Centrifugal Microfluidic Cell Culture Platform for Physiologically Relevant Virus Infection Studies: A Case Study with HSV-1 Infection of Periodontal Cells
Abstract
1. Introduction
2. Materials and Methods
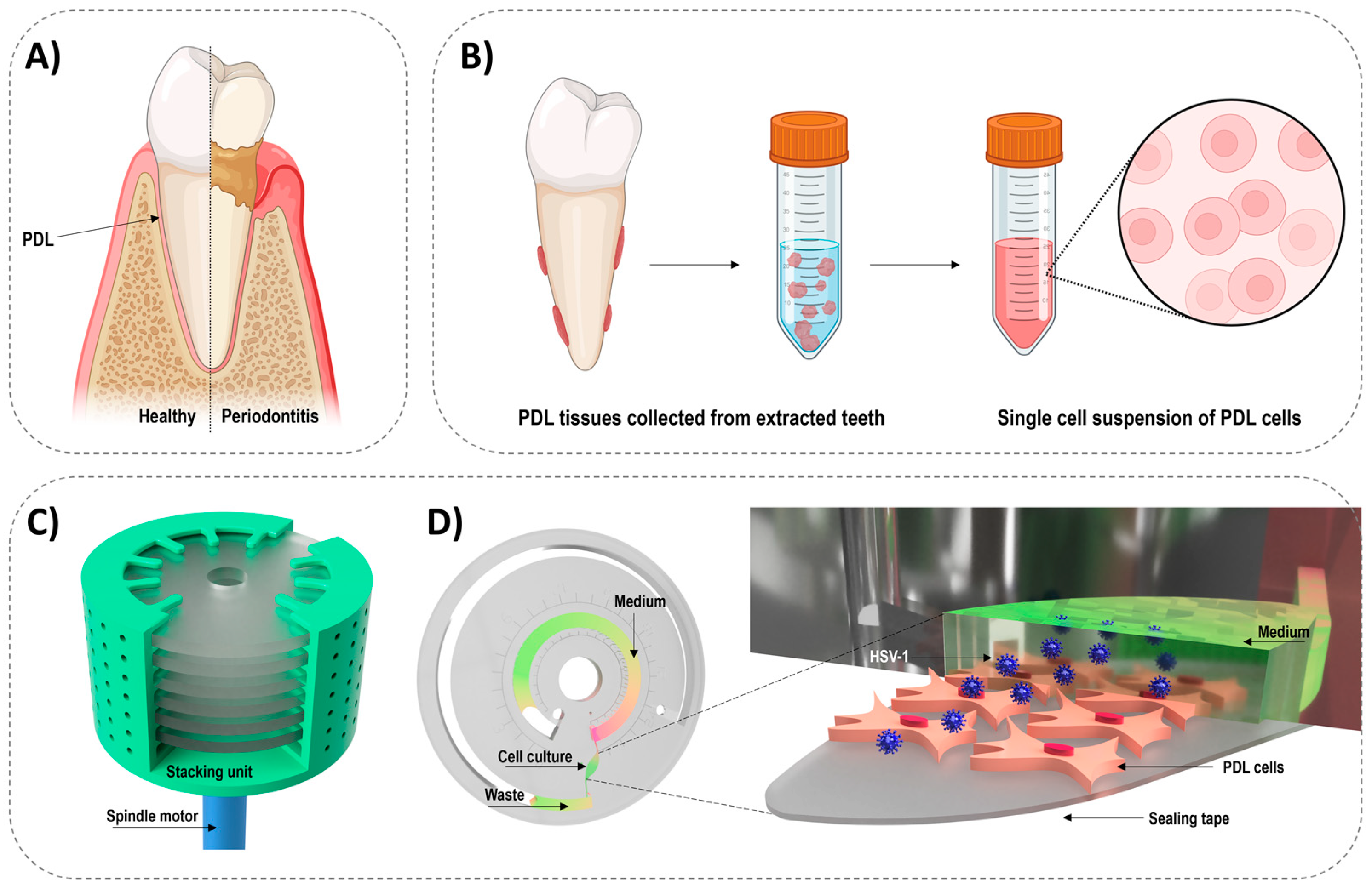
2.1. Design and Fabrication of the Centrifugal Microfluidic Cell Culture Platform
2.2. Preparation of PDL Single Cell Suspensions and Cell Culture
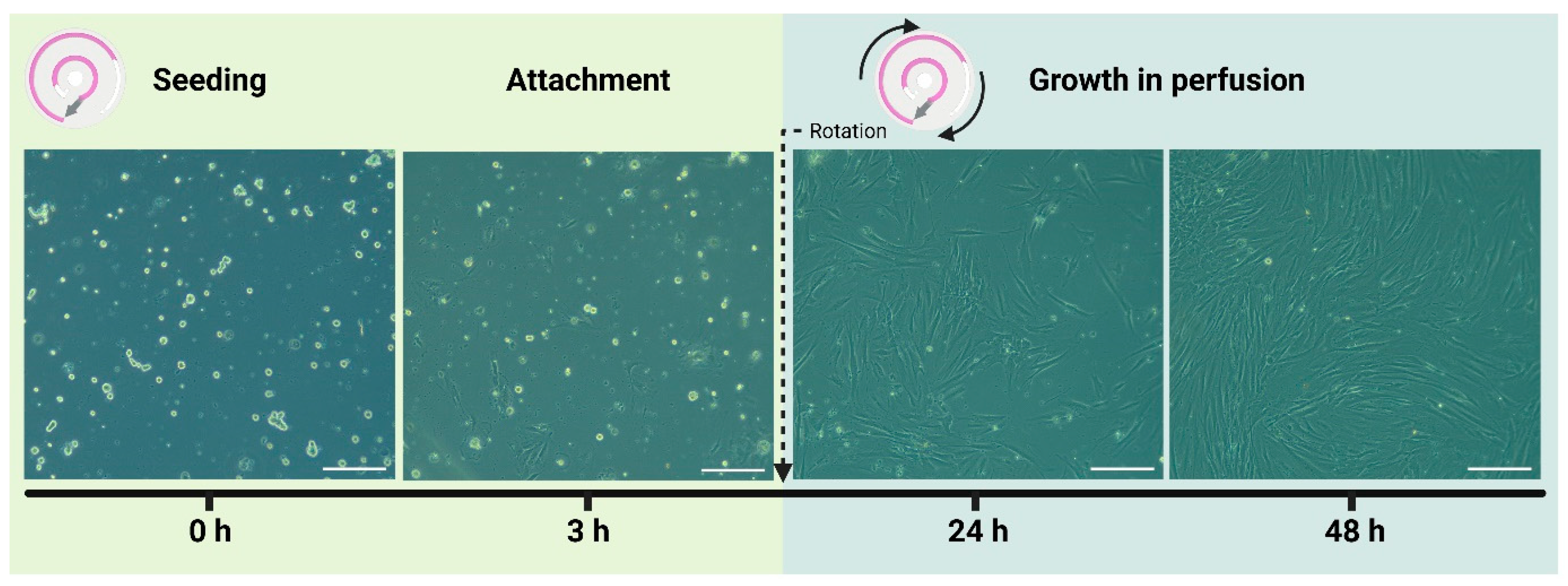
2.3. PDL Cell Seeding and Growth in the Microfluidic Chip
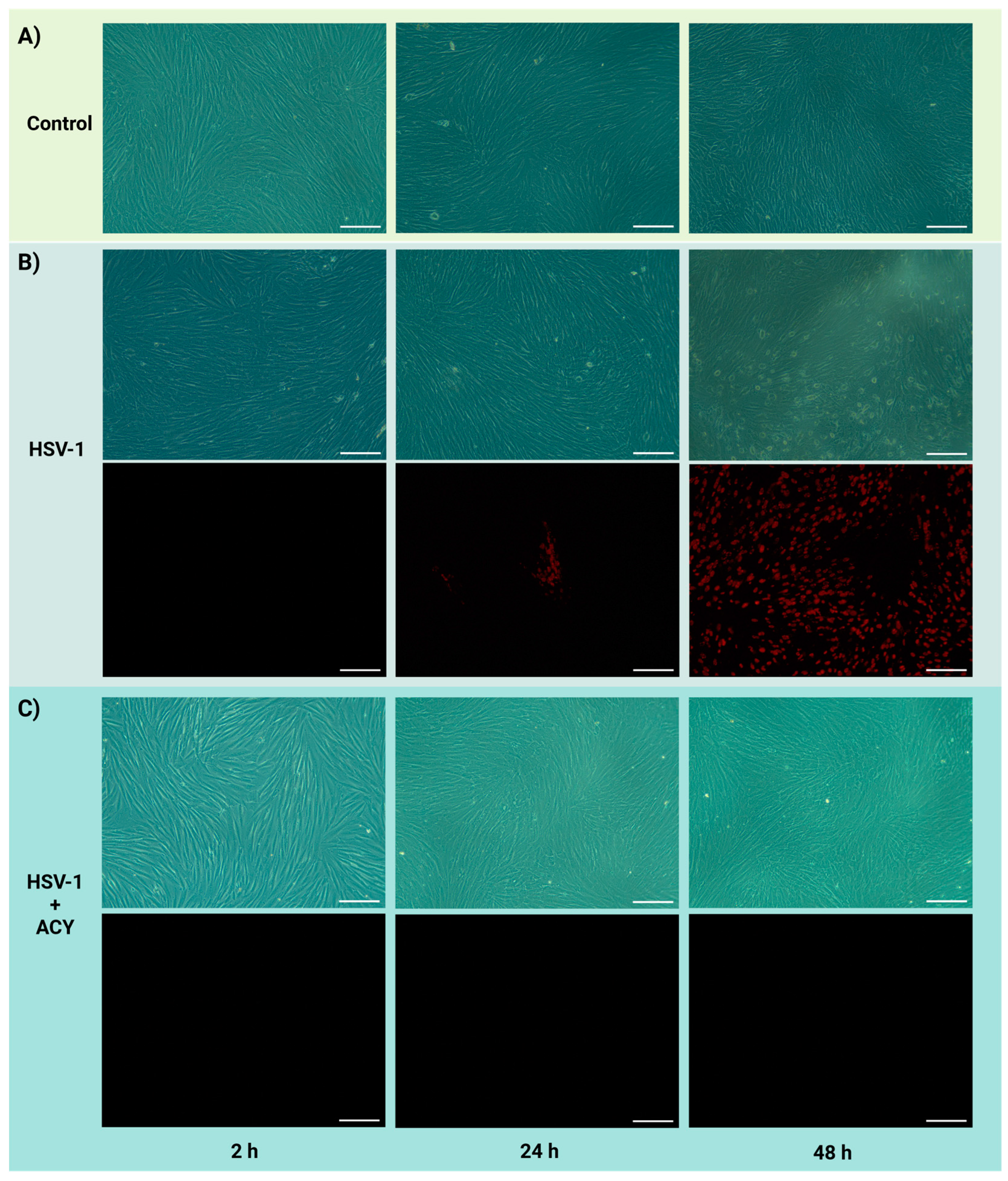
2.4. HSV-1 Infection of PDL Cells in the Microfluidic Chip
2.5. RNA Extraction and RT-qPCR
2.6. Data Analysis and Visualization
3. Results and Discussion
3.1. Centrifugal Microfluidic Chip Design
3.2. Culturing of Periodontal Ligament Cells in the Microfluidic Chip
3.3. HSV-1 Infection of PDL Cells and Treatment with Acyclovir
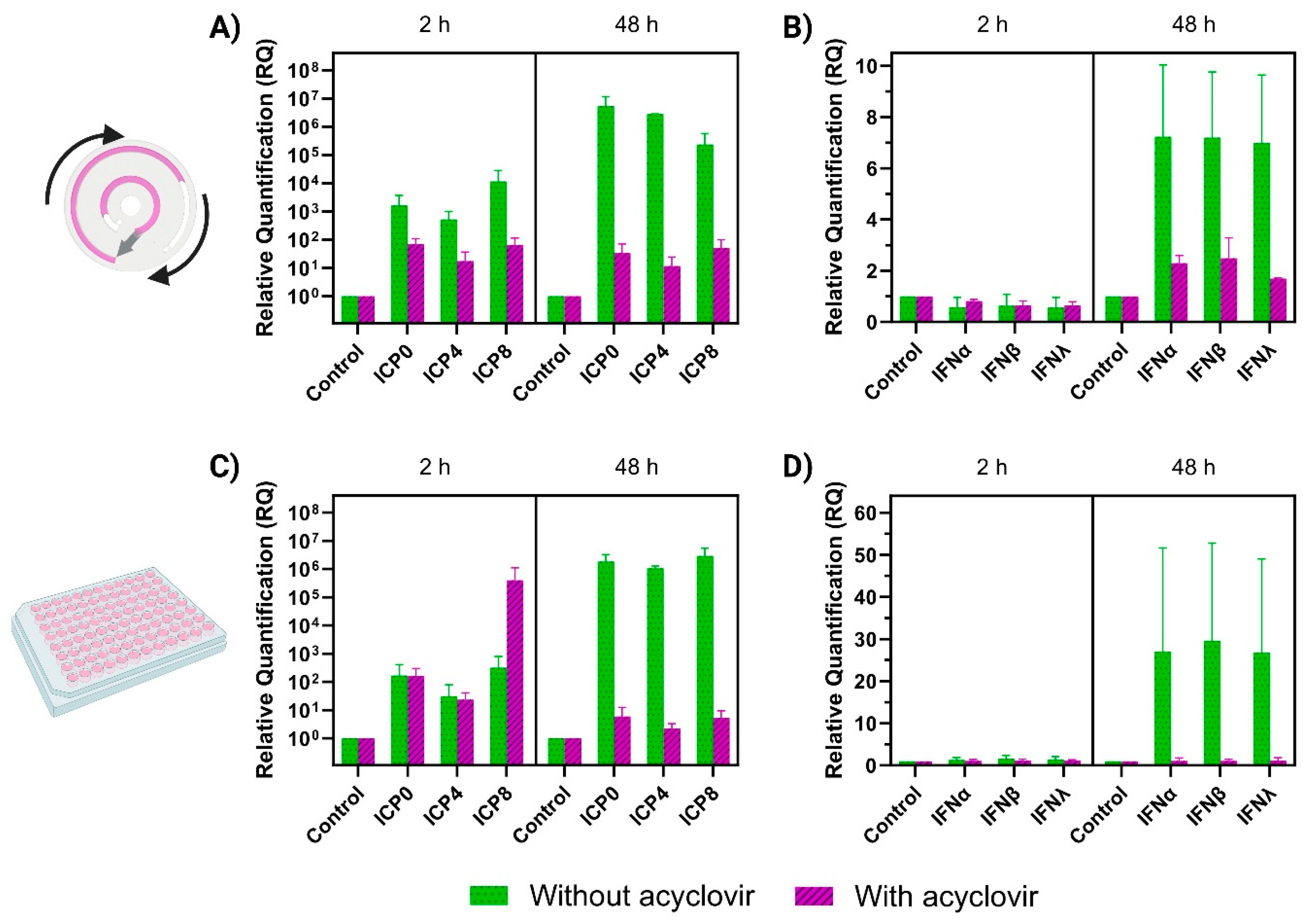
3.4. RT-qPCR Analysis of HSV-1 Gene Expression and Interferon Response in the Microfluidic Chip and Static Conditions
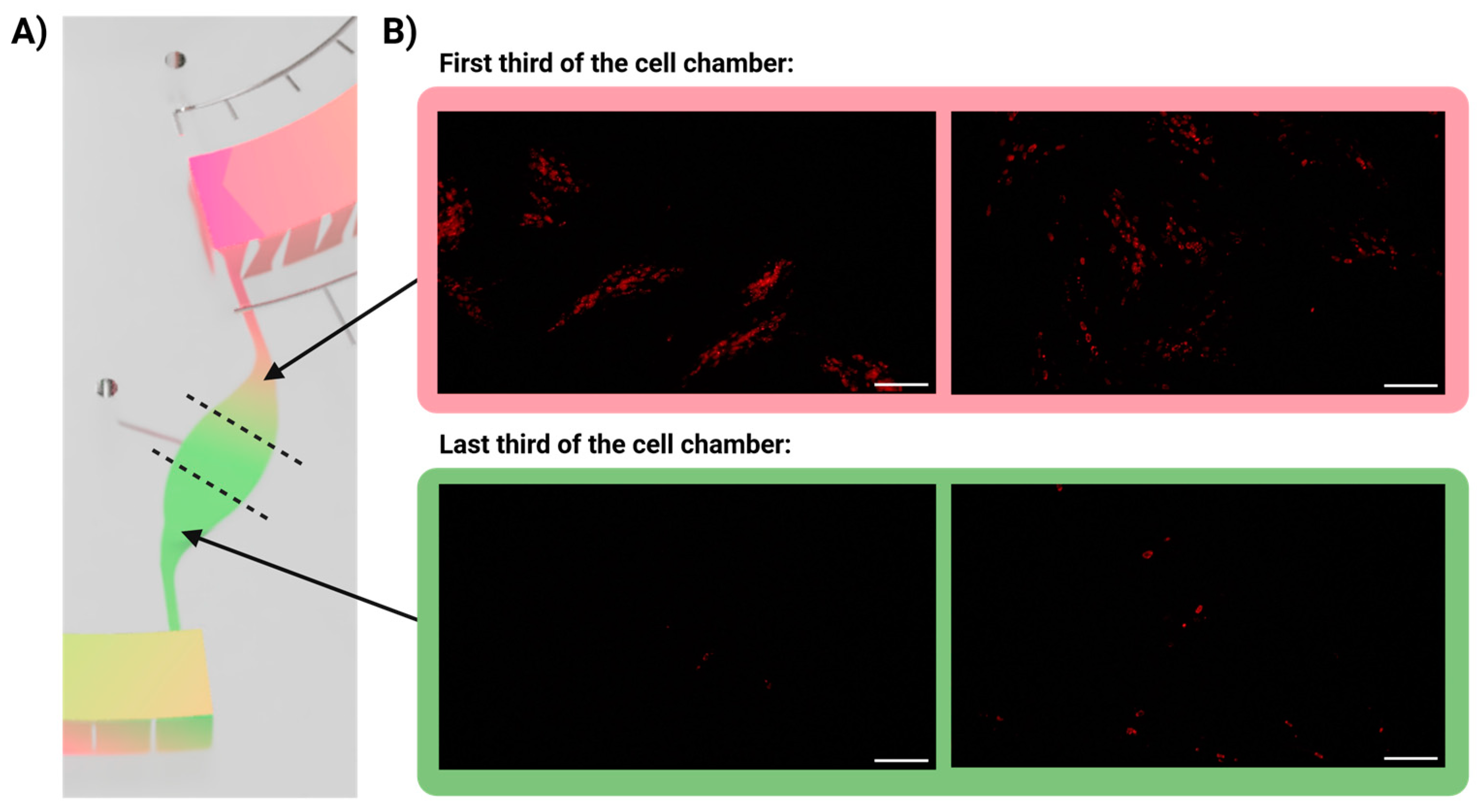
3.5. Infection Gradient
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ingber, D.E. Human Organs-on-Chips for Disease Modelling, Drug Development and Personalized Medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Chi, C.W.; Ahmed, A.R.; Dereli-Korkut, Z.; Wang, S. Microfluidic Cell Chips for High-Throughput Drug Screening. Bioanalysis 2016, 8, 921. [Google Scholar] [CrossRef]
- Kim, H.J.; Ingber, D.E. Gut-on-a-Chip Microenvironment Induces Human Intestinal Cells to Undergo Villus Differentiation. Integr. Biol. 2013, 5, 1130–1140. [Google Scholar] [CrossRef]
- Thomas, D.P.; Zhang, J.; Nguyen, N.T.; Ta, H.T. Microfluidic Gut-on-a-Chip: Fundamentals and Challenges. Biosensors 2023, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Fritz, J.V.; Glaab, E.; Desai, M.S.; Greenhalgh, K.; Frachet, A.; Niegowska, M.; Estes, M.; Jäger, C.; Seguin-Devaux, C.; et al. A Microfluidics-Based in Vitro Model of the Gastrointestinal Human-Microbe Interface. Nat. Commun. 2016, 7, 11535. [Google Scholar] [CrossRef] [PubMed]
- Serioli, L.; Laksafoss, T.Z.; Haagensen, J.A.J.; Sternberg, C.; Soerensen, M.P.; Molin, S.; Zór, K.; Boisen, A. Bacterial Cell Cultures in a Lab-on-a-Disc: A Simple and Versatile Tool for Quantification of Antibiotic Treatment Efficacy. Anal. Chem. 2020, 92, 13871–13879. [Google Scholar] [CrossRef]
- Schneider, S.; Erdemann, F.; Schneider, O.; Hutschalik, T.; Loskill, P. Organ-on-a-Disc: A Platform Technology for the Centrifugal Generation and Culture of Microphysiological 3D Cell Constructs Amenable for Automation and Parallelization. APL Bioeng. 2020, 4, 046101. [Google Scholar] [CrossRef]
- Zhao, Q.; Yuan, D.; Zhang, J.; Li, W. A Review of Secondary Flow in Inertial Microfluidics. Micromachines 2020, 11, 461. [Google Scholar] [CrossRef]
- Gorkin, R.; Park, J.; Siegrist, J.; Amasia, M.; Lee, B.S.; Park, J.M.; Kim, J.; Kim, H.; Madou, M.; Cho, Y.K. Centrifugal Microfluidics for Biomedical Applications. Lab Chip 2010, 10, 1758–1773. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 513823. [Google Scholar]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D Cell Cultures—A Comparison of Different Types of Cancer Cell Cultures. Arch. Med. Sci. 2018, 14, 910. [Google Scholar] [CrossRef]
- Baddal, B.; Marrazzo, P. Refining Host-Pathogen Interactions: Organ-on-Chip Side of the Coin. Pathogens 2021, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Prieto, A.M.; Skelton, J.K.; Wai, S.N.; Large, E.; Lussignol, M.; Vizcay-Barrena, G.; Hughes, D.; Fleck, R.A.; Thursz, M.; Catanese, M.T.; et al. 3D Microfluidic Liver Cultures as a Physiological Preclinical Tool for Hepatitis B Virus Infection. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Villenave, R.; Wales, S.Q.; Hamkins-Indik, T.; Papafragkou, E.; Weaver, J.C.; Ferrante, T.C.; Bahinski, A.; Elkins, C.A.; Kulka, M.; Ingber, D.E. Human Gut-On-A-Chip Supports Polarized Infection of Coxsackie B1 Virus In Vitro. PLoS ONE 2017, 12, e0169412. [Google Scholar] [CrossRef]
- Johnson, B.N.; Lancaster, K.Z.; Hogue, I.B.; Meng, F.; Kong, Y.L.; Enquist, L.W.; McAlpine, M.C. 3D Printed Nervous System on a Chip. Lab Chip 2016, 16, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Bai, H.; Rodas, M.; Cao, W.; Oh, C.Y.; Jiang, A.; Moller, R.; Hoagland, D.; Oishi, K.; Horiuchi, S.; et al. A Human-Airway-on-a-Chip for the Rapid Identification of Candidate Antiviral Therapeutics and Prophylactics. Nat. Biomed. Eng. 2021, 5, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Junaid, A.; Tang, H.; van Reeuwijk, A.; Abouleila, Y.; Wuelfroth, P.; van Duinen, V.; Stam, W.; van Zonneveld, A.J.; Hankemeier, T.; Mashaghi, A. Ebola Hemorrhagic Shock Syndrome-on-a-Chip. iScience 2020, 23, 100765. [Google Scholar] [CrossRef]
- Jain, N.; Dutt, U.; Radenkov, I.; Jain, S. WHO’s Global Oral Health Status Report 2022: Actions, Discussion and Implementation. Oral Dis. 2024, 30, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Chavakis, T. Local and Systemic Mechanisms Linking Periodontal Disease and Inflammatory Comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Mark Welch, J.L.; Kauffman, K.M.; McLean, J.S.; He, X. The Oral Microbiome: Diversity, Biogeography and Human Health. Nat. Rev. Microbiol. 2023, 22, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Miles, B.; Zakhary, I.; El-Awady, A.; Scisci, E.; Carrion, J.; O’Neill, J.C.; Rawlings, A.; Kobi Stern, J.; Susin, C.; Cutler, C.W. Secondary Lymphoid Organ Homing Phenotype of Human Myeloid Dendritic Cells Disrupted by an Intracellular Oral Pathogen. Infect. Immun. 2014, 82, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi, M.; Crincoli, V.; Laino, L.; Alovisi, M.; Sovereto, D.; Mastrangelo, F.; Lo Russo, L.; Lo Muzio, L. The Role of Periodontitis and Periodontal Bacteria in the Onset and Progression of Alzheimer’s Disease: A Systematic Review. J. Clin. Med. 2020, 9, 495. [Google Scholar] [CrossRef]
- Atarashi, K.; Suda, W.; Luo, C.; Kawaguchi, T.; Motoo, I.; Narushima, S.; Kiguchi, Y.; Yasuma, K.; Watanabe, E.; Tanoue, T.; et al. Ectopic Colonization of Oral Bacteria in the Intestine Drives TH1 Cell Induction and Inflammation. Science 2017, 358, 359–365. [Google Scholar] [CrossRef]
- Kitamoto, S.; Nagao-Kitamoto, H.; Jiao, Y.; Gillilland, M.G.; Hayashi, A.; Imai, J.; Sugihara, K.; Miyoshi, M.; Brazil, J.C.; Kuffa, P.; et al. The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont-Driven Colitis. Cell 2020, 182, 447–462.e14. [Google Scholar] [CrossRef]
- Huang, C.; Sanaei, F.; Verdurmen, W.P.R.; Yang, F.; Ji, W.; Walboomers, X.F. The Application of Organs-on-a-Chip in Dental, Oral, and Craniofacial Research. J. Dent. Res. 2023, 102, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Pierfelice, T.V.; D’Amico, E.; Petrini, M.; Romano, M.; D’Arcangelo, C.; Sbordone, L.; Barone, A.; Plebani, R.; Iezzi, G. A Systematic Review on Organ-on-a-Chip in PDMS or Hydrogel in Dentistry: An Update of the Literature. Gels 2024, 10, 102. [Google Scholar] [CrossRef]
- Rahimi, C.; Rahimi, B.; Padova, D.; Rooholghodos, S.A.; Bienek, D.R.; Luo, X.; Kaufman, G.; Raub, C.B. Oral Mucosa-on-a-Chip to Assess Layer-Specific Responses to Bacteria and Dental Materials. Biomicrofluidics 2018, 12, 054106. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal Diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial Complexes in Subgingival Plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Slots, J. Human Viruses in Periodontitis. Periodontol 2000 2010, 53, 89–110. [Google Scholar] [CrossRef]
- Slots, J.; Slots, H. Periodontal Herpesvirus Morbidity and Treatment. Periodontol 2000 2019, 79, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.I. Subgingival Fungi, Archaea, and Viruses under the Omics Loupe. Periodontol 2000 2021, 85, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Arduino, P.G.; Cabras, M.; Lodi, G.; Petti, S. Herpes Simplex Virus Type 1 in Subgingival Plaque and Periodontal Diseases. Meta-Analysis of Observational Studies. J. Periodontal. Res. 2022, 57, 256–268. [Google Scholar] [CrossRef]
- Song, Y.; Liu, N.; Gao, L.; Yang, D.; Liu, J.; Xie, L.; Dan, H.; Chen, Q. Association between Human Herpes Simplex Virus and Periodontitis: Results from the Continuous National Health and Nutrition Examination Survey 2009–2014. BMC Oral Health 2023, 23, 675. [Google Scholar]
- Ortis, M.; Chevalier, M.; Olivieri, C.-V.; Vitale, S.; Paul, A.; Tonoyan, L.; Doglio, A.; Marsault, R. Herpes Simplex Virus Type 1 Infection of Human Periodontal Ligament. Int. J. Mol. Sci. 2024, 25, 8466. [Google Scholar] [CrossRef] [PubMed]
- Park, O.J.; Kim, A.R.; So, Y.J.; Im, J.; Ji, H.J.; Ahn, K.B.; Seo, H.S.; Yun, C.H.; Han, S.H. Induction of Apoptotic Cell Death by Oral Streptococci in Human Periodontal Ligament Cells. Front. Microbiol. 2021, 12, 738047. [Google Scholar]
- Nilsson, B.O. Mechanisms Involved in Regulation of Periodontal Ligament Cell Production of Pro-Inflammatory Cytokines: Implications in Periodontitis. J. Periodontal. Res. 2021, 56, 249–255. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, M.; Hao, M.; Tang, J. Periodontal Ligament Stem Cells in the Periodontitis Niche: Inseparable Interactions and Mechanisms. J. Leukoc. Biol. 2021, 110, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Chaikeawkaew, D.; Everts, V.; Pavasant, P. TLR3 Activation Modulates Immunomodulatory Properties of Human Periodontal Ligament Cells. J. Periodontol. 2020, 91, 1225–1236. [Google Scholar] [CrossRef]
- Serioli, L.; Gruzinskyte, L.; Zappalà, G.; Te Hwu, E.; Laksafoss, T.Z.; Jensen, P.L.; Demarchi, D.; Müllertz, A.; Boisen, A.; Zór, K. Moving Perfusion Culture and Live-Cell Imaging from Lab to Disc: Proof of Concept Toxicity Assay with AI-Based Image Analysis. Lab Chip 2023, 23, 1603–1612. [Google Scholar] [CrossRef]
- Etienne, L.; Joshi, P.; Dingle, L.; Huang, E.; Grzesik, P.; Desai, P.J. Visualization of Herpes Simplex Virus Type 1 Virions Using Fluorescent Colors. J. Virol. Methods 2017, 241, 46–51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, H.J.; Huh, D.; Hamilton, G.; Ingber, D.E. Human Gut-on-a-Chip Inhabited by Microbial Flora That Experiences Intestinal Peristalsis-like Motions and Flow. Lab Chip 2012, 12, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, P.; Banks, J.M.; Rahat, R.; Brandini, D.A.; Naqvi, A.R. Viruses of the Oral Cavity: Prevalence, Pathobiology and Association with Oral Diseases. Rev. Med. Virol. 2022, 32, e2311. [Google Scholar] [PubMed]
- Kausar, S.; Said Khan, F.; Ishaq Mujeeb Ur Rehman, M.; Akram, M.; Riaz, M.; Rasool, G.; Hamid Khan, A.; Saleem, I.; Shamim, S.; Malik, A. A Review: Mechanism of Action of Antiviral Drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211002621. [Google Scholar] [CrossRef]
- Katze, M.G.; He, Y.; Gale, M. Viruses and Interferon: A Fight for Supremacy. Nat. Rev. Immunol. 2002, 2, 675–687. [Google Scholar] [CrossRef]
| Forward Primer (5′ → 3′) | Reverse Primer (5′ → 3′) | |
|---|---|---|
| GAPDH | GGTGGTCTCCTCTGACTTCAACA | GTTGCTGTAGCCAAATTCGTTGT |
| ICP0 | GTCGCCTTACGTGAACAAGAC | GTCGCCATGTTTCCCGTCTG |
| ICP4 | CGACACGGATCCACGACCC | GATCCCCCTCCCGCGCTTCGTCCG |
| ICP8 | CGACAGTAACGCCAGAAG | GGAGACAAAGCCCAAGAC |
| IFNα | AGAAGGCTCCAGCCATCTCTGT | TGCTGGTAGAGTTCGGTGCAGA |
| IFNβ | CTTGGATTCCTACAAAGAAGCAGC | TCCTCCTTCTGGAACTGCTGCA |
| IFNλ | AACTGGGAAGGGCTGCCACATT | GGAAGACAGGAGAGCTGCAACT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christfort, J.F.; Ortis, M.; Nguyen, H.V.; Marsault, R.; Doglio, A. Centrifugal Microfluidic Cell Culture Platform for Physiologically Relevant Virus Infection Studies: A Case Study with HSV-1 Infection of Periodontal Cells. Biosensors 2024, 14, 401. https://doi.org/10.3390/bios14080401
Christfort JF, Ortis M, Nguyen HV, Marsault R, Doglio A. Centrifugal Microfluidic Cell Culture Platform for Physiologically Relevant Virus Infection Studies: A Case Study with HSV-1 Infection of Periodontal Cells. Biosensors. 2024; 14(8):401. https://doi.org/10.3390/bios14080401
Chicago/Turabian StyleChristfort, Juliane Fjelrad, Morgane Ortis, Hau Van Nguyen, Robert Marsault, and Alain Doglio. 2024. "Centrifugal Microfluidic Cell Culture Platform for Physiologically Relevant Virus Infection Studies: A Case Study with HSV-1 Infection of Periodontal Cells" Biosensors 14, no. 8: 401. https://doi.org/10.3390/bios14080401
APA StyleChristfort, J. F., Ortis, M., Nguyen, H. V., Marsault, R., & Doglio, A. (2024). Centrifugal Microfluidic Cell Culture Platform for Physiologically Relevant Virus Infection Studies: A Case Study with HSV-1 Infection of Periodontal Cells. Biosensors, 14(8), 401. https://doi.org/10.3390/bios14080401





