A Cost-Effective and Easy-to-Fabricate Conductive Velcro Dry Electrode for Durable and High-Performance Biopotential Acquisition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Impedance Measurement
2.3. SNR Calculation
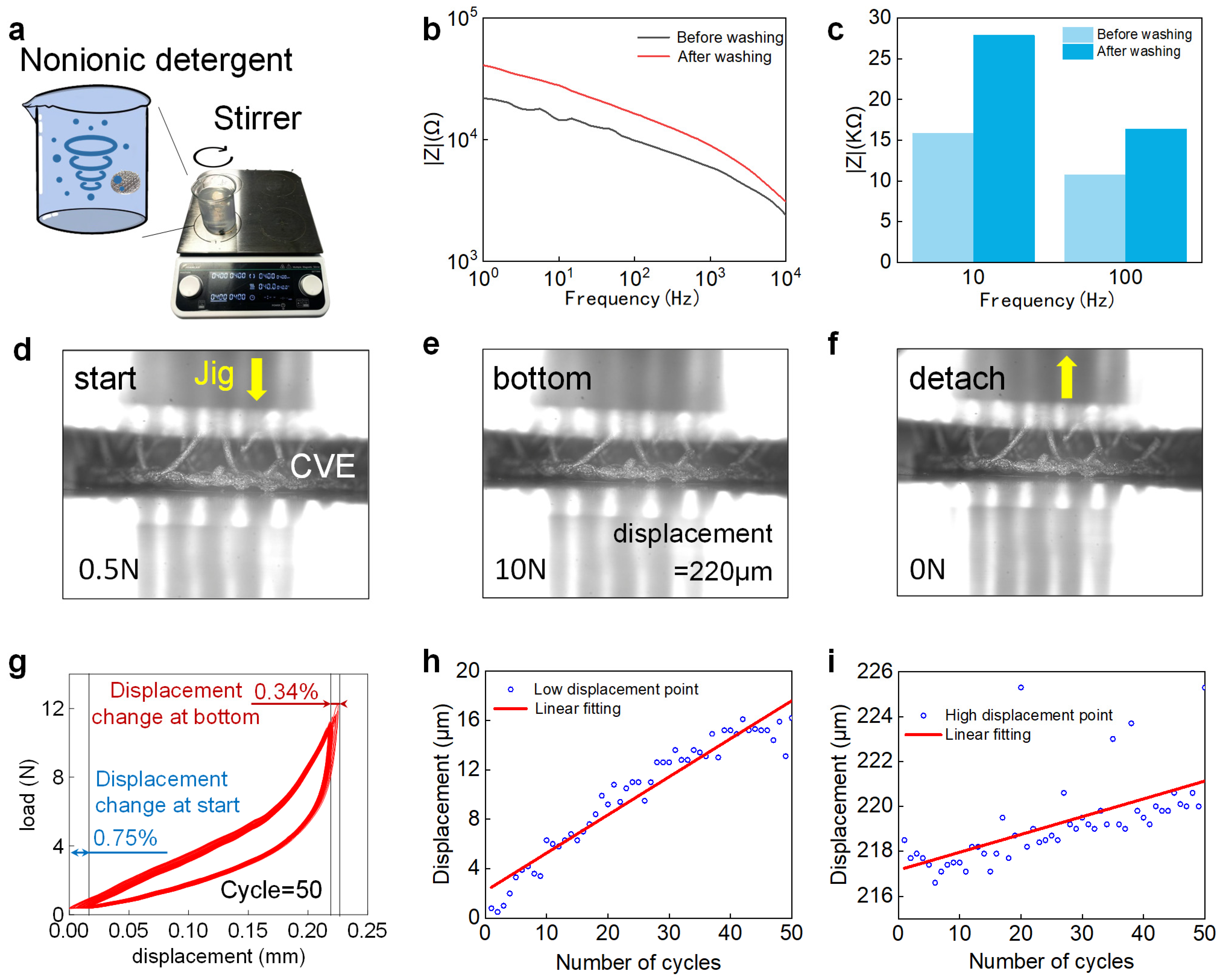
2.4. Water Resistance Test and Compression Resistance Testing
2.5. Gesture Recognition Method
3. Results
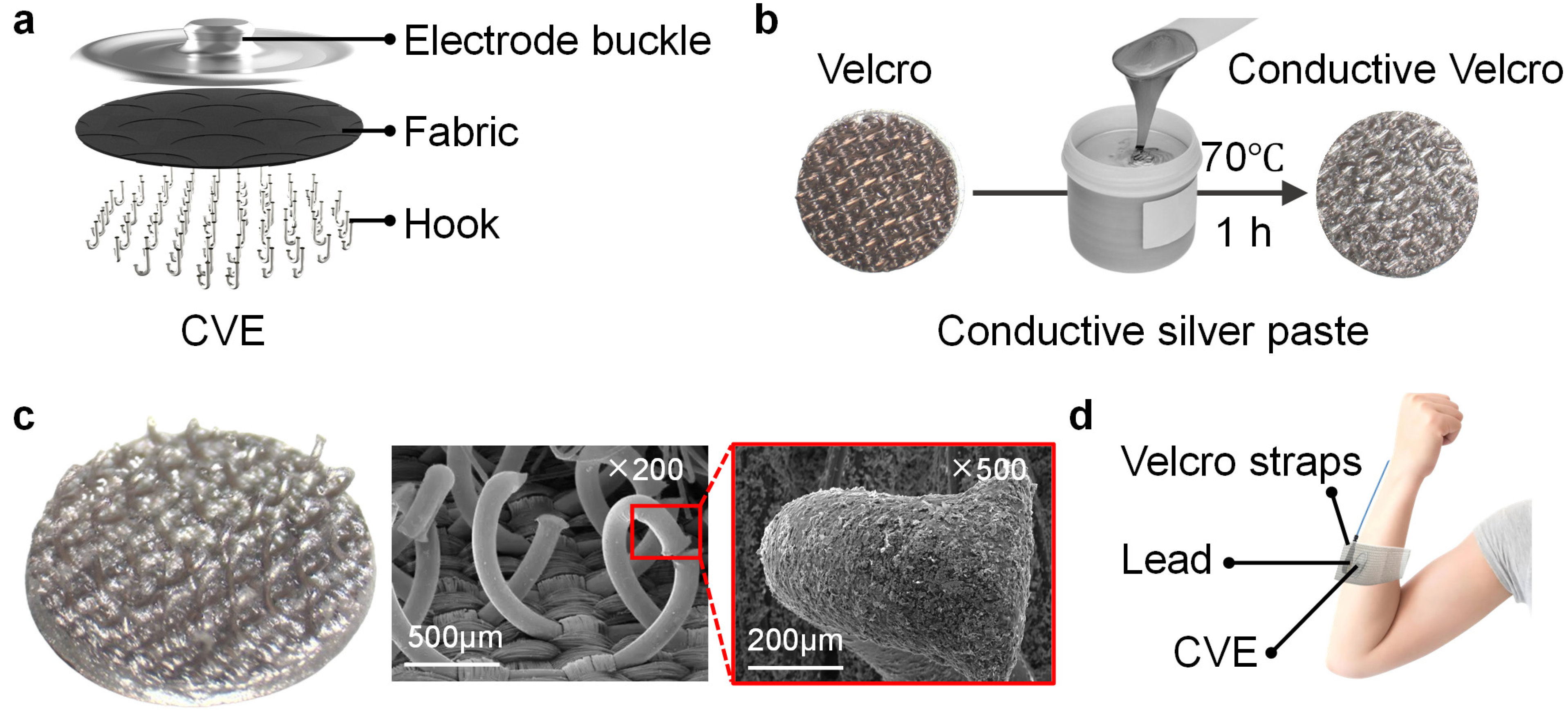
3.1. Fabrication Processes of the CVE
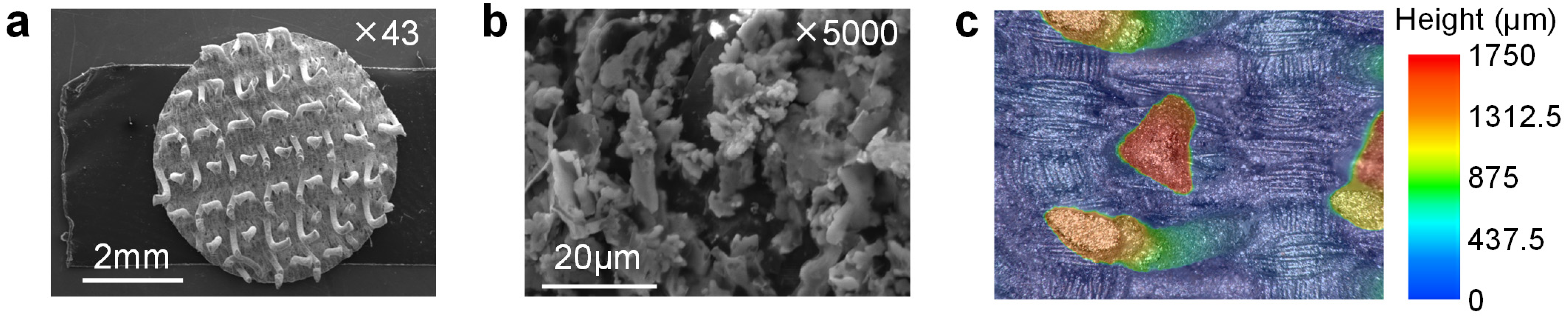
3.2. Microstructure of the CVE
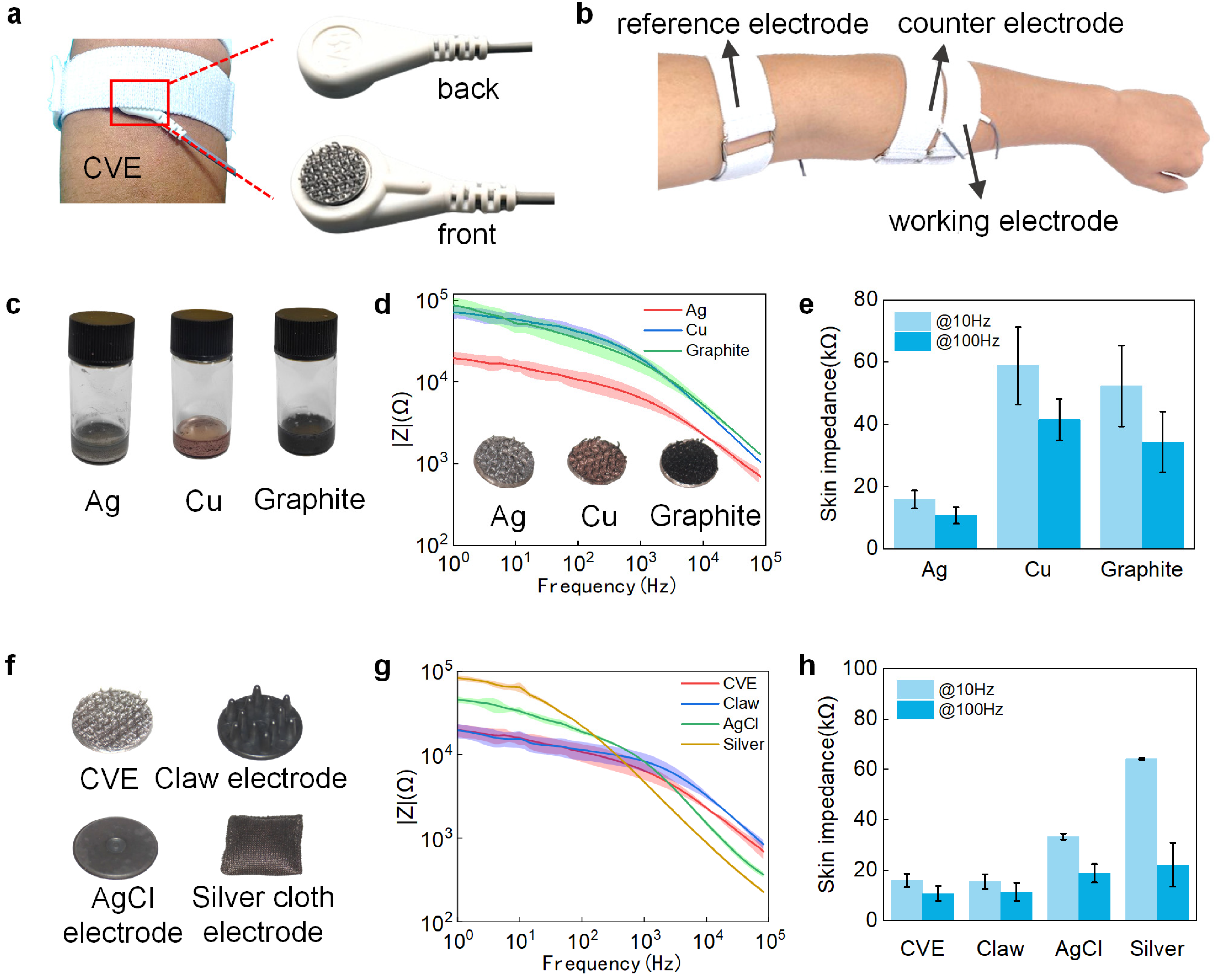
3.3. Selection of CVE
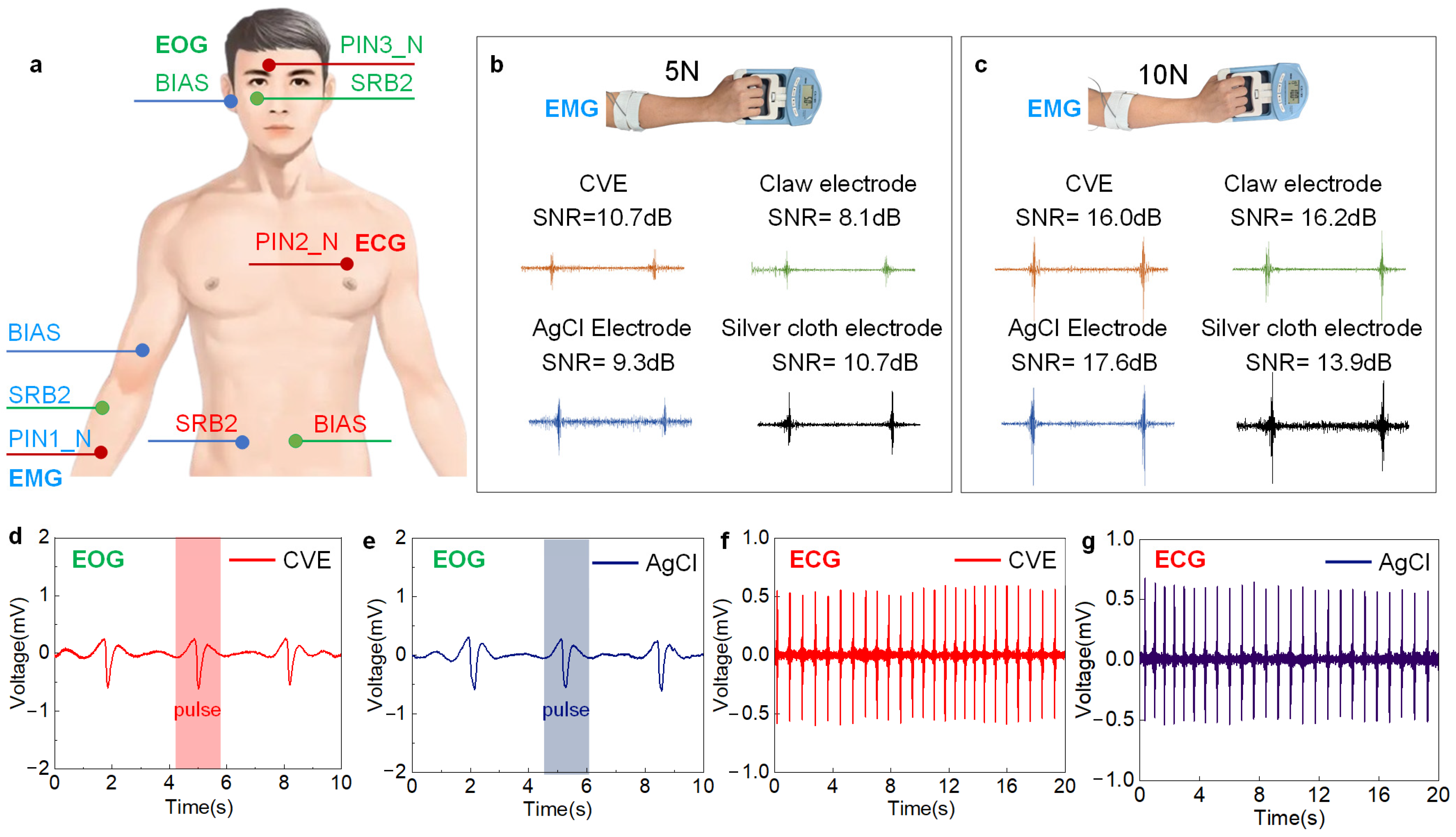
3.4. Biopotential Acquisition
3.4.1. sEMG Signal Acquisition
3.4.2. EOG Signal Acquisition
3.4.3. ECG Signal Acquisition
3.5. Reliability Testing
3.5.1. Water-Resistance Performance
3.5.2. Compression-Resistance Performance
3.6. sEMG-Based Gesture Recognition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, F.; Wang, T.; Liu, G.; Liu, H.; Xie, X.; Wei, Z.; Li, J.; Jiang, C.; He, Y.; Xu, F. Materials with tunable optical properties for wearable epidermal sensing in health monitoring. Adv. Mater. 2022, 34, 2109055. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Zheng, Q.; Zou, Y.; Shi, B.; Jiang, D.; Qu, X.; Han, O.; Zhao, C.; Yu, C.; Fan, Y.; et al. A battery-like self-charge universal module for motional energy harvest. Adv. Energy Mater. 2019, 9, 1901875. [Google Scholar] [CrossRef]
- Frediani, G.; Mazzei, D.; De Rossi, D.E.; Carpi, F. Wearable wireless tactile display for virtual interactions with soft bodies. Front. Bioeng. Biotech. 2014, 2, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, S.; Yu, T.; Zhang, Y.; Ye, G.; Cui, H.; He, C.; Jiang, W.; Zhai, Y.; Lu, C.; et al. Ultra-conformal skin electrodes with synergistically enhanced conductivity for long-time and low-motion artifact epidermal electrophysiology. Nat. Commun. 2021, 12, 4880. [Google Scholar] [CrossRef] [PubMed]
- Yapici, M.K.; Alkhidir, T.; Samad, Y.A.; Liao, K. Graphene-clad textile electrodes for electrocardiogram monitoring. Sens. Actuators B Chem. 2015, 221, 1469–1474. [Google Scholar] [CrossRef]
- Tian, L.; Zimmerman, B.; Akhtar, A.; Yu, K.J.; Moore, M.; Wu, J.; Larsen, R.J.; Lee, J.W.; Li, J.; Liu, Y.; et al. Large-area MRI-compatible epidermal electronic interfaces for prosthetic control and cognitive monitoring. Nat. Biomed. Eng. 2019, 3, 194–205. [Google Scholar] [CrossRef]
- Wang, K.; Yap, L.W.; Gong, S.; Wang, R.; Wang, S.J.; Cheng, W. Nanowire-based soft wearable human–machine interfaces for future virtual and augmented reality applications. Adv. Funct. Mater. 2021, 31, 2008347. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, Z.; Wang, H.; Ji, R. Data-driven design for targeted regulation of heat transfer in carbon/carbon composite structure. J. Therm. Sci. 2024, 33, 648–657. [Google Scholar] [CrossRef]
- Yin, E.; Zhou, Z.; Jiang, J.; Yu, Y.; Hu, D. A dynamically optimized SSVEP brain–computer interface (BCI) speller. IEEE T. Bio-Med. Eng. 2014, 62, 1447–1456. [Google Scholar] [CrossRef]
- Yin, E.; Zhou, Z.; Jiang, J.; Chen, F.; Liu, Y.; Hu, D. A novel hybrid BCI speller based on the incorporation of SSVEP into the P300 paradigm. J. Neural Eng. 2013, 10, 026012. [Google Scholar] [CrossRef]
- Alba, N.A.; Sclabassi, R.J.; Sun, M.; Cui, X.T. Novel hydrogel-based preparation-free EEG electrode. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, S.; Duan, Y.Y. Towards conductive-gel-free electrodes: Understanding the wet electrode, semi-dry electrode and dry electrode-skin interface impedance using electrochemical impedance spectroscopy fitting. Sens. Actuators B Chem. 2018, 277, 250–260. [Google Scholar] [CrossRef]
- Yin, E.; Zhou, Z.; Jiang, J.; Chen, F.; Liu, Y.; Hu, D. A speedy hybrid BCI spelling approach combining P300 and SSVEP. IEEE Trans. Biomed. Eng. 2013, 61, 473–483. [Google Scholar]
- Ye, Y.; Guo, J.; Wang, A.; Zheng, C.; Wu, T.; Chen, Z.; Wang, X.; Chu, Y.; Bai, R.; Liang, Z.; et al. Starfish tube feet inspired hydrogel electrode for durable underwater sEMG acquisition. Chem. Eng. J. 2024, 496, 153882. [Google Scholar] [CrossRef]
- Hargreaves, D.; Mates, E.; Menon, P.; Alderman, H.; Devakumar, D.; Fawzi, W.; Greenfield, G.; Hammoudeh, W.; He, S.; Lahiri, A.; et al. Strategies and interventions for healthy adolescent growth, nutrition, and development. Lancet 2022, 399, 198–210. [Google Scholar] [CrossRef]
- Reis Carneiro, M.; Majidi, C.; Tavakoli, M. Multi-electrode printed bioelectronic patches for long-term electrophysiological monitoring. Adv. Funct. Mater. 2022, 32, 2205956. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, X.; Guo, J.; Ye, Y.; Zhang, H.; Xie, L.; Tao, K.; Zeng, W.; Yin, E.; Ji, B. A wireless, high-quality, soft and portable wrist-worn system for sEMG signal detection. Micromachines 2023, 14, 1085. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Ershad, F.; Almasri, A.; Gonzalez, L.; Wu, X.; Yu, C. Soft electronics for the skin: From health monitors to human–machine interfaces. Adv. Mater. Technol. 2020, 5, 2000233. [Google Scholar] [CrossRef]
- Uyanga, K.A.; Li, W.; Daoud, W.A. Exploiting cellulose-based hydrogels for sustainable, intelligent wearables in pandemic preparedness and control. Eur. Polym. J. 2024, 212, 113041. [Google Scholar] [CrossRef]
- Costanzo, I.; Sen, D.; Rhein, L.; Guler, U. Respiratory monitoring: Current state of the art and future roads. IEEE Rev. Biomed. Eng. 2020, 15, 103–121. [Google Scholar] [CrossRef]
- Niu, X.; Gao, X.; Liu, Y.; Liu, H. Surface bioelectric dry Electrodes: A review. Measurement 2021, 183, 109774. [Google Scholar] [CrossRef]
- Li, Z.; Guo, W.; Huang, Y.; Zhu, K.; Yi, H.; Wu, H. On-skin graphene electrodes for large area electrophysiological monitoring and human-machine interfaces. Carbon 2020, 164, 164–170. [Google Scholar] [CrossRef]
- Wu, H.; Yang, G.; Zhu, K.; Liu, S.; Guo, W.; Jiang, Z.; Li, Z. Materials, devices, and systems of on-skin electrodes for electrophysiological monitoring and human–machine interfaces. Adv. Sci. 2021, 8, 2001938. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Xue, J.; Li, X.; Chan, C.P.Y.; Li, Z.; Li, P.; Yang, Z.; Lai, K.W.C. High-fidelity sEMG signals recorded by an on-skin electrode based on AgNWs for hand gesture classification using machine learning. ACS Appl. Mater. Interfaces 2023, 15, 19374–19383. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, H.; Li, S.; Liang, X.; Zhang, M.; Dai, X.; Zhang, Y. Flexible electrodes for in vivo and in vitro electrophysiological signal recording. Adv. Healthc. Mater. 2021, 10, 2100646. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Jiang, X.; Wang, W.; Li, Z. A microneedle electrode array on flexible substrate for long-term EEG monitoring. Sens. Actuators B Chem. 2017, 244, 750–758. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Ren, L.; Huang, P.; Yu, M.; Tian, L.; Li, X.; Xie, J.; Fang, P.; Li, G. Towards improving the quality of electrophysiological signal recordings by using microneedle electrode arrays. IEEE Trans. Biomed. Eng. 2021, 68, 3327–3335. [Google Scholar] [CrossRef]
- Yin, E.; Zeyl, T.; Saab, R.; Chau, T.; Hu, D.; Zhou, Z. A hybrid brain–computer interface based on the fusion of P300 and SSVEP scores. IEEE Trans. Neur. Sys. Reh. 2015, 23, 693–701. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, H.; Tian, Y.; Gao, K.; Wang, M.; Wang, X.; Liang, Z.; You, X.; Gao, S.; Shao, D.; et al. Smart epidermal electrophysiological electrode: Materials, structures, and algorithms. Nanotech. Precis. Eng. 2023, 6, 045001. [Google Scholar] [CrossRef]
- Todorova, K.; Mandinova, A. Novel approaches for managing aged skin and nonmelanoma skin cancer. Adv. Drug Deliv. Rev. 2020, 153, 18–27. [Google Scholar] [CrossRef]
- Wang, J.; Wang, T.; Liu, H.; Wang, K.; Moses, K.; Feng, Z.; Li, P.; Huang, W. Flexible electrodes for brain–computer interface system. Adv. Mater. 2023, 35, 2211012. [Google Scholar] [CrossRef] [PubMed]
- Rayhana, R.; Xiao, G.G.; Liu, Z. Printed sensor technologies for monitoring applications in smart farming: A review. IEEE Trans. Instrum. Meas. 2021, 70, 1–19. [Google Scholar] [CrossRef]
- Li, G.L.; Wu, J.T.; Xia, Y.H.; He, Q.G.; Jin, H.G. Review of semi-dry electrodes for EEG recording. J. Neural Eng. 2020, 17, 051004. [Google Scholar] [CrossRef]
- Paul, A.; Lee, M.S.; Xu, Y.; Deiss, S.R.; Cauwenberghs, G. A versatile in-ear biosensing system and body-area network for unobtrusive continuous health monitoring. IEEE Trans. Biomed. Circuits Syst. 2023, 17, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Ko, L.W.; Su, C.H.; Liao, P.L.; Liang, J.T.; Tseng, Y.H.; Chen, S.H. Flexible graphene/GO electrode for gel-free EEG. J. Neural Eng. 2021, 18, 046060. [Google Scholar] [CrossRef] [PubMed]
- Farago, E.; MacIsaac, D.; Suk, M.; Chan, A.D. A review of techniques for surface electromyography signal quality analysis. IEEE Rev. Biomed. Eng. 2022, 16, 472–486. [Google Scholar] [CrossRef] [PubMed]
- Yin, E.; Zeyl, T.; Saab, R.; Hu, D.; Zhou, Z.; Chau, T. An auditory-tactile visual saccade-independent P300 brain–computer interface. Int. J. Neural Syst. 2016, 26, 1650001. [Google Scholar] [CrossRef]
- Cay, G.; Solanki, D.; Al Rumon, M.A.; Ravichandran, V.; Fapohunda, K.O.; Mankodiya, K. SolunumWear: A smart textile system for dynamic respiration monitoring across various postures. iScience 2024, 27, 110223. [Google Scholar] [CrossRef]
- Almohammed, B.; Ismail, A.; Sali, A. Electro-textile wearable antennas in wireless body area networks: Materials, antenna design, manufacturing techniques, and human body consideration—A review. Text. Res. J. 2021, 91, 646–663. [Google Scholar] [CrossRef]
- Kairn, T.; Maxwell, S.K.; Trapp, J.V.; Crowe, S.B. Assessment of integrity and lead-equivalence of shielded garments using two-dimensional X-ray images from a computed tomography scanner. Radiat. Prot. Dosim. 2021, 193, 155–164. [Google Scholar] [CrossRef]
- Gao, K.; Wu, N.; Ji, B.; Liu, J. A film electrode upon nanoarchitectonics of bacterial cellulose and conductive fabric for forehead electroencephalogram measurement. Sensors 2023, 23, 7887. [Google Scholar] [CrossRef] [PubMed]
- Tsolis, A.; Michalopoulou, A.; Alexandridis, A. Use of conductive zip and Velcro as a polarisation reconfiguration means of a textile patch antenna. IET Microw. Antennas Propag. 2020, 14, 684–693. [Google Scholar] [CrossRef]
- Wu, S.J.; Zhao, X. Bioadhesive technology platforms. Chem. Rev. 2023, 123, 14084–14118. [Google Scholar] [CrossRef]
- Cheng, L.; Guo, A.; Li, J.; Li, M.; Lei, Q.; Xu, W.; Guo, X.; Zhang, J. Three-dimensional MXene/carbon nanotube composite electrodes in flexible 64-channel arrays for noninvasive electromyography signal acquisition. Sci. China Mater. 2024, 67, 2977–2984. [Google Scholar] [CrossRef]
- Zhang, Q.; Qu, M.; Liu, X.; Cui, Y.; Hu, H.; Li, Q.; Jin, M.; Xian, J.; Nie, Z.; Zhang, C. Three-in-one portable electronic sensory system based on low-impedance laser-induced graphene on-skin electrode sensors for electrophysiological signal monitoring. Adv. Mater. Interfaces 2022, 10, 2201735. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, J.; Liang, W.; Zhao, L.; Dong, Y.; Wang, X.; Lin, L. Water-retentive, 3D knitted textile electrode for long-term and motion state bioelectrical signal acquisition. Compos. Sci. Technol. 2022, 227, 109606. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.; Gong, Z.; Lei, Y.; OuYang, X.; Chan, C.C.; Ruan, S. Wearable physiological monitoring system based on electrocardiography and electromyography for upper limb rehabilitation training. Sensors 2020, 20, 4861. [Google Scholar] [CrossRef]
- Chen, X.; Morales-Gregorio, A.; Sprenger, J.; Kleinjohann, A.; Sridhar, S.; van Albada, S.J.; Grün, S.; Roelfsema, P.R. 1024-channel electrophysiological recordings in macaque V1 and V4 during resting state. Sci. Data 2022, 9, 77. [Google Scholar] [CrossRef]
- Botros, F.S.; Phinyomark, A.; Scheme, E.J. Electromyography-based gesture recognition: Is it time to change focus from the forearm to the wrist? IEEE Trans. Ind. Inform. 2020, 18, 174–184. [Google Scholar] [CrossRef]
- Ding, H.; Gu, Y.; Ren, Y.; Hu, C.; Qiu, Q.; Wu, D.; Mou, J.; Wu, Z.; Zhou, H. The latest research progress of conductive hydrogels in the field of electrophysiological signal acquisition. J. Mater. Chem. C 2024, 12, 3030–3052. [Google Scholar] [CrossRef]
- Afroj, S.; Tan, S.; Abdelkader, A.M.; Novoselov, K.S.; Karim, N. Highly conductive, scalable, and machine washable graphene-based E-textiles for multifunctional wearable electronic applications. Adv. Funct. Mater. 2020, 30, 2000293. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Zheng, Y.; Zhao, S.; Wei, W.; Zhang, D.; Fu, X.; Jiang, K.; Shen, G.; Han, W. Highly-stable polymer-crosslinked 2D MXene-based flexible biocompatible electronic skins for in vivo biomonitoring. Nano Energy 2021, 84, 105921. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Hu, R.; Zhang, X.; Chen, X. Hand gesture recognition based on surface electromyography using convolutional neural network with transfer learning method. IEEE J. Biomed. Health 2020, 25, 1292–1304. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Gao, Q.; Liu, H.; Shull, P.B. A novel, co-located EMG-FMG-sensing wearable armband for hand gesture recognition. Sensor. Actuat. A Phys. 2020, 301, 111738. [Google Scholar] [CrossRef]
- Kim, I.; Lee, T.; Lee, S.H. Ankle intention detection algorithm using electromyography signal. J. Comput. Des. Eng. 2021, 8, 1234–1242. [Google Scholar] [CrossRef]
- Etana, B.B.; Dawud, A.A.; Malengier, B.; Sitek, W.; Gemechu, W.F.; Krishnamoorthy, J.; Langenhove, L.V. Discrete wavelet transform based processing of embroidered textile-electrode electromyography signal acquired with load and pressure effect. J. Ind. Text. 2024, 54, 15280837241232449. [Google Scholar] [CrossRef]
- Pourmohammadi, S.; Maleki, A. Stress detection using ECG and EMG signals: A comprehensive study. Comput. Meth. Prog. Bio. 2020, 193, 105482. [Google Scholar] [CrossRef] [PubMed]
- Campanini, I.; Merlo, A.; Disselhorst-Klug, C.; Mesin, L.; Muceli, S.; Merletti, R. Fundamental concepts of bipolar and high-Density surface EMG understanding and teaching for clinical, occupational, and sport applications: Origin, detection, and main errors. Sensors 2022, 22, 4150. [Google Scholar] [CrossRef]
- Milanizadeh, S.; Safaie, J. EOG-based HCI system for quadcopter navigation. IEEE Trans. Instrum. Meas. 2020, 69, 8992–8999. [Google Scholar] [CrossRef]
- Li, H.; Lin, Z.; Zhang, L.; Cao, L.; Ren, F.; Meng, W.; Wang, Y.; Zhang, C.; Chen, L.; Zhang, S.; et al. Low half-wave voltage polymeric electro-optic modulator using CLD-1/PMMA for electrocardiogram (ECG) signal acquisition. Opt. Express 2023, 31, 12072–12082. [Google Scholar] [CrossRef]
- Galante, A.J.; Pilsbury, B.C.; Li, M.; LeMieux, M.; Liu, Q.; Leu, P.W. Achieving highly conductive, stretchable, and washable fabric from reactive silver ink and increased interfacial adhesion. ACS Appl. Polym. Mater. 2022, 4, 5253–5260. [Google Scholar] [CrossRef]
- Kang, Z.; He, Y.; Sang, J.; Hirahara, H.; Chen, D. Superhydrophobic and conductive cotton fabric composite with excellent corrosion resistance for wearable electronics. Adv. Mater. Interfaces 2021, 8, 2100651. [Google Scholar] [CrossRef]
- Fang, C.; He, B.; Wang, Y.; Cao, J.; Gao, S. EMG-centered multisensory based technologies for pattern recognition in rehabilitation: State of the art and challenges. Biosensors 2020, 10, 85. [Google Scholar] [CrossRef]
- Shen, C.; Pei, Z.; Chen, W.; Wang, J.; Zhang, J.; Chen, Z. Toward generalization of sEMG-based pattern recognition: A novel feature extraction for gesture recognition. IEEE Trans. Instrum. Meas. 2022, 71, 2501412. [Google Scholar] [CrossRef]
- Fatayer, A.; Gao, W.; Fu, Y. sEMG-Based gesture recognition using deep learning from noisy labels. IEEE J. Biomed. Health 2022, 26, 4462–4473. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, H.; Chen, X. Integrating social neuroscience into human-machine mutual behavioral understanding for autonomous driving. Innovation 2023, 4, 100455. [Google Scholar] [CrossRef] [PubMed]
- Shan, G.; Li, X.; Huang, W. AI-enabled wearable and flexible electronics for assessing full personal exposures. Innovation 2020, 1, 100031. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Wang, X.; Bai, R.; Zhang, Z.; Chen, H.; Xue, K.; Ma, C.; Zang, D.; Yin, E.; Gao, K.; et al. A Cost-Effective and Easy-to-Fabricate Conductive Velcro Dry Electrode for Durable and High-Performance Biopotential Acquisition. Biosensors 2024, 14, 432. https://doi.org/10.3390/bios14090432
Guo J, Wang X, Bai R, Zhang Z, Chen H, Xue K, Ma C, Zang D, Yin E, Gao K, et al. A Cost-Effective and Easy-to-Fabricate Conductive Velcro Dry Electrode for Durable and High-Performance Biopotential Acquisition. Biosensors. 2024; 14(9):432. https://doi.org/10.3390/bios14090432
Chicago/Turabian StyleGuo, Jun, Xuanqi Wang, Ruiyu Bai, Zimo Zhang, Huazhen Chen, Kai Xue, Chuang Ma, Dawei Zang, Erwei Yin, Kunpeng Gao, and et al. 2024. "A Cost-Effective and Easy-to-Fabricate Conductive Velcro Dry Electrode for Durable and High-Performance Biopotential Acquisition" Biosensors 14, no. 9: 432. https://doi.org/10.3390/bios14090432
APA StyleGuo, J., Wang, X., Bai, R., Zhang, Z., Chen, H., Xue, K., Ma, C., Zang, D., Yin, E., Gao, K., & Ji, B. (2024). A Cost-Effective and Easy-to-Fabricate Conductive Velcro Dry Electrode for Durable and High-Performance Biopotential Acquisition. Biosensors, 14(9), 432. https://doi.org/10.3390/bios14090432





