Ultrasensitive Electrochemical Biosensors Based on Allosteric Transcription Factors (aTFs) for Pb2+ Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Apparatus
2.3. Construction of Electrochemical Biosensors
2.4. Optimization of Conditions for the Construction of Biosensors
2.5. Pb2+ Quantitative and Selectivity Evaluation
2.6. Regeneration and Stability of Biosensors
2.7. Application of Biosensor for Pb2+ Detection in Actual Water Samples
2.8. Statistics
3. Results and Discussion
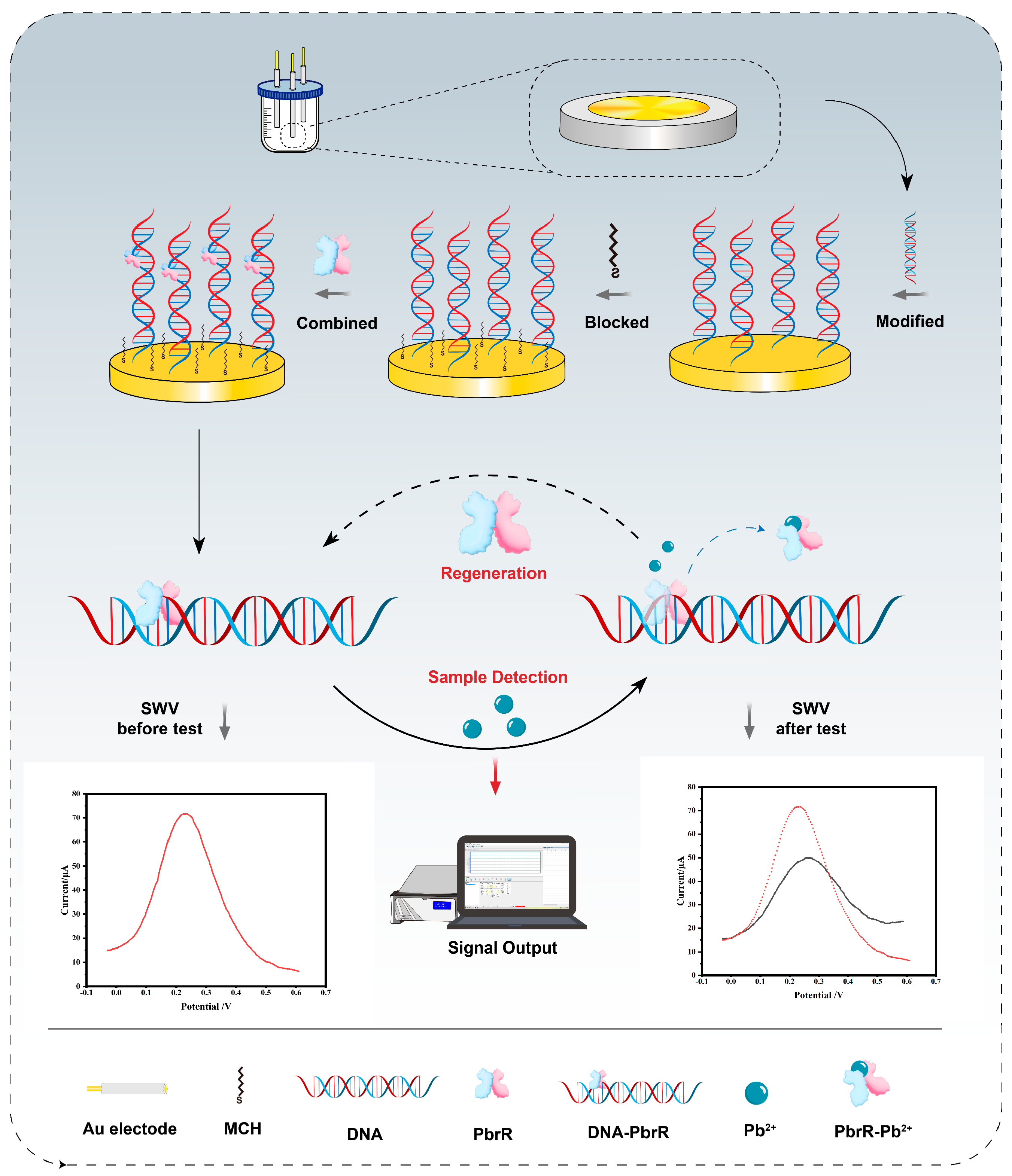
3.1. Principle and Construction of the Electrochemical Biosensors
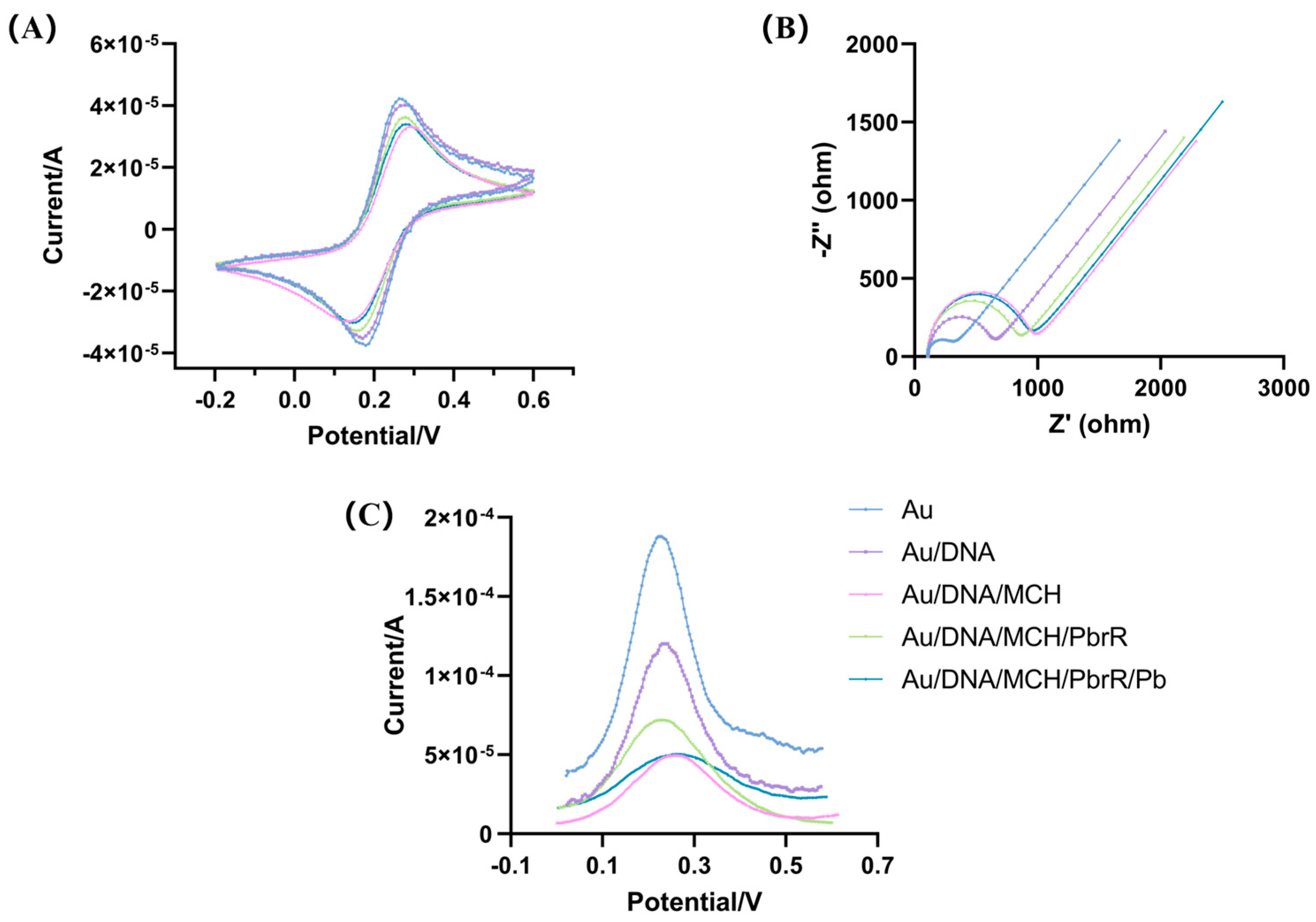
3.2. Optimization of the Electrochemical Biosensors
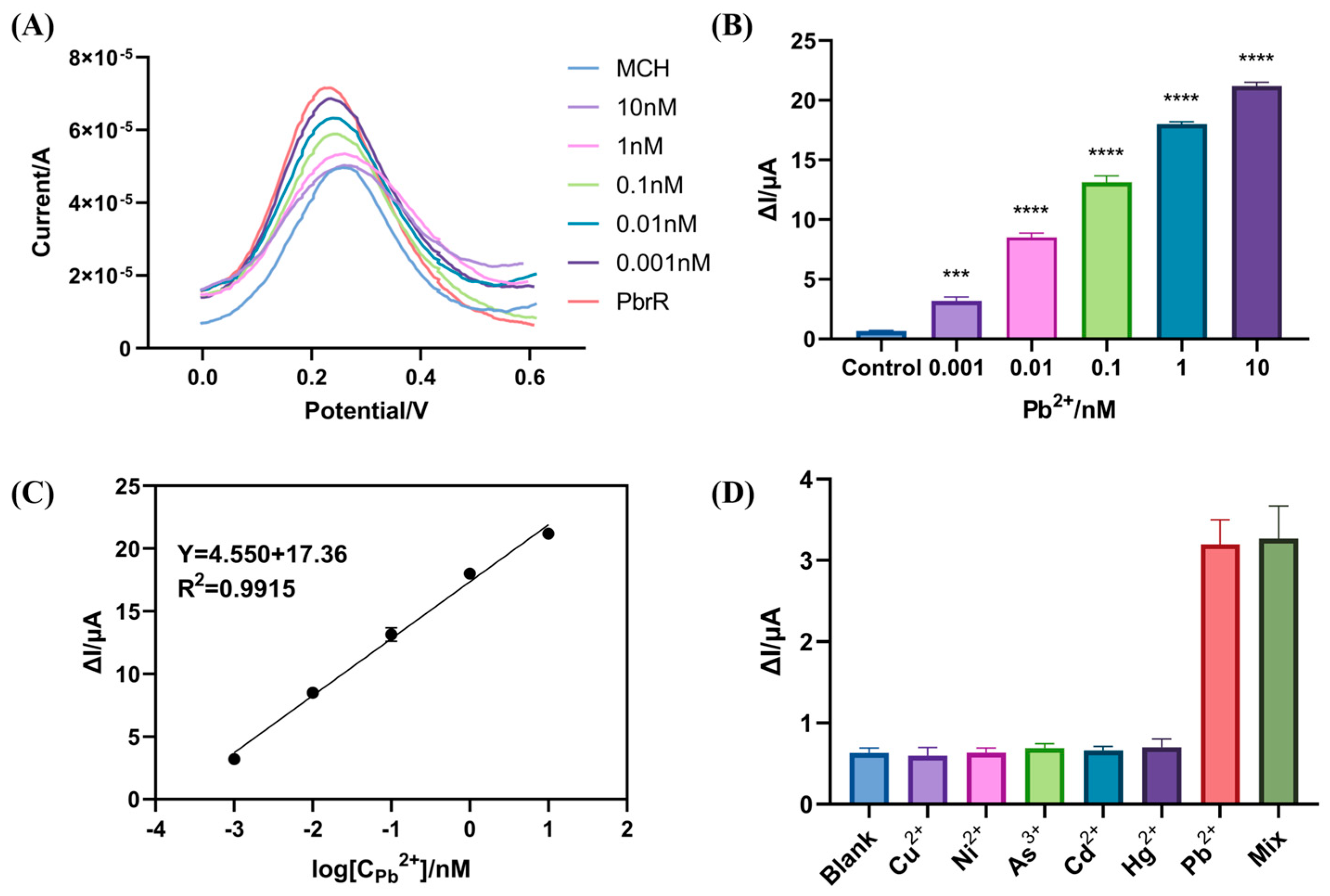
3.3. Sensitivity and Selectivity of the Electrochemical Biosensors
3.4. Regeneration and Stability of the Electrochemical Biosensors
3.5. Application of Biosensor for Pb2+ Detection in Actual Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Zhang, Z.; Qi, J.; You, J.; Ma, J.; Chen, L. Colorimetric detection of heavy metal ions with various chromogenic materials: Strategies and applications. J. Hazard. Mater. 2023, 441, 129889. [Google Scholar] [CrossRef] [PubMed]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and removal of heavy metal ions: A review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Zhang, S.; Han, X.; Cai, H.; Wu, X.; Yuan, Y.; Zhang, Y. Aramid nanofibers/WS2 nanosheets co-assembled aerogels for efficient and stable Pb(II) adsorption in harsh environments. Chem. Eng. J. 2022, 450, 138268. [Google Scholar] [CrossRef]
- Somayaji, A.; Sarkar, S.; Balasubramaniam, S.; Raval, R. Synthetic biology techniques to tackle heavy metal pollution and poisoning. Synth. Syst. Biotechnol. 2022, 7, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.Y.; Ma, B.C.; Wang, Y.Q.; Yang, X.Q.; Cai, J.M. Designed bacteria based on natural pbr operons for detecting and detoxifying environmental lead: A mini-review. Ecotoxicol. Environ. Saf. 2023, 267, 115662. [Google Scholar] [CrossRef]
- Wang, L.; Wen, Y.; Li, L.; Yang, X.; Jia, N.; Li, W.; Meng, J.; Duan, M.; Sun, X.; Liu, G. Sensitive and label-free electrochemical lead ion biosensor based on a DNAzyme triggered G-quadruplex/hemin conformation. Biosens. Bioelectron. 2018, 115, 91–96. [Google Scholar] [CrossRef]
- Zhou, J.; Li, B.; Qi, A.; Shi, Y.; Qi, J.; Xu, H.; Chen, L. ZnSe quantum dot based ion imprinting technology for fluorescence detecting cadmium and lead ions on a three-dimensional rotary paper-based microfluidic chip. Sens. Actuators B Chem. 2020, 305, 127462. [Google Scholar] [CrossRef]
- Milne, A.; Landing, W.; Bizimis, M.; Morton, P. Determination of Mn, Fe, Co, Ni, Cu, Zn, Cd and Pb in seawater using high resolution magnetic sector inductively coupled mass spectrometry (HR-ICP-MS). Anal. Chim. Acta 2010, 665, 200–207. [Google Scholar] [CrossRef]
- Guérin, T.; Chekri, R.; Vastel, C.; Sirot, V.; Volatier, J.-L.; Leblanc, J.-C.; Noël, L. Determination of 20 trace elements in fish and other seafood from the French market. Food Chem. 2011, 127, 934–942. [Google Scholar] [CrossRef]
- Ghaedi, M.; Ahmadi, F.; Shokrollahi, A. Simultaneous preconcentration and determination of copper, nickel, cobalt and lead ions content by flame atomic absorption spectrometry. J. Hazard. Mater. 2007, 142, 272–278. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Sankar, K.; Kuzmanović, U.; Schaus, S.E.; Galagan, J.E.; Grinstaff, M.W. Strategy, Design, and Fabrication of Electrochemical Biosensors: A Tutorial. ACS Sens. 2024, 9, 2254–2274. [Google Scholar] [CrossRef] [PubMed]
- Bakirhan, N.K.; Topal, B.D.; Ozcelikay, G.; Karadurmus, L.; Ozkan, S.A. Current Advances in Electrochemical Biosensors and Nanobiosensors. Crit. Rev. Anal. Chem. 2022, 52, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Peng, X.; Fu, H.; Huang, C.; Li, Y.; Liu, Z. Recent advances in the development of electrochemical aptasensors for detection of heavy metals in food. Biosens. Bioelectron. 2020, 147, 111777. [Google Scholar] [CrossRef]
- Li, D.; Xu, Y.; Fan, L.; Shen, B.; Ding, X.; Yuan, R.; Li, X.; Chen, W. Target-driven rolling walker based electrochemical biosensor for ultrasensitive detection of circulating tumor DNA using doxorubicin@tetrahedron-Au tags. Biosens. Bioelectron. 2020, 148, 111826. [Google Scholar] [CrossRef]
- Vanova, V.; Mitrevska, K.; Milosavljevic, V.; Hynek, D.; Richtera, L.; Adam, V. Peptide-based electrochemical biosensors utilized for protein detection. Biosens. Bioelectron. 2021, 180, 113087. [Google Scholar] [CrossRef]
- Kudr, J.; Michalek, P.; Ilieva, L.; Adam, V.; Zitka, O. COVID-19: A challenge for electrochemical biosensors. TrAC Trends Anal. Chem. 2021, 136, 116192. [Google Scholar] [CrossRef]
- Gao, F.; Zhan, F.; Li, S.; Antwi-Mensah, P.; Niu, L.; Wang, Q. Dual signal-based electrochemical aptasensor for simultaneous detection of Lead(II) and Mercury(II) in environmental water samples. Biosens. Bioelectron. 2022, 209, 114280. [Google Scholar] [CrossRef]
- Jalalian, S.H.; Karimabadi, N.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Electrochemical and optical aptamer-based sensors for detection of tetracyclines. Trends Food Sci. Technol. 2018, 73, 45–57. [Google Scholar] [CrossRef]
- Wang, J.; Cui, X.; Liang, L.; Li, J.; Pang, B.; Li, J. Advances in DNA-based electrochemical biosensors for the detection of foodborne pathogenic bacteria. Talanta 2024, 275, 126072. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Wu, G.; Xu, C.; Wu, J.; Zhang, X.; Liu, J. Applications of electrochemical biosensors based on functional antibody-modified screen-printed electrodes: A review. Anal. Methods 2022, 14, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Kilic, N.M.; Singh, S.; Keles, G.; Cinti, S.; Kurbanoglu, S.; Odaci, D. Novel Approaches to Enzyme-Based Electrochemical Nanobiosensors. Biosensors 2023, 13, 622. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Yan, X.; Zhu, C.; Du, D.; Lin, Y. Recent Advances in Electrochemical Immunosensors. Anal. Chem. 2017, 89, 138–156. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Liu, Y.; Jiang, Y. Recent Progress on Highly Selective and Sensitive Electrochemical Aptamer-based Sensors. Chem. Res. Chin. Univ. 2022, 38, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Z.; Tan, G.Y.; Xin, Z.; Wang, W. In vitro allosteric transcription factor-based biosensing. Trends Biotechnol. 2023, 41, 1080–1095. [Google Scholar] [CrossRef]
- Bi, H.; Zhao, C.; Zhang, Y.; Zhang, X.; Xue, B.; Li, C.; Wang, S.; Yang, X.; Li, C.; Qiu, Z.; et al. IVT cell-free biosensors for tetracycline and macrolide detection based on allosteric transcription factors (aTFs). Anal. Methods 2022, 14, 4545–4554. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; Bi, H.; Zhang, X.; Xue, B.; Li, C.; Wang, S.; Yang, X.; Qiu, Z.; Wang, J.; et al. A cell-free paper-based biosensor dependent on allosteric transcription factors (aTFs) for on-site detection of harmful metals Hg2+ and Pb2+ in water. J. Hazard. Mater. 2022, 438, 129499. [Google Scholar] [CrossRef]
- Browning, D.F.; Busby, S.J.W. The regulation of bacterial transcription initiation. Nat. Rev. Microbiol. 2004, 2, 57–65. [Google Scholar] [CrossRef]
- Brown, N.L.; Stoyanov, J.V.; Kidd, S.P.; Hobman, J.L. The MerR family of transcriptional regulators. FEMS Microbiol. Rev. 2003, 27, 145–163. [Google Scholar] [CrossRef]
- Mahr, R.; Frunzke, J. Transcription factor-based biosensors in biotechnology: Current state and future prospects. Appl. Microbiol. Biotechnol. 2015, 100, 79–90. [Google Scholar] [CrossRef]
- Jung, J.K.; Alam, K.K.; Verosloff, M.S.; Capdevila, D.A.; Desmau, M.; Clauer, P.R.; Lee, J.W.; Nguyen, P.Q.; Pastén, P.A.; Matiasek, S.J.; et al. Cell-free biosensors for rapid detection of water contaminants. Nat. Biotechnol. 2020, 38, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef] [PubMed]
- Villalonga, A.; Perez-Calabuig, A.M.; Villalonga, R. Electrochemical biosensors based on nucleic acid aptamers. Anal. Bioanal. Chem. 2020, 412, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Seok Kim, Y.; Ahmad Raston, N.H.; Bock Gu, M. Aptamer-based nanobiosensors. Biosens. Bioelectron. 2016, 76, 2–19. [Google Scholar] [CrossRef]
- Sankar, K.; Baer, R.; Grazon, C.; Sabatelle, R.C.; Lecommandoux, S.; Klapperich, C.M.; Galagan, J.E.; Grinstaff, M.W. An Allosteric Transcription Factor DNA-Binding Electrochemical Biosensor for Progesterone. ACS Sens. 2022, 7, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Libis, V.; Delépine, B.; Faulon, J.-L. Sensing new chemicals with bacterial transcription factors. Curr. Opin. Microbiol. 2016, 33, 105–112. [Google Scholar] [CrossRef]
- Blair, E.O.; Corrigan, D.K. A review of microfabricated electrochemical biosensors for DNA detection. Biosens. Bioelectron. 2019, 134, 57–67. [Google Scholar] [CrossRef]
- Stukovnik, Z.; Bren, U. Recent Developments in Electrochemical-Impedimetric Biosensors for Virus Detection. Int. J. Mol. Sci. 2022, 23, 15922. [Google Scholar] [CrossRef]
- Gorodetsky, A.A.; Dietrich, L.E.P.; Lee, P.E.; Demple, B.; Newman, D.K.; Barton, J.K. DNA binding shifts the redox potential of the transcription factor SoxR. Proc. Natl. Acad. Sci. USA 2008, 105, 3684–3689. [Google Scholar] [CrossRef]
- Xu, S.; Chen, X.; Peng, G.; Jiang, L.; Huang, H. An electrochemical biosensor for the detection of Pb2+ based on G-quadruplex DNA and gold nanoparticles. Anal. Bioanal. Chem. 2018, 410, 5879–5887. [Google Scholar] [CrossRef]
- Liang, R.; Dong, J.; Li, J.; Jin, H.; Wei, M.; Bai, T.; Ren, W.; Xu, Y.; He, B.; Suo, Z. DNAzyme-driven bipedal DNA walker and catalytic hairpin assembly multistage signal amplified electrochemical biosensor based on porous AuNPs@Zr-MOF for detection of Pb2+. Food Chem. 2024, 435, 137503. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Qian, S.; Cao, H.; Yu, J.; Ye, T.; Wu, X.; Chen, L.; Xu, F. An ultra-sensitive electrochemical aptasensor for simultaneous quantitative detection of Pb2+ and Cd2+ in fruit and vegetable. Food Chem. 2022, 382, 132173. [Google Scholar] [CrossRef] [PubMed]
- Ran, G.; Wu, F.; Ni, X.; Li, X.; Li, X.; Liu, D.; Sun, J.; Xie, C.; Yao, D.; Bai, W. A novel label-free electrochemical aptasensor with one-step assembly process for rapid detection of lead (II) ions. Sens. Actuators B Chem. 2020, 320, 128326. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, S.; Li, H.; Liu, H.; Pang, P.; Wang, H.; Wu, Z.; Yang, W. A Pb2+-ion electrochemical biosensor based on single-stranded DNAzyme catalytic beacon. Sens. Actuators B Chem. 2016, 222, 1083–1089. [Google Scholar] [CrossRef]
- Huang, S.; Liu, X.; Wang, D.; Chen, W.; Hu, Q.; Wei, T.; Zhou, W.; Gan, J.; Chen, H. Structural Basis for the Selective Pb(II) Recognition of Metalloregulatory Protein PbrR691. Inorg. Chem. 2016, 55, 12516–12519. [Google Scholar] [CrossRef]
- Chen, P.; He, C. A General Strategy to Convert the MerR Family Proteins into Highly Sensitive and Selective Fluorescent Biosensors for Metal Ions. J. Am. Chem. Soc. 2004, 126, 728–729. [Google Scholar] [CrossRef]
- Muller Jocelyn, F.; Stevens Ann, M.; Craig, J.; Love Nancy, G. Transcriptome Analysis Reveals that Multidrug Efflux Genes Are Upregulated to Protect Pseudomonas aeruginosa from Pentachlorophenol Stress. Appl. Environ. Microbiol. 2007, 73, 4550–4558. [Google Scholar] [CrossRef]


| Analytical Method | Recognition Element | Linear Range | Detection Limit | Detection Time | Reference | Notes |
|---|---|---|---|---|---|---|
| Electrochemical | aTFs | 1 pM–10 nM | 1 pM | 10 min | This work | Sensor regeneration time 1.5 h |
| Fluorescence | aTFs | 1–250 nM | 0.1 nM | 60 min | [27] | / |
| Electrochemical | aptamer | 0.1–1000 nM | 89.31 pM | 30 min | [42] | |
| Electrochemical | aptamer | 0.5 nM–5 µM | 0.14 nM | 15 min | [43] | |
| Electrochemical | G-quadruplex | 0.01–200 nM | 4.2 pM | 60 min | [40] | |
| Electrochemical | DNAzymes | 0.5 nM–5 µM | 0.25 nM | 25 min | [44] | |
| Electrochemical | DNA walker | 0.05–1000 nM | 4.65 pM | 1.5 h | [41] |
| Samples | Spiked (nM) | Detected (nM) | AAS (nM) | R.S.D. (%) |
|---|---|---|---|---|
| River water | 5 | 5.33 ± 0.11 | 5.87 ± 0.15 | 2.21% |
| 7 | 7.0 ± 0.10 | 6.81 ± 0.02 | 1.51% | |
| 10 | 10.24 ± 0.22 | 9.90 ± 0.03 | 2.25% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, N.; Zhao, C.; Kang, X.; Zhang, C.; Zhang, X.; Li, C.; Wang, S.; Xue, B.; Yang, X.; Li, C.; et al. Ultrasensitive Electrochemical Biosensors Based on Allosteric Transcription Factors (aTFs) for Pb2+ Detection. Biosensors 2024, 14, 446. https://doi.org/10.3390/bios14090446
Yu N, Zhao C, Kang X, Zhang C, Zhang X, Li C, Wang S, Xue B, Yang X, Li C, et al. Ultrasensitive Electrochemical Biosensors Based on Allosteric Transcription Factors (aTFs) for Pb2+ Detection. Biosensors. 2024; 14(9):446. https://doi.org/10.3390/bios14090446
Chicago/Turabian StyleYu, Ningkang, Chen Zhao, Xiaodan Kang, Cheng Zhang, Xi Zhang, Chenyu Li, Shang Wang, Bin Xue, Xiaobo Yang, Chao Li, and et al. 2024. "Ultrasensitive Electrochemical Biosensors Based on Allosteric Transcription Factors (aTFs) for Pb2+ Detection" Biosensors 14, no. 9: 446. https://doi.org/10.3390/bios14090446
APA StyleYu, N., Zhao, C., Kang, X., Zhang, C., Zhang, X., Li, C., Wang, S., Xue, B., Yang, X., Li, C., Qiu, Z., Wang, J., & Shen, Z. (2024). Ultrasensitive Electrochemical Biosensors Based on Allosteric Transcription Factors (aTFs) for Pb2+ Detection. Biosensors, 14(9), 446. https://doi.org/10.3390/bios14090446





