Revolutionizing Drug Discovery: The Impact of Distinct Designs and Biosensor Integration in Microfluidics-Based Organ-on-a-Chip Technology
Abstract
:1. Introduction
2. Fabrication and Sensors of Organ-on-a-Chip
2.1. OOC Fabrication
2.2. Sensing Systems Implemented in OOC
3. Design of Organ-on-a-Chip
3.1. Single-Organ-on-a-Chip Systems
3.1.1. Lung-on-a-Chip
3.1.2. Heart-on-a-Chip
3.1.3. Liver-on-a-Chip
3.1.4. Other Single-Organ-on-a-Chip Systems
3.2. Multi-Organ-on-a-Chip Systems
3.2.1. Horizontal Design
3.2.2. Vertical Design
4. Applications of OOC in Biomedicine and Clinics
4.1. Disease Modeling and Drug Evaluation
4.2. Drug Screening and Discovery
4.3. Preclinical Studies
4.3.1. PD-PK Testing
4.3.2. Toxicology Testing
4.4. Precision Medicine in Clinics
5. Technical Challenges and Future Prospects
5.1. Cost and Manufacturing
5.2. Sensing Systems in OOC
5.3. Cell Source and Variability
5.4. Integration of the Immune System
5.5. Influence of OOC Nanostructures
5.6. Prospects for the Commercialization of OOC Technology
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Low, L.A.; Sutherland, M.; Lumelsky, N.; Selimovic, S.; Lundberg, M.S.; Tagle, D.A. Organs-on-a-Chip. In Biomaterials- and Microfluidics-Based Tissue Engineered 3D Models; Oliveira, J.M., Reis, R.L., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 27–42. ISBN 978-3-030-36588-2. [Google Scholar]
- Kaitin, K. Deconstructing the Drug Development Process: The New Face of Innovation. Clin. Pharmacol. Ther. 2010, 87, 356–361. [Google Scholar] [CrossRef]
- Singh, N.; Vayer, P.; Tanwar, S.; Poyet, J.-L.; Tsaioun, K.; Villoutreix, B.O. Drug Discovery and Development: Introduction to the General Public and Patient Groups. Front. Drug Discov. 2023, 3, 1201419. [Google Scholar] [CrossRef]
- Berdigaliyev, N.; Aljofan, M. An Overview of Drug Discovery and Development. Future Med. Chem. 2020, 12, 939–947. [Google Scholar] [CrossRef]
- Ma, C.; Peng, Y.; Li, H.; Chen, W. Organ-on-a-Chip: A New Paradigm for Drug Development. Trends Pharmacol. Sci. 2021, 42, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Ingber, D.E. Microfluidic Organs-on-Chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Hamon, M.; Hong, J.W. New Tools and New Biology: Recent Miniaturized Systems for Molecular and Cellular Biology. Mol. Cells 2013, 36, 485–506. [Google Scholar] [CrossRef]
- Yoon, S.; Kilicarslan You, D.; Jeong, U.; Lee, M.; Kim, E.; Jeon, T.-J.; Kim, S.M. Microfluidics in High-Throughput Drug Screening: Organ-on-a-Chip and C. Elegans-Based Innovations. Biosensors 2024, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Polaris. Organ-On-Chip Market Size, Growth & Revenue Report, 2024–2032. Available online: https://www.polarismarketresearch.com/industry-analysis/organ-on-chip-market (accessed on 4 June 2024).
- Leung, C.M.; de Haan, P.; Ronaldson-Bouchard, K.; Kim, G.-A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A Guide to the Organ-on-a-Chip. Nat. Rev. Methods Primer 2022, 2, 33. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Angew. Chem. Int. Ed. 1998, 37, 550–575. [Google Scholar] [CrossRef]
- Chen, X.; Shen, J.; Zhou, M. Rapid Fabrication of a Four-Layer PMMA-Based Microfluidic Chip Using CO2-Laser Micromachining and Thermal Bonding. J. Micromech. Microeng. 2016, 26, 107001. [Google Scholar] [CrossRef]
- When PDMS Isn’t the Best. Anal. Chem. 2007, 79, 3248–3253. [CrossRef] [PubMed]
- Liu, S.; Kumari, S.; He, H.; Mishra, P.; Singh, B.N.; Singh, D.; Liu, S.; Srivastava, P.; Li, C. Biosensors Integrated 3D Organoid/Organ-on-a-Chip System: A Real-Time Biomechanical, Biophysical, and Biochemical Monitoring and Characterization. Biosens. Bioelectron. 2023, 231, 115285. [Google Scholar] [CrossRef] [PubMed]
- Oleaga, C.; Riu, A.; Rothemund, S.; Lavado, A.; McAleer, C.W.; Long, C.J.; Persaud, K.; Narasimhan, N.S.; Tran, M.; Roles, J.; et al. Investigation of the Effect of Hepatic Metabolism on Off-Target Cardiotoxicity in a Multi-Organ Human-on-a-Chip System. Biomaterials 2018, 182, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Sabaté del Río, J.; Ro, J.; Yoon, H.; Park, T.-E.; Cho, Y.-K. Integrated Technologies for Continuous Monitoring of Organs-on-Chips: Current Challenges and Potential Solutions. Biosens. Bioelectron. 2023, 224, 115057. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Wang, Y.I.; Sriram, N.N.; Jackson, M.; Long, C.; Hickman, J.J.; Shuler, M.L. Recent Advances in Body-on-a-Chip Systems. Anal. Chem. 2019, 91, 330–351. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, N.; Desai, R.R.; Fleischman, A.J.; Roy, S.; Humes, H.D.; Fissell, W.H. A Microfluidic Bioreactor with Integrated Transepithelial Electrical Resistance (TEER) Measurement Electrodes for Evaluation of Renal Epithelial Cells. Biotechnol. Bioeng. 2010, 107, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Douville, N.J.; Tung, Y.-C.; Li, R.; Wang, J.D.; El-Sayed, M.E.H.; Takayama, S. Fabrication of Two-Layered Channel System with Embedded Electrodes to Measure Resistance Across Epithelial and Endothelial Barriers. Anal. Chem. 2010, 82, 2505–2511. [Google Scholar] [CrossRef]
- Odijk, M.; van der Meer, A.D.; Levner, D.; Kim, H.J.; van der Helm, M.W.; Segerink, L.I.; Frimat, J.-P.; Hamilton, G.A.; Ingber, D.E.; Berg, A. van den Measuring Direct Current Trans-Epithelial Electrical Resistance in Organ-on-a-Chip Microsystems. Lab Chip 2015, 15, 745–752. [Google Scholar] [CrossRef]
- Wu, Q.; Wei, X.; Pan, Y.; Zou, Y.; Hu, N.; Wang, P. Bionic 3D Spheroids Biosensor Chips for High-Throughput and Dynamic Drug Screening. Biomed. Microdevices 2018, 20, 82. [Google Scholar] [CrossRef]
- An, Y.; Jin, T.; Zhang, F.; He, P. Electric Cell-Substrate Impedance Sensing (ECIS) for Profiling Cytotoxicity of Cigarette Smoke. J. Electroanal. Chem. 2019, 834, 180–186. [Google Scholar] [CrossRef]
- Giaever, I.; Keese, C.R. A Morphological Biosensor for Mammalian Cells. Nature 1993, 366, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Lind, J.U.; Busbee, T.A.; Valentine, A.D.; Pasqualini, F.S.; Yuan, H.; Yadid, M.; Park, S.-J.; Kotikian, A.; Nesmith, A.P.; Campbell, P.H.; et al. Instrumented Cardiac Microphysiological Devices via Multimaterial Three-Dimensional Printing. Nat. Mater. 2017, 16, 303–308. [Google Scholar] [CrossRef]
- Lind, J.U.; Yadid, M.; Perkins, I.; O’Connor, B.B.; Eweje, F.; Chantre, C.O.; Hemphill, M.A.; Yuan, H.; Campbell, P.H.; Vlassak, J.J.; et al. Cardiac Microphysiological Devices with Flexible Thin-Film Sensors for Higher-Throughput Drug Screening. Lab Chip 2017, 17, 3692–3703. [Google Scholar] [CrossRef] [PubMed]
- Koutsouras, D.A.; Perrier, R.; Villarroel Marquez, A.; Pirog, A.; Pedraza, E.; Cloutet, E.; Renaud, S.; Raoux, M.; Malliaras, G.G.; Lang, J. Simultaneous Monitoring of Single Cell and of Micro-Organ Activity by PEDOT:PSS Covered Multi-Electrode Arrays. Mater. Sci. Eng. C 2017, 81, 84–89. [Google Scholar] [CrossRef]
- Maoz, B.M.; Herland, A.; Henry, O.Y.F.; Leineweber, W.D.; Yadid, M.; Doyle, J.; Mannix, R.; Kujala, V.J.; FitzGerald, E.A.; Parker, K.K.; et al. Organs-on-Chips with Combined Multi-Electrode Array and Transepithelial Electrical Resistance Measurement Capabilities. Lab Chip 2017, 17, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Ştefănescu, D.M. Strain Gauges and Wheatstone Bridges—Basic Instrumentation and New Applications for Electrical Measurement of Non-Electrical Quantities. In Proceedings of the Eighth International Multi-Conference on Systems, Signals & Devices, Sousse, Tunisia, 22–25 March 2011; pp. 1–5. [Google Scholar]
- Bavli, D.; Prill, S.; Ezra, E.; Levy, G.; Cohen, M.; Vinken, M.; Vanfleteren, J.; Jaeger, M.; Nahmias, Y. Real-Time Monitoring of Metabolic Function in Liver-on-Chip Microdevices Tracks the Dynamics of Mitochondrial Dysfunction. Proc. Natl. Acad. Sci. USA 2016, 113, E2231–E2240. [Google Scholar] [CrossRef] [PubMed]
- Misun, P.M.; Rothe, J.; Schmid, Y.R.F.; Hierlemann, A.; Frey, O. Multi-Analyte Biosensor Interface for Real-Time Monitoring of 3D Microtissue Spheroids in Hanging-Drop Networks. Microsyst. Nanoeng. 2016, 2, 16022. [Google Scholar] [CrossRef]
- Moya, A.; Ortega-Ribera, M.; Guimerà, X.; Sowade, E.; Zea, M.; Illa, X.; Ramon, E.; Villa, R.; Gracia-Sancho, J.; Gabriel, G. Online Oxygen Monitoring Using Integrated Inkjet-Printed Sensors in a Liver-on-a-Chip System. Lab Chip 2018, 18, 2023–2035. [Google Scholar] [CrossRef]
- Zirath, H.; Rothbauer, M.; Spitz, S.; Bachmann, B.; Jordan, C.; Müller, B.; Ehgartner, J.; Priglinger, E.; Mühleder, S.; Redl, H.; et al. Every Breath You Take: Non-Invasive Real-Time Oxygen Biosensing in Two- and Three-Dimensional Microfluidic Cell Models. Front. Physiol. 2018, 9, 815. [Google Scholar] [CrossRef]
- Wang, X.; Wolfbeis, O.S. Optical Methods for Sensing and Imaging Oxygen: Materials, Spectroscopies and Applications. Chem. Soc. Rev. 2014, 43, 3666–3761. [Google Scholar] [CrossRef]
- Prill, S.; Bavli, D.; Levy, G.; Ezra, E.; Schmälzlin, E.; Jaeger, M.S.; Schwarz, M.; Duschl, C.; Cohen, M.; Nahmias, Y. Real-Time Monitoring of Oxygen Uptake in Hepatic Bioreactor Shows CYP450-Independent Mitochondrial Toxicity of Acetaminophen and Amiodarone. Arch. Toxicol. 2016, 90, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Rennert, K.; Steinborn, S.; Gröger, M.; Ungerböck, B.; Jank, A.-M.; Ehgartner, J.; Nietzsche, S.; Dinger, J.; Kiehntopf, M.; Funke, H.; et al. A Microfluidically Perfused Three Dimensional Human Liver Model. Biomaterials 2015, 71, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical Sensors: Definitions and Classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Shrestha, J.; Ryan, S.T.; Mills, O.; Zhand, S.; Bazaz, S.R.; Hansbro, P.M.; Ghadiri, M.; Warkiani, M.E. A 3D-Printed Microfluidic Platform for Simulating the Effects of CPAP on the Nasal Epithelium. Biofabrication 2021, 13, 035028. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sun, L.; Wang, Y.; Cai, L.; Zhang, Z.; Shang, Y.; Zhao, Y. A Biomimetic Human Lung-on-a-Chip with Colorful Display of Microphysiological Breath. Adv. Mater. 2022, 34, 2108972. [Google Scholar] [CrossRef]
- Najjar, D.; Rainbow, J.; Sharma Timilsina, S.; Jolly, P.; de Puig, H.; Yafia, M.; Durr, N.; Sallum, H.; Alter, G.; Li, J.Z.; et al. A Lab-on-a-Chip for the Concurrent Electrochemical Detection of SARS-CoV-2 RNA and Anti-SARS-CoV-2 Antibodies in Saliva and Plasma. Nat. Biomed. Eng. 2022, 6, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Soler, M.; Szydzik, C.; Khoshmanesh, K.; Schmidt, J.; Coukos, G.; Mitchell, A.; Altug, H. Label-Free Optofluidic Nanobiosensor Enables Real-Time Analysis of Single-Cell Cytokine Secretion. Small 2018, 14, 1800698. [Google Scholar] [CrossRef]
- Zhang, F.; Qu, K.-Y.; Zhou, B.; Luo, Y.; Zhu, Z.; Pan, D.-J.; Cui, C.; Zhu, Y.; Chen, M.-L.; Huang, N.-P. Design and Fabrication of an Integrated Heart-on-a-Chip Platform for Construction of Cardiac Tissue from Human iPSC-Derived Cardiomyocytes and in Situ Evaluation of Physiological Function. Biosens. Bioelectron. 2021, 179, 113080. [Google Scholar] [CrossRef]
- Meissner, R.; Eker, B.; Kasi, H.; Bertsch, A.; Renaud, P. Distinguishing Drug-Induced Minor Morphological Changes from Major Cellular Damage via Label-Free Impedimetric Toxicity Screening. Lab Chip 2011, 11, 2352–2361. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, J.; Chen, J.; Dou, W.; Zhao, Q.; Han, J.; Liu, J.; Su, W.; Li, A.; Liu, P.; et al. Advances in Reconstructing Intestinal Functionalities in Vitro: From Two/Three Dimensional-Cell Culture Platforms to Human Intestine-on-a-Chip. Talanta 2021, 226, 122097. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Su, W.; Tan, M. Advances of Microfluidic Intestine-on-a-Chip for Analyzing Anti-Inflammation of Food. Crit. Rev. Food Sci. Nutr. 2022, 62, 4418–4434. [Google Scholar] [CrossRef] [PubMed]
- Zirath, H.; Spitz, S.; Roth, D.; Schellhorn, T.; Rothbauer, M.; Müller, B.; Walch, M.; Kaur, J.; Wörle, A.; Kohl, Y.; et al. Bridging the Academic–Industrial Gap: Application of an Oxygen and pH Sensor-Integrated Lab-on-a-Chip in Nanotoxicology. Lab Chip 2021, 21, 4237–4248. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Han, J.; Su, W.; Li, A.; Zhang, W.; Li, H.; Hu, H.; Song, W.; Xu, C.; Chen, J. Gut-on-a-Chip for Exploring the Transport Mechanism of Hg(II). Microsyst. Nanoeng. 2023, 9, 2. [Google Scholar] [CrossRef]
- Marrero, D.; Pujol-Vila, F.; Vera, D.; Gabriel, G.; Illa, X.; Elizalde-Torrent, A.; Alvarez, M.; Villa, R. Gut-on-a-Chip: Mimicking and Monitoring the Human Intestine. Biosens. Bioelectron. 2021, 181, 113156. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-G.; Chen, M.; Zhao, S.; Wang, X. Intestinal Models for Personalized Medicine: From Conventional Models to Microfluidic Primary Intestine-on-a-Chip. Stem Cell Rev. Rep. 2022, 18, 2137–2151. [Google Scholar] [CrossRef] [PubMed]
- Trietsch, S.J.; Naumovska, E.; Kurek, D.; Setyawati, M.C.; Vormann, M.K.; Wilschut, K.J.; Lanz, H.L.; Nicolas, A.; Ng, C.P.; Joore, J.; et al. Membrane-Free Culture and Real-Time Barrier Integrity Assessment of Perfused Intestinal Epithelium Tubes. Nat. Commun. 2017, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Bein, A.; Shin, W.; Jalili-Firoozinezhad, S.; Park, M.H.; Sontheimer-Phelps, A.; Tovaglieri, A.; Chalkiadaki, A.; Kim, H.J.; Ingber, D.E. Microfluidic Organ-on-a-Chip Models of Human Intestine. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 659–668. [Google Scholar] [CrossRef]
- Sciurti, E.; Blasi, L.; Prontera, C.T.; Barca, A.; Giampetruzzi, L.; Verri, T.; Siciliano, P.A.; Francioso, L. TEER and Ion Selective Transwell-Integrated Sensors System for Caco-2 Cell Model. Micromachines 2023, 14, 496. [Google Scholar] [CrossRef]
- Soscia, D.A.; Lam, D.; Tooker, A.C.; Enright, H.A.; Triplett, M.; Karande, P.; Peters, S.K.G.; Sales, A.P.; Wheeler, E.K.; Fischer, N.O. A Flexible 3-Dimensional Microelectrode Array for in Vitro Brain Models. Lab Chip 2020, 20, 901–911. [Google Scholar] [CrossRef]
- Cecen, B.; Saygili, E.; Zare, I.; Nejati, O.; Khorsandi, D.; Zarepour, A.; Alarcin, E.; Zarrabi, A.; Topkaya, S.N.; Yesil-Celiktas, O.; et al. Biosensor Integrated Brain-on-a-Chip Platforms: Progress and Prospects in Clinical Translation. Biosens. Bioelectron. 2023, 225, 115100. [Google Scholar] [CrossRef]
- Weltin, A.; Slotwinski, K.; Kieninger, J.; Moser, I.; Jobst, G.; Wego, M.; Ehret, R.; Urban, G.A. Cell Culture Monitoring for Drug Screening and Cancer Research: A Transparent, Microfluidic, Multi-Sensor Microsystem. Lab Chip 2013, 14, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-H.; Lin, S.-P.; Liang, C.-K.; Chen, J.-J.J. Impedimetric Monitoring of IGF-1 Protection of in Vitro Cortical Neurons under Ischemic Conditions. Biomed. Microdevices 2013, 15, 135–143. [Google Scholar] [CrossRef]
- Wang, Y.I.; Abaci, H.E.; Shuler, M.L. Microfluidic Blood-Brain Barrier Model Provides In Vivo-Like Barrier Properties for Drug Permeability Screening. Biotechnol. Bioeng. 2017, 114, 184–194. [Google Scholar] [CrossRef]
- Gehre, C.; Flechner, M.; Kammerer, S.; Küpper, J.-H.; Coleman, C.D.; Püschel, G.P.; Uhlig, K.; Duschl, C. Real Time Monitoring of Oxygen Uptake of Hepatocytes in a Microreactor Using Optical Microsensors. Sci. Rep. 2020, 10, 13700. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Z.; Tang, X.; Qin, J.; Jiang, Z. Recent Advances in Sensor-Integrated Brain-on-a-Chip Devices for Real-Time Brain Monitoring. Colloids Surf. B Biointerfaces 2023, 229, 113431. [Google Scholar] [CrossRef] [PubMed]
- Spitz, S.; Schobesberger, S.; Brandauer, K.; Ertl, P. Sensor-integrated Brain-on-a-chip Platforms: Improving the Predictive Validity in Neurodegenerative Research. Bioeng. Transl. Med. 2023, 9, e10604. [Google Scholar] [CrossRef]
- Marino, A.; Battaglini, M.; Lefevre, M.C.; Ceccarelli, M.C.; Ziaja, K.; Ciofani, G. Sensorization of Microfluidic Brain-on-a-Chip Devices: Towards a New Generation of Integrated Drug Screening Systems. Trends Anal. Chem. TRAC 2023, 168, 117319. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Luo, Z.; Lin, X.; Zhu, Y.; Zhao, Y. Sensors-Integrated Organ-on-a-Chip for Biomedical Applications. Nano Res. 2023, 16, 10072–10099. [Google Scholar] [CrossRef]
- Sukhodub, L.F. Interactions between nucleotide bases in coplanar and stacking dimers under vacuum. Mass spectrometric study. Biofizika 1987, 32, 994–1005. [Google Scholar]
- Biswas, C.; Nugent, M.A. Membrane Association of Collagenase Stimulatory Factor(s) from B-16 Melanoma Cells. J. Cell. Biochem. 1987, 35, 247–258. [Google Scholar] [CrossRef]
- Sutterby, E.; Thurgood, P.; Baratchi, S.; Khoshmanesh, K.; Pirogova, E. Microfluidic Skin-on-a-Chip Models: Toward Biomimetic Artificial Skin. Small 2020, 16, 2002515. [Google Scholar] [CrossRef]
- Risueño, I.; Valencia, L.; Jorcano, J.L.; Velasco, D. Skin-on-a-Chip Models: General Overview and Future Perspectives. APL Bioeng. 2021, 5, 030901. [Google Scholar] [CrossRef] [PubMed]
- Zoio, P.; Oliva, A. Skin-on-a-Chip Technology: Microengineering Physiologically Relevant In Vitro Skin Models. Pharmaceutics 2022, 14, 682. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Nadda, R.; Repaka, R. Advances and Challenges in Organ-on-Chip Technology: Toward Mimicking Human Physiology and Disease in Vitro. Med. Biol. Eng. Comput. 2024, 62, 1925–1957. [Google Scholar] [CrossRef]
- Torisawa, Y.; Mammoto, T.; Jiang, E.; Jiang, A.; Mammoto, A.; Watters, A.L.; Bahinski, A.; Ingber, D.E. Modeling Hematopoiesis and Responses to Radiation Countermeasures in a Bone Marrow-on-a-Chip. Tissue Eng. Part C Methods 2016, 22, 509–515. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Yuan Hsin, H.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, Q.; Jiang, A.; Wen, A.M.; Mannix, R.J.; Man, Y.; Hall, S.; Javorsky, E.; Ingber, D.E. A Human Lung Alveolus-on-a-Chip Model of Acute Radiation-Induced Lung Injury. Nat. Commun. 2023, 14, 6506. [Google Scholar] [CrossRef]
- Jalili-Firoozinezhad, S.; Gazzaniga, F.S.; Calamari, E.L.; Camacho, D.M.; Fadel, C.W.; Bein, A.; Swenor, B.; Nestor, B.; Cronce, M.J.; Tovaglieri, A.; et al. A Complex Human Gut Microbiome Cultured in an Anaerobic Intestine-on-a-Chip. Nat. Biomed. Eng. 2019, 3, 520–531. [Google Scholar] [CrossRef]
- Bein, A.; Fadel, C.W.; Swenor, B.; Cao, W.; Powers, R.K.; Camacho, D.M.; Naziripour, A.; Parsons, A.; LoGrande, N.; Sharma, S.; et al. Nutritional Deficiency in an Intestine-on-a-Chip Recapitulates Injury Hallmarks Associated with Environmental Enteric Dysfunction. Nat. Biomed. Eng. 2022, 6, 1236–1247. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, X.; Wen, X.; Wu, T.; Wang, W.; Yang, M.; Wang, J.; Fang, M.; Lin, B.; Lin, H. Development of a Functional Glomerulus at the Organ Level on a Chip to Mimic Hypertensive Nephropathy. Sci. Rep. 2016, 6, 31771. [Google Scholar] [CrossRef]
- Jang, K.-J.; Suh, K.-Y. A Multi-Layer Microfluidic Device for Efficient Culture and Analysis of Renal Tubular Cells. Lab Chip 2010, 10, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Ya, S.; Ding, W.; Li, S.; Du, K.; Zhang, Y.; Li, C.; Liu, J.; Li, F.; Li, P.; Luo, T.; et al. On-Chip Construction of Liver Lobules with Self-Assembled Perfusable Hepatic Sinusoid Networks. ACS Appl. Mater. Interfaces 2021, 13, 32640–32652. [Google Scholar] [CrossRef]
- Jang, K.-J.; Otieno, M.A.; Ronxhi, J.; Lim, H.-K.; Ewart, L.; Kodella, K.R.; Petropolis, D.B.; Kulkarni, G.; Rubins, J.E.; Conegliano, D.; et al. Reproducing Human and Cross-Species Drug Toxicities Using a Liver-Chip. Sci. Transl. Med. 2019, 11, eaax5516. [Google Scholar] [CrossRef] [PubMed]
- Rigat-Brugarolas, L.G.; Elizalde-Torrent, A.; Bernabeu, M.; Niz, M.D.; Martin-Jaular, L.; Fernandez-Becerra, C.; Homs-Corbera, A.; Samitier, J.; Portillo, H.A. del A Functional Microengineered Model of the Human Splenon-on-a-Chip. Lab Chip 2014, 14, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, Y.G.; Jung, H.-I.; Lim, J.S.; Nam, K.C.; Choi, H.S.; Kwak, B.S. Bone-on-a-Chip Simulating Bone Metastasis in Osteoporosis. Biofabrication 2024, 16, 045025. [Google Scholar] [CrossRef] [PubMed]
- Park, T.-E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.; Mannix, R.; FitzGerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M.; et al. Hypoxia-Enhanced Blood-Brain Barrier Chip Recapitulates Human Barrier Function and Shuttling of Drugs and Antibodies. Nat. Commun. 2019, 10, 2621. [Google Scholar] [CrossRef]
- Goyal, G.; Prabhala, P.; Mahajan, G.; Bausk, B.; Gilboa, T.; Xie, L.; Zhai, Y.; Lazarovits, R.; Mansour, A.; Kim, M.S.; et al. Ectopic Lymphoid Follicle Formation and Human Seasonal Influenza Vaccination Responses Recapitulated in an Organ-on-a-Chip. Adv. Sci. 2022, 9, 2103241. [Google Scholar] [CrossRef]
- Hansen, J.E.; Ampaya, E.P.; Bryant, G.H.; Navin, J.J. Branching Pattern of Airways and Air Spaces of a Single Human Terminal Bronchiole. J. Appl. Physiol. 1975, 38, 983–989. [Google Scholar] [CrossRef]
- Manickavel, S. Pathophysiology of Respiratory Failure and Physiology of Gas Exchange during ECMO. Indian J. Thorac. Cardiovasc. Surg. 2021, 37, 203–209. [Google Scholar] [CrossRef]
- Shrestha, J.; Razavi Bazaz, S.; Aboulkheyr Es, H.; Yaghobian Azari, D.; Thierry, B.; Ebrahimi Warkiani, M.; Ghadiri, M. Lung-on-a-Chip: The Future of Respiratory Disease Models and Pharmacological Studies. Crit. Rev. Biotechnol. 2020, 40, 213–230. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Han, M.; Long, M.; Li, T.; Hu, L.; Wang, L.; Huang, W.; Wu, Y. Engineering Cardiac Tissue for Advanced Heart-on-a-Chip Platforms. Adv. Healthc. Mater. 2024, 13, 2301338. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.W.; Lee, W.H.; Kim, B.-S.; Kim, D.-H. Sensors in Heart-on-a-Chip: A Review on Recent Progress. Talanta 2020, 219, 121269. [Google Scholar] [CrossRef]
- Criscione, J.; Rezaei, Z.; Hernandez Cantu, C.M.; Murphy, S.; Shin, S.R.; Kim, D.-H. Heart-on-a-Chip Platforms and Biosensor Integration for Disease Modeling and Phenotypic Drug Screening. Biosens. Bioelectron. 2023, 220, 114840. [Google Scholar] [CrossRef]
- Zimmermann, W.-H.; Schneiderbanger, K.; Schubert, P.; Didié, M.; Münzel, F.; Heubach, J.F.; Kostin, S.; Neuhuber, W.L.; Eschenhagen, T. Tissue Engineering of a Differentiated Cardiac Muscle Construct. Circ. Res. 2002, 90, 223–230. [Google Scholar] [CrossRef]
- Ošt̀ádalová, I.; Kolář, F.; Ošt̀ádal, B.; Rohlíček, V.; Rohlíček, J.; Procházka, J. Early Postnatal Development of Contractile Performance and Responsiveness to Ca2+, Verapamil and Ryanodine in the Isolated Rat Heart. J. Mol. Cell. Cardiol. 1993, 25, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Bannerman, D.; Pascual-Gil, S.; Wu, Q.; Fernandes, I.; Zhao, Y.; Wagner, K.T.; Okhovatian, S.; Landau, S.; Rafatian, N.; Bodenstein, D.F.; et al. Heart-on-a-Chip Model of Epicardial–Myocardial Interaction in Ischemia Reperfusion Injury. Adv. Healthc. Mater. 2024, 13, e2302642. [Google Scholar] [CrossRef]
- Abdel-Misih, S.R.Z.; Bloomston, M. Liver Anatomy. Surg. Clin. N. Am. 2010, 90, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Kruepunga, N.; Hakvoort, T.B.M.; Hikspoors, J.P.J.M.; Köhler, S.E.; Lamers, W.H. Anatomy of Rodent and Human Livers: What Are the Differences? Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2019, 1865, 869–878. [Google Scholar] [CrossRef]
- Liu, M.; Xiang, Y.; Yang, Y.; Long, X.; Xiao, Z.; Nan, Y.; Jiang, Y.; Qiu, Y.; Huang, Q.; Ai, K. State-of-the-Art Advancements in Liver-on-a-Chip (LOC): Integrated Biosensors for LOC. Biosens. Bioelectron. 2022, 218, 114758. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.; Xie, L.; Xing, W. A Pumpless Liver-on-a-Chip for Drug Hepatotoxicity Analysis. Analyst 2024. advance article. [Google Scholar] [CrossRef]
- Wang, D.; Gust, M.; Ferrell, N. Kidney-on-a-Chip: Mechanical Stimulation and Sensor Integration. Sensors 2022, 22, 6889. [Google Scholar] [CrossRef] [PubMed]
- de Mello, C.P.P.; Carmona-Moran, C.; McAleer, C.W.; Perez, J.; Coln, E.A.; Long, C.J.; Oleaga, C.; Riu, A.; Note, R.; Teissier, S.; et al. Microphysiological Heart-Liver Body-on-a-Chip System with Skin Mimic for Evaluating Topical Drug Delivery. Lab Chip 2020, 20, 749–759. [Google Scholar] [CrossRef]
- Maschmeyer, I.; Lorenz, A.K.; Schimek, K.; Hasenberg, T.; Ramme, A.P.; Hübner, J.; Lindner, M.; Drewell, C.; Bauer, S.; Thomas, A.; et al. A Four-Organ-Chip for Interconnected Long-Term Co-Culture of Human Intestine, Liver, Skin and Kidney Equivalents. Lab Chip 2015, 15, 2688–2699. [Google Scholar] [CrossRef]
- Theobald, J.; Ghanem, A.; Wallisch, P.; Banaeiyan, A.A.; Andrade-Navarro, M.A.; Taškova, K.; Haltmeier, M.; Kurtz, A.; Becker, H.; Reuter, S.; et al. Liver-Kidney-on-Chip To Study Toxicity of Drug Metabolites. ACS Biomater. Sci. Eng. 2018, 4, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Beger, R.D.; Sun, J.; Schnackenberg, L.K. Metabolomics Approaches for Discovering Biomarkers of Drug-Induced Hepatotoxicity and Nephrotoxicity. Toxicol. Appl. Pharmacol. 2010, 243, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The Liver. Curr. Biol. CB 2017, 27, R1147–R1151. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, T.; Song, Y.; Sun, W.; Xu, J.; Cheng, Y.; Yin, R.; Zhu, L.; Zhang, M.; Ma, L.; et al. A Three-Dimensional (3D) Liver–Kidney on a Chip with a Biomimicking Circulating System for Drug Safety Evaluation. Lab Chip 2024, 24, 1715–1726. [Google Scholar] [CrossRef] [PubMed]
- Monteduro, A.G.; Rizzato, S.; Caragnano, G.; Trapani, A.; Giannelli, G.; Maruccio, G. Organs-on-Chips Technologies—A Guide from Disease Models to Opportunities for Drug Development. Biosens. Bioelectron. 2023, 231, 115271. [Google Scholar] [CrossRef]
- Jie, M.; Mao, S.; Liu, H.; He, Z.; Li, H.-F.; Lin, J.-M. Evaluation of Drug Combination for Glioblastoma Based on an Intestine–Liver Metabolic Model on Microchip. Analyst 2017, 142, 3629–3638. [Google Scholar] [CrossRef]
- Tanimizu, N.; Ichinohe, N.; Sasaki, Y.; Itoh, T.; Sudo, R.; Yamaguchi, T.; Katsuda, T.; Ninomiya, T.; Tokino, T.; Ochiya, T.; et al. Generation of Functional Liver Organoids on Combining Hepatocytes and Cholangiocytes with Hepatobiliary Connections Ex Vivo. Nat. Commun. 2021, 12, 3390. [Google Scholar] [CrossRef]
- Zhou, S.-F.; Zhong, W.-Z. Drug Design and Discovery: Principles and Applications. Mol. J. Synth. Chem. Nat. Prod. Chem. 2017, 22, 279. [Google Scholar] [CrossRef] [PubMed]
- Marturano-Kruik, A.; Nava, M.M.; Yeager, K.; Chramiec, A.; Hao, L.; Robinson, S.; Guo, E.; Raimondi, M.T.; Vunjak-Novakovic, G. Human Bone Perivascular Niche-on-a-Chip for Studying Metastatic Colonization. Proc. Natl. Acad. Sci. USA 2018, 115, 1256–1261. [Google Scholar] [CrossRef]
- Toh, Y.-C.; Raja, A.; Yu, H.; van Noort, D. A 3D Microfluidic Model to Recapitulate Cancer Cell Migration and Invasion. Bioengineering 2018, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Imparato, G.; Urciuolo, F.; Netti, P.A. Organ on Chip Technology to Model Cancer Growth and Metastasis. Bioengineering 2022, 9, 28. [Google Scholar] [CrossRef]
- Regmi, S.; Poudel, C.; Adhikari, R.; Luo, K.Q. Applications of Microfluidics and Organ-on-a-Chip in Cancer Research. Biosensors 2022, 12, 459. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.T.; Dolgalev, I.; Evensen, N.A.; Ma, C.; Chambers, T.; Roberts, K.G.; Sreeram, S.; Dai, Y.; Tikhonova, A.N.; Lasry, A.; et al. Extensive Remodeling of the Immune Microenvironment in B-Cell Acute Lymphoblastic Leukemia. Cancer Cell 2020, 37, 867–882.e12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Aleman, J.; Shin, S.R.; Kilic, T.; Kim, D.; Mousavi Shaegh, S.A.; Massa, S.; Riahi, R.; Chae, S.; Hu, N.; et al. Multisensor-Integrated Organs-on-Chips Platform for Automated and Continual in Situ Monitoring of Organoid Behaviors. Proc. Natl. Acad. Sci. USA 2017, 114, E2293–E2302. [Google Scholar] [CrossRef]
- Jalili-Firoozinezhad, S.; Prantil-Baun, R.; Jiang, A.; Potla, R.; Mammoto, T.; Weaver, J.C.; Ferrante, T.C.; Kim, H.J.; Cabral, J.M.S.; Levy, O.; et al. Modeling Radiation Injury-Induced Cell Death and Countermeasure Drug Responses in a Human Gut-on-a-Chip. Cell Death Dis. 2018, 9, 223. [Google Scholar] [CrossRef]
- Liu, Y.; Sakolish, C.; Chen, Z.; Phan, D.T.T.; Bender, R.H.F.; Hughes, C.C.W.; Rusyn, I. Human In Vitro Vascularized Micro-Organ and Micro-Tumor Models Are Reproducible Organ-on-a-Chip Platforms for Studies of Anticancer Drugs. Toxicology 2020, 445, 152601. [Google Scholar] [CrossRef]
- Herland, A.; Maoz, B.M.; Das, D.; Somayaji, M.R.; Prantil-Baun, R.; Novak, R.; Cronce, M.; Huffstater, T.; Jeanty, S.S.F.; Ingram, M.; et al. Quantitative Prediction of Human Pharmacokinetic Responses to Drugs via Fluidically Coupled Vascularized Organ Chips. Nat. Biomed. Eng. 2020, 4, 421–436. [Google Scholar] [CrossRef]
- Yan, J.; Li, Z.; Guo, J.; Liu, S.; Guo, J. Organ-on-a-Chip: A New Tool for in Vitro Research. Biosens. Bioelectron. 2022, 216, 114626. [Google Scholar] [CrossRef] [PubMed]
- Ajalik, R.E.; Alenchery, R.G.; Cognetti, J.S.; Zhang, V.Z.; McGrath, J.L.; Miller, B.L.; Awad, H.A. Human Organ-on-a-Chip Microphysiological Systems to Model Musculoskeletal Pathologies and Accelerate Therapeutic Discovery. Front. Bioeng. Biotechnol. 2022, 10, 846230. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Mishra, A.; Mathur, A.; Shastri, S.; Nizam, A.; Rizwan, A.; Dadial, A.S.; Firdous, A.; Hassan, H. Advancement of Organ-on-Chip towards next Generation Medical Technology. Biosens. Bioelectron. X 2024, 18, 100480. [Google Scholar] [CrossRef]
- Novak, R.; Ingram, M.; Marquez, S.; Das, D.; Delahanty, A.; Herland, A.; Maoz, B.M.; Jeanty, S.S.F.; Somayaji, M.R.; Burt, M.; et al. Robotic Fluidic Coupling and Interrogation of Multiple Vascularized Organ Chips. Nat. Biomed. Eng. 2020, 4, 407–420. [Google Scholar] [CrossRef]
- Kratz, S.R.A.; Höll, G.; Schuller, P.; Ertl, P.; Rothbauer, M. Latest Trends in Biosensing for Microphysiological Organs-on-a-Chip and Body-on-a-Chip Systems. Biosensors 2019, 9, 110. [Google Scholar] [CrossRef]
- Olsson, B.; Bondesson, E.; Borgström, L.; Edsbäcker, S.; Eirefelt, S.; Ekelund, K.; Gustavsson, L.; Hegelund-Myrbäck, T. Pulmonary Drug Metabolism, Clearance, and Absorption. In Controlled Pulmonary Drug Delivery; Smyth, H.D.C., Hickey, A.J., Eds.; Springer: New York, NY, USA, 2011; pp. 21–50. ISBN 978-1-4419-9745-6. [Google Scholar]
- Barros, A.S.; Costa, A.; Sarmento, B. Building Three-Dimensional Lung Models for Studying Pharmacokinetics of Inhaled Drugs. Adv. Drug Deliv. Rev. 2021, 170, 386–395. [Google Scholar] [CrossRef]
- Ingber, D.E. Human Organs-on-Chips for Disease Modelling, Drug Development and Personalized Medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef] [PubMed]
- Abaci, H.E.; Shuler, M.L. Human-on-a-Chip Design Strategies and Principles for Physiologically Based Pharmocokinetics/Pharmacodynamics Modeling. Integr. Biol. Quant. Biosci. Nano Macro 2015, 7, 383–391. [Google Scholar] [CrossRef]
- Sung, J.H.; Kam, C.; Shuler, M.L. A Microfluidic Device for a Pharmacokinetic–Pharmacodynamic (PK–PD) Model on a Chip. Lab Chip 2010, 10, 446–455. [Google Scholar] [CrossRef]
- Day, C.-P.; Merlino, G.; Van Dyke, T. Preclinical Mouse Cancer Models: A Maze of Opportunities and Challenges. Cell 2015, 163, 39–53. [Google Scholar] [CrossRef]
- Ahmed, T. Organ-on-a-Chip Microengineering for Bio-Mimicking Disease Models and Revolutionizing Drug Discovery. Biosens. Bioelectron. X 2022, 11, 100194. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, L.; Zhou, E.-M.; Xu, J.; Shen, S.; Wang, J. On-Chip Construction of Liver Lobule-like Microtissue and Its Application for Adverse Drug Reaction Assay. Anal. Chem. 2016, 88, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Benam, K.H.; Villenave, R.; Lucchesi, C.; Varone, A.; Hubeau, C.; Lee, H.-H.; Alves, S.E.; Salmon, M.; Ferrante, T.C.; Weaver, J.C.; et al. Small Airway-on-a-Chip Enables Analysis of Human Lung Inflammation and Drug Responses in Vitro. Nat. Methods 2016, 13, 151–157. [Google Scholar] [CrossRef]
- Vatine, G.D.; Barrile, R.; Workman, M.J.; Sances, S.; Barriga, B.K.; Rahnama, M.; Barthakur, S.; Kasendra, M.; Lucchesi, C.; Kerns, J.; et al. Human iPSC-Derived Blood-Brain Barrier Chips Enable Disease Modeling and Personalized Medicine Applications. Cell Stem Cell 2019, 24, 995–1005.e6. [Google Scholar] [CrossRef]
- van den Berg, A.; Mummery, C.L.; Passier, R.; van der Meer, A.D. Personalised Organs-on-Chips: Functional Testing for Precision Medicine. Lab Chip 2019, 19, 198–205. [Google Scholar] [CrossRef]
- Cloughesy, T.F.; Mochizuki, A.Y.; Orpilla, J.R.; Hugo, W.; Lee, A.H.; Davidson, T.B.; Wang, A.C.; Ellingson, B.M.; Rytlewski, J.A.; Sanders, C.M.; et al. Neoadjuvant Anti-PD-1 Immunotherapy Promotes a Survival Benefit with Intratumoral and Systemic Immune Responses in Recurrent Glioblastoma. Nat. Med. 2019, 25, 477–486. [Google Scholar] [CrossRef]
- Cui, X.; Ma, C.; Vasudevaraja, V.; Serrano, J.; Tong, J.; Peng, Y.; Delorenzo, M.; Shen, G.; Frenster, J.; Morales, R.-T.T.; et al. Dissecting the Immunosuppressive Tumor Microenvironments in Glioblastoma-on-a-Chip for Optimized PD-1 Immunotherapy. eLife 2020, 9, e52253. [Google Scholar] [CrossRef]
- Wang, X.; Scarfò, I.; Schmidts, A.; Toner, M.; Maus, M.V.; Irimia, D. Dynamic Profiling of Antitumor Activity of CAR T Cells Using Micropatterned Tumor Arrays. Adv. Sci. 2019, 6, 1901829. [Google Scholar] [CrossRef]
- Chliara, M.A.; Elezoglou, S.; Zergioti, I. Bioprinting on Organ-on-Chip: Development and Applications. Biosensors 2022, 12, 1135. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy—Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef]
- Ramme, A.P.; Koenig, L.; Hasenberg, T.; Schwenk, C.; Magauer, C.; Faust, D.; Lorenz, A.K.; Krebs, A.-C.; Drewell, C.; Schirrmann, K.; et al. Autologous Induced Pluripotent Stem Cell-Derived Four-Organ-Chip. Future Sci. OA 2019, 5, FSO413. [Google Scholar] [CrossRef] [PubMed]
- Lucendo-Villarin, B.; Meseguer-Ripolles, J.; Drew, J.; Fischer, L.; Ma, E.; Flint, O.; Simpson, K.J.; Machesky, L.M.; Mountford, J.C.; Hay, D.C. Development of a Cost-Effective Automated Platform to Produce Human Liver Spheroids for Basic and Applied Research. Biofabrication 2020, 13, 015009. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-P.; Li, Y.-M.; Guo, N.-N.; Li, S.; Ma, X.; Zhang, Y.-X.; Gao, Y.; Huang, J.-L.; Zheng, D.-X.; Wang, L.-Y.; et al. Therapeutic Potential of Patient iPSC-Derived iMelanocytes in Autologous Transplantation. Cell Rep. 2019, 27, 455–466.e5. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Liu, C.-L.; Ting, C.-Y.; Chiu, Y.-T.; Cheng, Y.-C.; Nicholson, M.W.; Hsieh, P.C.H. Human iPSC Banking: Barriers and Opportunities. J. Biomed. Sci. 2019, 26, 87. [Google Scholar] [CrossRef]
- Ferrari, E.; Palma, C.; Vesentini, S.; Occhetta, P.; Rasponi, M. Integrating Biosensors in Organs-on-Chip Devices: A Perspective on Current Strategies to Monitor Microphysiological Systems. Biosensors 2020, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of Surface Energy and Roughness on Cell Adhesion and Growth–Facile Surface Modification for Enhanced Cell Culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lian, M.; Wu, Q.; Qiao, Z.; Sun, B.; Dai, K. Effect of Pore Size on Cell Behavior Using Melt Electrowritten Scaffolds. Front. Bioeng. Biotechnol. 2021, 9, 629270. [Google Scholar] [CrossRef]
- Busek, M.; Aizenshtadt, A.; Amirola-Martinez, M.; Delon, L.; Krauss, S. Academic User View: Organ-on-a-Chip Technology. Biosensors 2022, 12, 126. [Google Scholar] [CrossRef]
- da Silva, R.G.L.; Blasimme, A. Organ Chip Research in Europe: Players, Initiatives, and Policies. Front. Bioeng. Biotechnol. 2023, 11, 1237561. [Google Scholar] [CrossRef]
- Nolan, J.; Pearce, O.M.T.; Screen, H.R.C.; Knight, M.M.; Verbruggen, S.W. Organ-on-a-Chip and Microfluidic Platforms for Oncology in the UK. Cancers 2023, 15, 635. [Google Scholar] [CrossRef]
- Directive-2010/63-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32010L0063 (accessed on 19 August 2024).

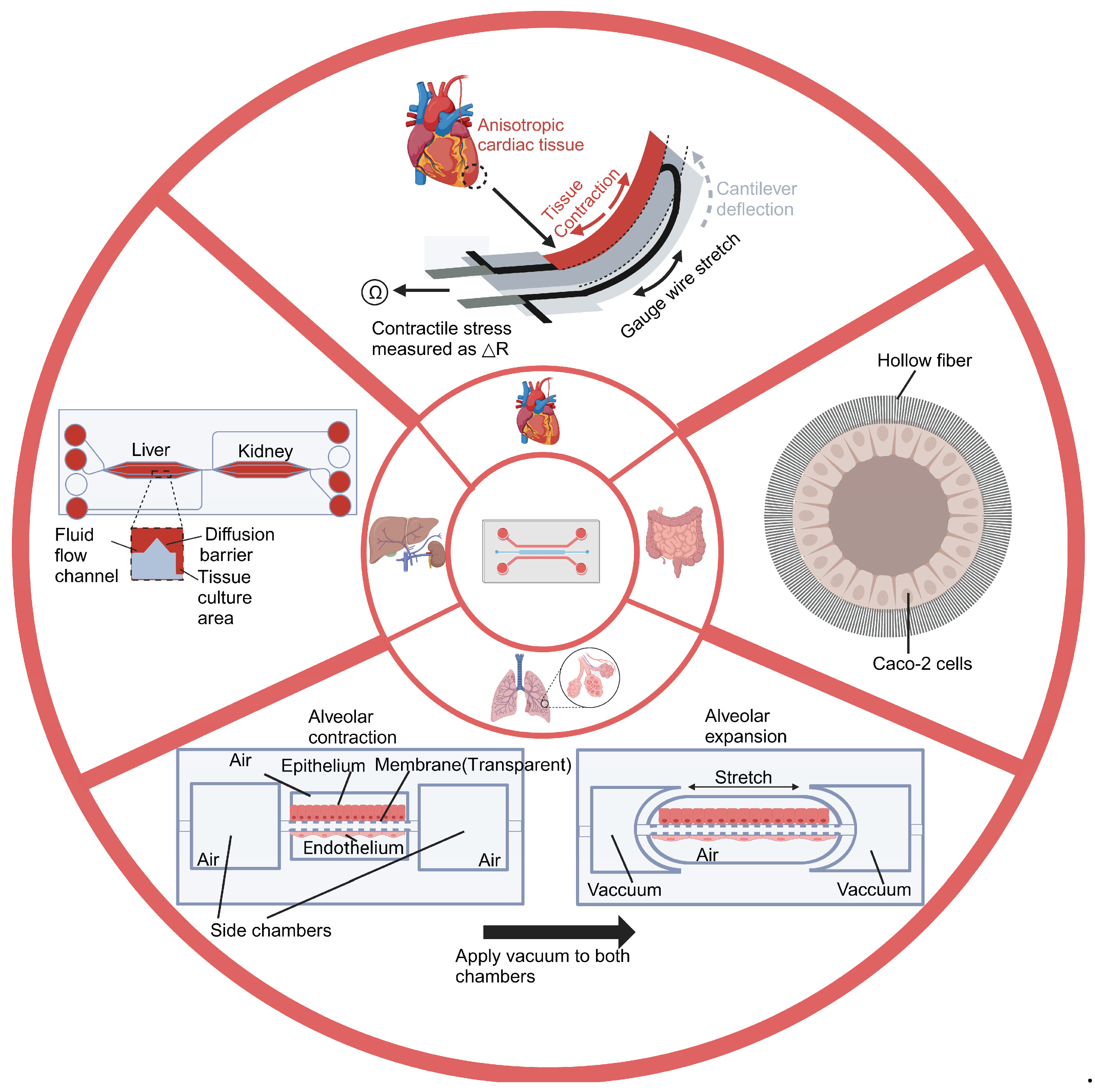



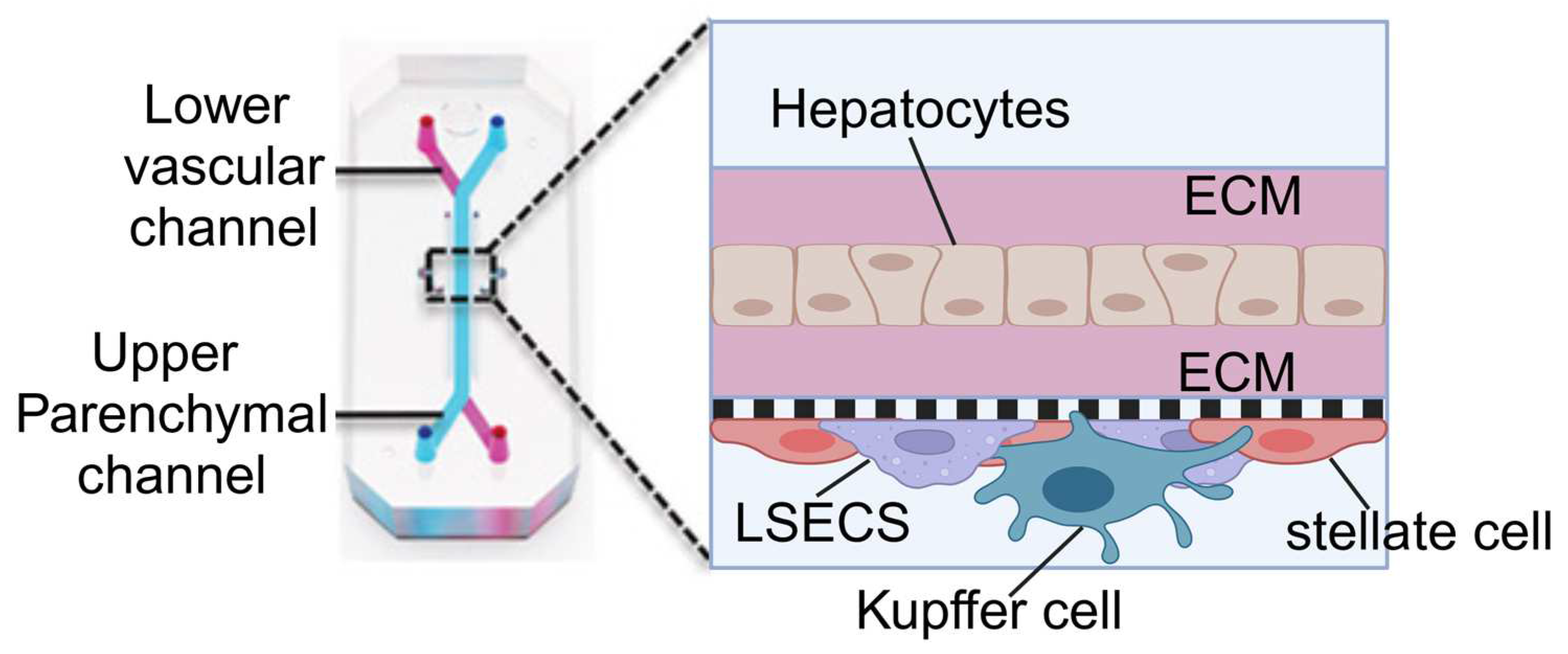

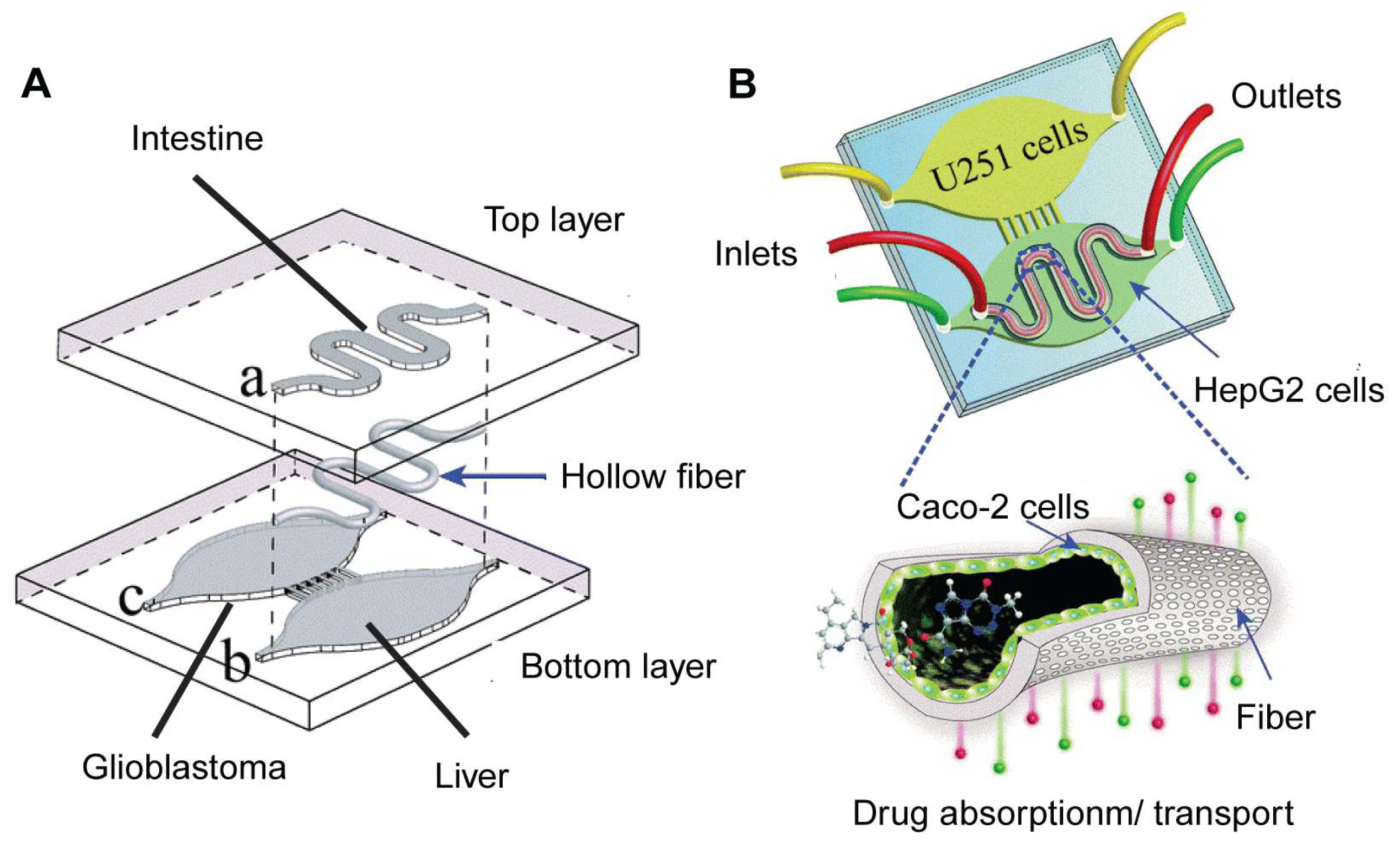
| OOC Platform | Measurements | Sensors | Applications | Reference |
|---|---|---|---|---|
| Lung | The barrier integrity of the cells, the secretion of inflammatory markers, Mechanical stress, and changes in cell mechanics | Trans epithelial electrical resistance (TEER) measurement, sodium fluorescein permeability test, ELISA and ATP luminescence assay, and special material that changes color in sync with air pressure | Lung disease models, drug evaluation, mechanical stretching effect | [37,38,39] |
| Heart | Electrophysiological signals and mechanical contractions of cardiac tissue, and dynamic tissue beating pulse | Microelectrode arrays (MEAs), piezoresistive sensors, calcium transient dye, optical sensing technology, and nanowire probe | Drug evaluation, cardiotoxicity detection | [24,25,40,41] |
| Liver | Oxygen concentration, cell growth population | Electrochemical dissolved oxygen sensors produced by inkjet printing technology, electrochemical impedance spectroscopy, and amperometric sensors | Metabolic activity monitoring, hepatotoxicity tests | [29,30,31,42] |
| Intestine | pH, oxygen, temperature, barrier integrity, ion flow resistance, sequential impedance measurement and cell migration | Fluorescent probes, TEER sensors, electrochemical sensors, electrical cell-impedance sensors, monitoring sensors | Barrier function test, ion transport monitoring, anti-inflammation test, human disease models | [43,44,45,46,47,48,49,50,51] |
| Brain | pH, oxygen, temperature, shear stress, secreted molecules (e.g., cytokines, insulin), blood flow, cell viability, cell-cell interactions, and BBB crossing of drugs and nanoparticles | MEA, External sensor-integrated BOC (TEER measurement and multi-parameter measurement), and internal sensor-integrated BOC (microelectrode arrays and multi-sensor integration platform) | Real-time brain activity monitoring, neurodegenerative disease model, drug development and screening, pre-clinical test of novel therapies | [32,52,53,54,55,56,57,58,59,60,61] |
| Skin | pH, oxygen, temperature, tight junction formation | Optical pH, oxygen and temperature monitors, TEER sensors, electrochemically activated immune biosensors attached to physical microelectrodes | Skin barrier function test, drug evaluation, toxicity test, biomimetic artificial skin model | [62,63,64,65,66] |
| Organ Type | Special Structure | Morphological Simulation | Environmental Simulation | Special Indicator Tests | General Indicator Tests | References |
|---|---|---|---|---|---|---|
| Lung | Alveoli | Dynamic deformation and gas exchange between alveoli and capillaries | Simulating the gas exchange environment during respiration | Gas exchange efficiency | Temperature, pH, oxygen concentration, cell viability, etc. | [69,70] |
| Heart | Myocardial tissue | Periodic mechanical contraction of heart tissue | Simulating the electrophysiological environment and mechanical stress during heartbeats | Contraction stress of heart tissue, electrophysiological parameters | [24] | |
| Intestine | Intestinal epithelial cells | Periodic mechanical contraction of the intestine; interaction among intestinal epithelial cells, vascular endothelial cells, and microbiome | Simulating the chemical environment inside the intestine, including pH and microbial communities | Barrier function, microbiome balance, inflammation markers | [71,72] | |
| Kidney | Glomerulus | Imitates the filtering action of the kidney glomerulus | Simulating fluid flow, electrolyte concentration gradient, and pressure changes | Glomerular filtration rate, metabolite concentration, renal tubule reabsorption function | [73,74] | |
| Renal tubules | Imitating the reabsorption function of the nephron | |||||
| Liver | Liver lobule | Imitating the special shape of the liver lobules and the multiple blood vessels through the liver lobules | Simulating the liver’s metabolic environment, including oxygen concentration, nutrient, and metabolite concentrations | Metabolic activity, toxicity response, liver enzyme activity | [75,76] | |
| Hepatic Sinus | Cultivation of endothelial cells from perforated, discontinuous hepatic sinusoids and associated macrophages | |||||
| Spleen | Spleen red pulp | Imitates the red bone marrow, stores red blood cells and white blood cells, and screens for healthy red blood cells | Simulating the closed-fast and open-slow microcirculation in the spleen | Mechanical and physiological responses of red blood cells | [77] | |
| Bone | Bone marrow | Three-dimensional bone tissue and bone marrow cavities to mimic the spatial layout of bone | The hematopoietic microenvironment includes stromal cells that support hematopoietic stem cells, vascular networks, signaling molecules and cytokines that regulate cell activity, marrow signaling molecules, and cytokines that regulate cell activity. | Hematopoietic function, cell type, cytokine level | [68] | |
| Osteoblasts, osteocytes, and osteoclasts | By adjusting the ratio of osteoblasts, osteocytes, and osteoclasts, different bone conditions can be simulated. | The permeability of the vascular system under different bone conditions is simulated through a simulated vascular channel lined with endothelial cells. | Cell co-culture ratio, vascular permeability, tissue mineralization level | [78] | ||
| Brain | Blood–brain barrier | Cultured human brain microvascular endothelial cells, human brain astrocytes, and pericytes formed a blood–brain barrier | Simulates the hypoxic microenvironment with less oxygen that the blood–brain barrier is exposed to during development. | TEER, apparent permeability, tight junction protein expression, efflux pump function, | [79] | |
| Lymphatic system | Lymphoid follicle | Using B and T cells, ectopic lymphoid follicles were simulated in 3D extracellular matrix gel | 3D extracellular matrix gel as a platform for the spontaneous assembly of ectopic lymphoid follicles. | Lymphoid follicle formation and number, B cell activation status, cytokine secretion | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.; Yuan, H.; Hay, D.C.; Hu, H.; Wang, C. Revolutionizing Drug Discovery: The Impact of Distinct Designs and Biosensor Integration in Microfluidics-Based Organ-on-a-Chip Technology. Biosensors 2024, 14, 425. https://doi.org/10.3390/bios14090425
Yuan S, Yuan H, Hay DC, Hu H, Wang C. Revolutionizing Drug Discovery: The Impact of Distinct Designs and Biosensor Integration in Microfluidics-Based Organ-on-a-Chip Technology. Biosensors. 2024; 14(9):425. https://doi.org/10.3390/bios14090425
Chicago/Turabian StyleYuan, Sheng, Huipu Yuan, David C. Hay, Huan Hu, and Chaochen Wang. 2024. "Revolutionizing Drug Discovery: The Impact of Distinct Designs and Biosensor Integration in Microfluidics-Based Organ-on-a-Chip Technology" Biosensors 14, no. 9: 425. https://doi.org/10.3390/bios14090425
APA StyleYuan, S., Yuan, H., Hay, D. C., Hu, H., & Wang, C. (2024). Revolutionizing Drug Discovery: The Impact of Distinct Designs and Biosensor Integration in Microfluidics-Based Organ-on-a-Chip Technology. Biosensors, 14(9), 425. https://doi.org/10.3390/bios14090425







