Resonant Young’s Slit Interferometer for Sensitive Detection of Low-Molecular-Weight Biomarkers
Abstract
:1. Introduction
2. Results and Discussion
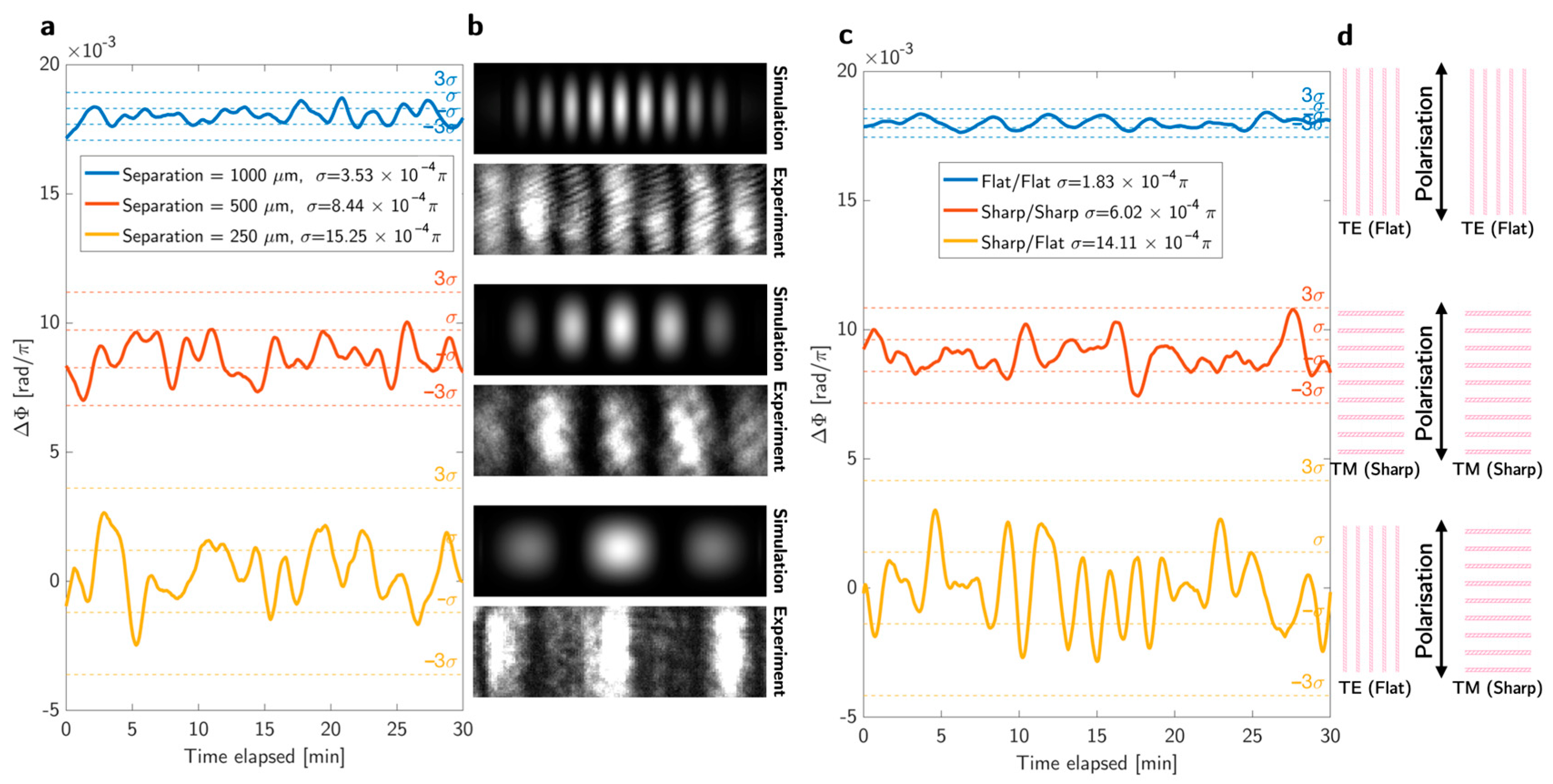
2.1. Approaches to Noise Reduction
2.2. Protein Sensing
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Sensor Fabrication
Appendix B. Optical Setup and Data Processing
Appendix C. Surface Chemistry
References
- Khani, S.; Hayati, M. Optical Biosensors Using Plasmonic and Photonic Crystal Band-Gap Structures for the Detection of Basal Cell Cancer. Sci. Rep. 2022, 12, 5246. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, J. Optical Biosensors: An Exhaustive and Comprehensive Review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef] [PubMed]
- Wawro, D.; Ding, Y.; Gimlin, S.; Zimmerman, S.; Kearney, C.; Pawlowski, K.; Magnusson, R. Guided-Mode Resonance Sensors for Rapid Medical Diagnostic Testing Applications; Gannot, I., Ed.; SPIE: San Jose, CA, USA, 2009; p. 717303. [Google Scholar]
- Cu, D.T.; Wu, H.-W.; Chen, H.-P.; Su, L.-C.; Kuo, C.-C. Exploiting Thin-Film Properties and Guided-Mode Resonance for Designing Ultrahigh-Figure-of-Merit Refractive Index Sensors. Sensors 2024, 24, 960. [Google Scholar] [CrossRef] [PubMed]
- Barth, I.; Conteduca, D.; Reardon, C.; Johnson, S.; Krauss, T.F. Common-Path Interferometric Label-Free Protein Sensing with Resonant Dielectric Nanostructures. Light Sci. Appl. 2020, 9, 96. [Google Scholar] [CrossRef]
- Hermannsson, P.G. Design and Use of Guided Mode Resonance Filters for Refractive Index Sensing. Ph.D. Thesis, Technical University of Denmark, Lyngby, Denmark, July 2015. [Google Scholar]
- Triggs, G.J.; Wang, Y.; Reardon, C.P.; Fischer, M.; Evans, G.J.O.; Krauss, T.F. Chirped Guided-Mode Resonance Biosensor. Optica 2017, 4, 229. [Google Scholar] [CrossRef]
- Drayton, A.; Barth, I.; Krauss, T.F. Guided Mode Resonances and Photonic Crystals for Biosensing and Imaging. In Semiconductors and Semimetals; Elsevier: Amsterdam, The Netherlands, 2019; Volume 100, pp. 115–148. ISBN 978-0-12-817542-2. [Google Scholar]
- Drayton, A.; Li, K.; Simmons, M.; Reardon, C.; Krauss, T.F. Performance Limitations of Resonant Refractive Index Sensors with Low-Cost Components. Opt. Express 2020, 28, 32239. [Google Scholar] [CrossRef]
- Kenaan, A.; Li, K.; Barth, I.; Johnson, S.; Song, J.; Krauss, T.F. Guided Mode Resonance Sensor for the Parallel Detection of Multiple Protein Biomarkers in Human Urine with High Sensitivity. Biosens. Bioelectron. 2020, 153, 112047. [Google Scholar] [CrossRef]
- Jack, C.R.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised Criteria for Diagnosis and Staging of Alzheimer’s Disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef]
- Raskatov, J.A. What Is the “Relevant” Amyloid Β42 Concentration? ChemBioChem 2019, 20, 1725–1726. [Google Scholar] [CrossRef]
- Blennow, K.; Mattsson, N.; Schöll, M.; Hansson, O.; Zetterberg, H. Amyloid Biomarkers in Alzheimer’s Disease. Trends Pharmacol. Sci. 2015, 36, 297–309. [Google Scholar] [CrossRef]
- Chen, S.; Cao, Z.; Nandi, A.; Counts, N.; Jiao, L.; Prettner, K.; Kuhn, M.; Seligman, B.; Tortorice, D.; Vigo, D.; et al. The Global Macroeconomic Burden of Alzheimer’s Disease and Other Dementias: Estimates and Projections for 152 Countries or Territories. Lancet Glob. Health 2024, 12, e1534–e1543. [Google Scholar] [CrossRef] [PubMed]
- Arslan, B.; Zetterberg, H.; Ashton, N.J. Blood-Based Biomarkers in Alzheimer’s Disease—Moving towards a New Era of Diagnostics. Clin. Chem. Lab. Med. CCLM 2024, 62, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s Disease: The Amyloid Cascade Hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Toledo, J.B.; Shaw, L.M.; Trojanowski, J.Q. Plasma Amyloid Beta Measurements—A Desired but Elusive Alzheimer’s Disease Biomarker. Alzheimers Res. Ther. 2013, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.P.; Loo, F.C.; Wu, S.Y.; Kong, S.K.; Wu, C.M.L.; Ho, H.P. Common-Path Spectral Interferometry with Temporal Carrier for Highly Sensitive Surface Plasmon Resonance Sensing. Opt. Express 2013, 21, 20268. [Google Scholar] [CrossRef]
- Bläsi, J.; Gerken, M. Multiplex Optical Biosensors Based on Multi-Pinhole Interferometry. Biomed. Opt. Express 2021, 12, 4265–4275. [Google Scholar] [CrossRef]
- Gupta, R.; Labella, E.; Goddard, N.J. An Optofluidic Young Interferometer Sensor for Real-Time Imaging of Refractive Index in μTAS Applications. Sens. Actuators B Chem. 2020, 321, 128491. [Google Scholar] [CrossRef]
- Labella, E.; Gupta, R. An Optofluidic Young Interferometer for Electrokinetic Transport-Coupled Biosensing. Micromachines 2024, 15, 861. [Google Scholar] [CrossRef]
- Barth, I.; Conteduca, D.; Dong, P.; Wragg, J.; Sahoo, P.K.; Arruda, G.S.; Martins, E.R.; Krauss, T.F. Phase Noise Matching in Resonant Metasurfaces for Intrinsic Sensing Stability. Optica 2024, 11, 354. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Sarkar, S.; Joseph, J. High Sensitivity Guided-Mode-Resonance Optical Sensor Employing Phase Detection. Sci. Rep. 2017, 7, 7607. [Google Scholar] [CrossRef]
- Lavín, Á.; Vicente, J.D.; Holgado, M.; Laguna, M.F.; Casquel, R.; Santamaría, B.; Maigler, M.V.; Hernández, A.L.; Ramírez, Y. On the Determination of Uncertainty and Limit of Detection in Label-Free Biosensors. Sensors 2018, 18, 2038. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, C.E.; Verberk, I.M.W.; Thijssen, E.H.; Vermunt, L.; Hansson, O.; Zetterberg, H.; Van Der Flier, W.M.; Mielke, M.M.; Del Campo, M. Blood-Based Biomarkers for Alzheimer’s Disease: Towards Clinical Implementation. Lancet Neurol. 2022, 21, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Altay, D.N.; Yagar, H.; Ozcan, H.M. A New ITO-Based Aβ42 Biosensor for Early Detection of Alzheimer’s Disease. Bioelectrochemistry 2023, 153, 108501. [Google Scholar] [CrossRef] [PubMed]
- Krauss, T.F.; Miller, L.; Wälti, C.; Johnson, S. Photonic and Electrochemical Biosensors for Near-Patient Tests–a Critical Comparison. Optica 2024, 11, 1408. [Google Scholar] [CrossRef]
- Supraja, P.; Tripathy, S.; Singh, R.; Singh, V.; Chaudhury, G.; Singh, S.G. Towards Point-of-Care Diagnosis of Alzheimer’s Disease: Multi-Analyte Based Portable Chemiresistive Platform for Simultaneous Detection of β-Amyloid (1–40) and (1–42) in Plasma. Biosens. Bioelectron. 2021, 186, 113294. [Google Scholar] [CrossRef]
- Sung, W.-H.; Hung, J.-T.; Lu, Y.-J.; Cheng, C.-M. Paper-Based Detection Device for Alzheimer’s Disease—Detecting β-Amyloid Peptides (1–42) in Human Plasma. Diagnostics 2020, 10, 272. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijaya, S.R.; Martins, A.; Morris, K.; Quinn, S.D.; Krauss, T.F. Resonant Young’s Slit Interferometer for Sensitive Detection of Low-Molecular-Weight Biomarkers. Biosensors 2025, 15, 50. https://doi.org/10.3390/bios15010050
Wijaya SR, Martins A, Morris K, Quinn SD, Krauss TF. Resonant Young’s Slit Interferometer for Sensitive Detection of Low-Molecular-Weight Biomarkers. Biosensors. 2025; 15(1):50. https://doi.org/10.3390/bios15010050
Chicago/Turabian StyleWijaya, Stefanus Renaldi, Augusto Martins, Katie Morris, Steven D. Quinn, and Thomas F. Krauss. 2025. "Resonant Young’s Slit Interferometer for Sensitive Detection of Low-Molecular-Weight Biomarkers" Biosensors 15, no. 1: 50. https://doi.org/10.3390/bios15010050
APA StyleWijaya, S. R., Martins, A., Morris, K., Quinn, S. D., & Krauss, T. F. (2025). Resonant Young’s Slit Interferometer for Sensitive Detection of Low-Molecular-Weight Biomarkers. Biosensors, 15(1), 50. https://doi.org/10.3390/bios15010050




