. Wellness 2022, 11, 1156–1170. [Google Scholar] [CrossRef]
- Cvjetinovic, J.; Bedoshvili, Y.; Nozdriukhin, D.; Efimova, O.; Salimon, A.; Volokitina, N.; Korsunsky, A.; Gorin, D. In Situ Fluorescence/Photoacoustic Monitoring of Diatom Algae. In Proceedings of the Dynamics and Fluctuations in Biomedical Photonics XVIII, Online, 5 March 2021; Tuchin, V.V., Leahy, M.J., Wang, R.K., Eds.; SPIE: Paris, France, 2021; p. 8. [Google Scholar]
- Fang, H. Photoacoustic Doppler Effect and Flow Sensing; Taylor & Francis: Abingdon, UK, 2009; pp. 19–24. [Google Scholar]
- Liu, Y.; Fang, H.; Farooq, T.; Yuan, X. Multispectral Photoacoustic Doppler Velocimetry with Intensity Modulated High Repetition Supercontinnum Laser Pulses. In Proceedings of the SPIE—The International Society for Optical Engineering, Beijing, China, 12 March 2020; Volume 11436. [Google Scholar]
- van den Berg, P.J.; Daoudi, K.; Steenbergen, W. Review of Photoacoustic Flow Imaging: Its Current State and Its Promises. Photoacoustics 2015, 3, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Sheinfeld, A.; Gilead, S.; Eyal, A. Photoacoustic Doppler Measurement of Flow Using Tone Burst Excitation. Opt. Express 2010, 18, 4212–4221. [Google Scholar] [CrossRef] [PubMed]
- Paschotta, R. Supercontinuum Generation—An Encyclopedia Article. In RP Photonics Encyclopedia; RP Photonics AG: Frauenfeld, Switzerland, 2008. [Google Scholar]
- Liu, M.; Buma, T. Wavelength Agile Photoacoustic Microscopy with a Pulsed Supercontinuum Source. In Proceedings of the 2010 IEEE International Ultrasonics Symposium, San Diego, CA, USA, 11–14 October 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 382–385. [Google Scholar]
- Ishihara, M.; Hirasawa, T.; Le, Z.; Miyashita, M.; Matsuda, T.; Murakoshi, H.; Nagai, T. Photoacoustic Microscopy Using Supercontinuum Light for Imaging of Chromoproteins in Living Cells. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2022, San Francisco, CA, USA, 7 March 2022; Oraevsky, A.A., Wang, L.V., Eds.; SPIE: Paris, France, 2022; p. 153. [Google Scholar]
- Lange, F.; Giannoni, L.; Tachtsidis, I. The Use of Supercontinuum Laser Sources in Biomedical Diffuse Optics: Unlocking the Power of Multispectral Imaging. Appl. Sci. 2021, 11, 4616. [Google Scholar] [CrossRef]
- Yavas, S.; Kipergil, E.A.; Akcaalan, O.; Eldeniz, Y.B.; Arabul, U.; Erkol, H.; Unlu, M.B.; Ilday, F.O. All-Fiber Nanosecond Laser System Generating Supercontinuum Spectrum for Photoacoustic Imaging. In Proceedings of the 2013 Conference on Lasers & Electro-Optics Europe & International Quantum Electronics Conference CLEO EUROPE/IQEC, Munich, Germany, 12–16 May 2013; IEEE: Piscataway, NJ, USA, 2013; p. CL_P_16. [Google Scholar]
- Shu, X.; Bondu, M.; Dong, B.; Podoleanu, A.; Leick, L.; Zhang, H.F. Single All-Fiber-Based Nanosecond-Pulsed Supercontinuum Source for Multispectral Photoacoustic Microscopy and Optical Coherence Tomography. Opt. Lett. 2016, 41, 2743–2746. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Farooq, T.; Li, L.; Yan, S.; Fang, H. Photoacoustic Property of Gallium-Based Liquid Metals Measured with Intensity Modulated High Repetition Supercontinnum Laser. In Proceedings of the SPIE—The International Society for Optical Engineering, Xi’an, China, 24 November 2023; Volume 12935. [Google Scholar]
- Kosumi, D.; Fujii, R.; Sugisaki, M.; Oka, N.; Iha, M.; Hashimoto, H. Characterization of the Intramolecular Transfer State of Marine Carotenoid Fucoxanthin by Femtosecond Pump–Probe Spectroscopy. Photosynth. Res. 2014, 121, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Bhuyar, P.; Yusoff, M.; Ab Rahim, M.H.; Pragas Maniam, G.; Ramaraj, R.; Govindan, N. Antimicrobial Study of Algal Enzymes Extracted from Microalgae by Ultrasonication. SSRN Electron. J. 2019. [Google Scholar] [CrossRef]
- Rao, N.R.H.; Henderson, R.K. Unit Operations Applied for Microalgae-Based Solid–Liquid Separation. In 3rd Generation Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 175–212. [Google Scholar]
- Tzang, O.; Piestun, R. Lock-in Detection of Photoacoustic Feedback Signal for Focusing through Scattering Media Using Wave-Front Shaping. Opt. Express 2016, 24, 28122–28130. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Hajireza, P.; Zemp, R. Photoacoustic Imaging Using Lock-in Amplification and Pulsed Fiber Lasers. In Proceedings of the SPIE BiOS, San Diego, CA, USA, 15 March 2016; Oraevsky, A.A., Wang, L.V., Eds.; SPIE: Paris, France, 2016; p. 97084I. [Google Scholar]
- Tong, Y.; Zhao, H.; Fang, H.; Zhao, Y.; Yuan, X. Flow Angle Dependent Photoacoustic Doppler Power Spectra under Intensity-Modulated Continuous Wave Laser Excitation. AIP Adv. 2016, 6, 025109. [Google Scholar] [CrossRef]
- Solomon, O.M., Jr. PSD Computations Using Welch’s Method; NASA STI/Recon Technical Report N; Sandia National Laboratory: Albuquerque, NM, USA, 1991; Volume 92, p. 23584. [Google Scholar]
- Büchel, C. Light-Harvesting Complexes of Diatoms: Fucoxanthin-Chlorophyll Proteins; Springer: Berlin/Heidelberg, Germany, 2020; pp. 441–457. [Google Scholar]
- Klünemann, H.-H.; Arnolds, B.J.; von Reutern, G.-M. Angle Dependence and Transit Time Effect in Doppler Flow Measurement. Eur. J. Ultrasound 1996, 3, 261–266. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes; Elsevier: Amsterdam, The Netherlands, 1987; pp. 350–382. [Google Scholar]
- Baránková, B.; Lazár, D.; Nauš, J.; Solovchenko, A.; Gorelova, O.; Baulina, O.; Huber, G.; Nedbal, L. Light Absorption and Scattering by High Light-Tolerant, Fast-Growing Chlorella Vulgaris IPPAS C-1 Cells. Algal Res. 2020, 49, 101881. [Google Scholar] [CrossRef]
- Ikeda, Y.; Yamagishi, A.; Komura, M.; Suzuki, T.; Dohmae, N.; Shibata, Y.; Itoh, S.; Koike, H.; Satoh, K. Two Types of Fucoxanthin-Chlorophyll-Binding Proteins I Tightly Bound to the Photosystem I Core Complex in Marine Centric Diatoms. Biochim. Biophys. Acta BBA—Bioenerg. 2013, 1827, 529–539. [Google Scholar] [CrossRef] [PubMed]


| Average Flow Speed | (V2/Hz) | (Hz) | (Hz) | A (V2) | FWHM (Hz) |
|---|---|---|---|---|---|
| 0.1 mm/s | 1.42985 × 10−9 | −4.99822 | 0.06144 | 2.2015 × 10−10 | 0.14468 |
| 0.2 mm/s | 6.09522 × 10−10 | −4.99926 | 0.16522 | 2.5237 × 10−10 | 0.389063 |
| 0.4 mm/s | 2.31676 × 10−10 | −4.99959 | 0.41571 | 2.4135 × 10−10 | 0.978922 |
| 0.6 mm/s | 1.81575 × 10−10 | −4.99964 | 0.557 | 2.5345 × 10−10 | 1.311635 |
| 1.0 mm/s | 1.30865 × 10−10 | −4.99701 | 0.74828 | 2.4540 × 10−10 | 1.762065 |
| 2.0 mm/s | 5.89998 × 10−11 | −4.99464 | 1.56694 | 2.3168 × 10−10 | 3.689862 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, T.; Wu, X.; Yan, S.; Fang, H. Multiwavelength Photoacoustic Doppler Flowmetry of Living Microalgae Cells. Biosensors 2024, 14, 397. https://doi.org/10.3390/bios14080397
Farooq T, Wu X, Yan S, Fang H. Multiwavelength Photoacoustic Doppler Flowmetry of Living Microalgae Cells. Biosensors. 2024; 14(8):397. https://doi.org/10.3390/bios14080397
Chicago/Turabian StyleFarooq, Tayyab, Xiuru Wu, Sheng Yan, and Hui Fang. 2024. "Multiwavelength Photoacoustic Doppler Flowmetry of Living Microalgae Cells" Biosensors 14, no. 8: 397. https://doi.org/10.3390/bios14080397
APA StyleFarooq, T., Wu, X., Yan, S., & Fang, H. (2024). Multiwavelength Photoacoustic Doppler Flowmetry of Living Microalgae Cells. Biosensors, 14(8), 397. https://doi.org/10.3390/bios14080397







