Emerging Biomarkers and Nanobiosensing Strategies in Diabetes
Abstract
1. Diabetes Mellitus: An Overview
1.1. Type 1 Diabetes (T1D)
1.2. Type 2 Diabetes (T2D)
1.3. Other Types
1.4. Secondary Complications
1.5. Diabetes Symptoms
2. Diabetes Screening and Diagnosis
2.1. T1D Screening and Diagnosis
2.2. T2D Screening and Diagnosis
3. Emerging Biomarkers in Diabetes
3.1. Emerging Biomarkers in T1D
| miRNA | Diseases |
|---|---|
| miR-145-5p | T1D [51], T2D [60,61,62], diabetic nephropathy [63], microvascular complications [64,65], macrovascular complications [66], diabetic foot ulcer [67,68], gestational diabetes [69] |
| miR-30e-3p | T1D [70], prediabetes [71], T2D [72], microvascular complication [73], macrovascular complication [74], retinopathy [75], diabetic nephropathy [76] |
| miR-30c-5p | T2D [77], diabetic macrovascular complication [78], diabetic nephropathy [79,80] |
| miR-148a-3p | T1D [70,81,82], T2D [83], diabetic retinopathy [75] |
| miR-155-5p | T1D [84,85], T2D [86], diabetic dyslipidaemia [87], diabetic foot ulcer [88] |
| miR-25-3p | T1D [82], diabetic nephropathy [89], diabetic retinopathy [90] |
| miR-625-5p | Diabetic cognitive impairment [91] |
| miR-20a-5p | Diabetic foot ulcer [92], gestational diabetes [93], diabetic macrovascular complication [94,95], non-alcoholic fatty liver disease [96,97] |
| miR-326 | T1D [51,98], T2D [90] |
| miR-24-3p | T1D [51,81,82,99], T2D [83], gestational diabetes [100], diabetic retinopathy [101,102] |
| miR-301b-3p | T1D [51], diabetic wound healing [103] |
| miR-223-3p | T1D [51], T2D [104], diabetic microvascular complication [105], gestational diabetes [106] |
| miR-21-5p | T2D [83], diabetic nephropathy [89,107], diabetic foot ulcer [92] |
| miR-30a-5p | T1D [51,82], T2D [108] and prediabetes [109], diabetic nephropathy [76,110] |
| miR-186-5p | T1D [51], diabetic peripheral neuropathy [111], diabetic macrovascular complication [112,113], diabetic retinopathy [114] |
| miR-26a-5p | T1D [51,82], T2D [60,83], diabetic nephropathy [89] |
| miR-126-3p | T2D [60,83,115,116,117], diabetic retinopathy [118,119], diabetic nephropathy [76,89] |
| miR-199a-3p | T1D [51], T2D [60], diabetic macrovascular complication [78], diabetic retinopathy [75] |
| miR-127-3p | T1D [51,120], T2D and prediabetes [121] |
| let-7e-5p | T1D [51,99], T2D [83] and prediabetes [121], diabetic retinopathy [75], diabetic nephropathy [107] |
| miR-146a-5p | T1D [51,81], T2D [83,122,123] and prediabetes [124] |
| miR-200a-3p | T1D [51,82,125], diabetic retinopathy [126], diabetic nephropathy [127,128], diabetic macrovascular complication [129] |
| miR-34a-5p | T1D [51,130], T2D [122,124], diabetic cognitive impairment [131], diabetic cataractogenesis [132] |
| miR-181a-5p | T1D [51,81,82], T2D [133], diabetic nephropathy [134] |
| miR-22-5p | T1D [51], macrovascular complications [135] |
| miR-93-5p | T1D [70], diabetic macrovascular complication [136] |
3.2. Emerging Biomarkers in T2D
4. Biosensor in Diabetes
4.1. Historical Account of Biosensors in Diabetes
4.2. Glucose Biosensor Market
4.3. Scope for Potential Biosensors
5. Nanobiosensors in Diabetes
5.1. Nanobiosensors in miRNA Detection
5.1.1. EC Nanobiosensors in miRNA Detection
5.1.2. Optical Nanobiosensors in miRNA Detection
5.2. Nanobiosensors in Adipokine Detection
5.2.1. EC Nanobiosensors in Adipokine Detection
5.2.2. Optical Nanobiosensors in Adipokine Detection
6. Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1,5-AHG | 1,5-anhydroglucitol |
| 2 h PG | 2 h postprandial plasma glucose |
| ADA | American Diabetes Association |
| Ag/Pt NCs | Ag/Pt nanoclusters |
| AgNWs | Silver nanowires |
| AIE | Aggregation-induced emission |
| ALP | Alkaline phosphatase |
| AuNPs | Gold nanoparticles |
| AuNPs | Gold nanorods |
| AuNP-VS4 NS NR | AuNP-vanadium sulphide nanosheet self-assembled nanorod |
| AuNTs | Gold nanotriangle |
| BCCAs | Branched-chain amino acids |
| BMI | Body-mass-index |
| CA | Chronoamperometry |
| CAGR | Compound annual growth rate |
| CDs | Carbon quantum dots |
| CGM | Continuous glucose monitoring |
| CHA | Catalytic hairpin assembly |
| CP | Carbon paper |
| CRET | Chemiluminescence resonance energy transfer |
| CRP | C-reactive protein |
| CV | Cyclic voltammetry |
| DALY | Disability-adjusted life year |
| DKA | Diabetic ketoacidosis |
| DPV | Differential pulse voltammetry |
| EC | Electrochemical |
| ECL | Electrochemiluminescence |
| EIS | Electrochemical impedance spectroscopy |
| ELISA | Enzyme-linked immunosorbent assay |
| Fe3O4 NPs | Iron oxide nanoparticles |
| Fe-Ni NPs | Iron-nickel nanoparticles |
| FGF-21 | Fibroblast growth factor-21 |
| FPG | Fasting plasma glucose |
| FRET | Fluorescence resonance energy transfer |
| GADA | Glutamic acid decarboxylase antibodies |
| GDM | Gestational diabetes mellitus |
| GNPs | Gold nanoparticles |
| GO | Graphene oxide |
| GOx | Glucose oxidase |
| H2O2 | Hydrogen peroxide |
| HbA1c | Glycated haemoglobin |
| HCR | Hybridisation chain reaction |
| HDL | High-density lipoprotein |
| HLA | Human leukocyte antigen |
| HRP | Horseradish peroxidase |
| IA-2A | Islet antigen-2 antibodies |
| IAA | Insulin autoantibodies |
| IDF | International Diabetes Federation |
| IL-6 | Interleukin-6 |
| iSDA | Isothermal strand displacement amplification |
| LCR | Ligase chain reaction |
| LDL | Low-density lipoprotein |
| LFD | Lateral flow device |
| LOD | Limit of detection |
| LSPR | Localised surface plasmon resonance |
| MB | Methylene blue |
| MCH | 6-mercapto-1-hexanol |
| MCP-1 | Monocyte chemoattractant protein-1 |
| miRNA | microRNA |
| MNPs | Magnetic nanoparticles |
| MOFs | Metal–organic frameworks |
| MRE | Biomolecular recognition element |
| MW | Methylene white |
| NCQ | Nitrogen-doped carbon quantum dots |
| NGAL | Neutrophil gelatinase-associated lipocalin |
| NIH | National Institute of Health |
| NR | Not reported |
| OCV | Open circuit voltage |
| OGTT | Oral glucose tolerance test |
| Pd@Pt NDs | Pd@Pt core@shell nanodendrites |
| PET | Photoinduced electron transfer |
| POC | Point-of-care |
| PPY | Polypyrrole |
| PtNP-Ce-MOF | PtNP-modified Cerium MOF |
| QDs | Quantum dots |
| qRT-PCR | Quantitative reverse transcription polymerase chain reaction |
| RBP-4 | Retinol binding protein-4 |
| RCA | Rolling circle amplification |
| rGO | Reduced graphene oxide |
| RPG | Random plasma glucose |
| SDA | Strand displacement amplification |
| SERS | Surface-enhanced Raman scattering |
| SHBG | Sex hormone-binding globulin |
| Si QDs | Silicon quantum dots |
| SMBG | Self-monitoring blood glucose |
| SPCE | Screen-printed carbon electrode |
| SPE | Screen-printed electrode |
| SPR | Surface plasmon resonance |
| SWV | Square wave voltammetry |
| ssDNA | Single-stranded DNA |
| T1D | Type 1 diabetes |
| T2D | Type 2 diabetes |
| TCNQ | 7,7,8,8-tetracyanoquinodimethane |
| TFO | Triplex-forming oligonucleotide |
| TiO2NPs | Titanium dioxide nanoparticles |
| TMB | 3,3’,5,5’-tetramethylbenzidine |
| tMB | Triplex molecular beacon |
| TNF-α | Tumour necrosis factor-α |
| TTF | Tetrathiafulvalene |
| UCNPs | Upconverting nanoparticles |
| WHO | World Health Organization |
| ZnO NPs | Zinc oxide nanoparticles |
| ZnO NRs | Zinc oxide nanorods |
| ZnT8A | Zinc transporter 8 antibodies |
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013, 36 (Suppl. S1), S67–S74. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33 (Suppl. S1), S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19. [Google Scholar]
- World Health Organization. Diabetes: Overview and Key Facts; World Health Organization: Geneva, Switzerland, 14 November 2024; Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 3 December 2024).
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef]
- A Gregory, G.; Robinson, T.I.G.; E Linklater, S.; Wang, F.; Colagiuri, S.; de Beaufort, C.; Donaghue, K.C.; Magliano, D.J.; Maniam, J.; Orchard, T.J.; et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: A modelling study. Lancet Diabetes Endocrinol. 2022, 10, 741–760. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, Å. Type 1 diabetes mellitus. Nat. Rev. Dis. Primers 2017, 3, 17016. [Google Scholar] [CrossRef]
- Herold, K.C.; Delong, T.; Perdigoto, A.L.; Biru, N.; Brusko, T.M.; Walker, L.S. The immunology of type 1 diabetes. Nat. Rev. Immunol. 2024, 24, 435–451. [Google Scholar] [CrossRef]
- Evans-Molina, C.; Sims, E.K.; DiMeglio, L.A.; Ismail, H.M.; Steck, A.K.; Palmer, J.P.; Krischer, J.P.; Geyer, S.; Xu, P.; Sosenko, J.M. β Cell dysfunction exists more than 5 years before type 1 diabetes diagnosis. J. Clin. Investig. 2018, 3, e120877. [Google Scholar] [CrossRef]
- Lu, X.; Xie, Q.; Pan, X.; Zhang, R.; Zhang, X.; Peng, G.; Zhang, Y.; Shen, S.; Tong, N. Type 2 diabetes mellitus in adults: Pathogenesis, prevention and therapy. Signal Transduct. Target. Ther. 2024, 9, 262. [Google Scholar] [CrossRef]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas: 2021, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Bonnefond, A.; Froguel, P. Rare and common genetic events in type 2 diabetes: What should biologists know? Cell Metab. 2015, 21, 357–368. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 2019, 5, 47. [Google Scholar] [CrossRef]
- Bonnefond, A.; Unnikrishnan, R.; Doria, A.; Vaxillaire, M.; Kulkarni, R.N.; Mohan, V.; Trischitta, V.; Froguel, P. Monogenic diabetes. Nat. Rev. Dis. Primers 2023, 9, 12. [Google Scholar] [CrossRef]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Tan, T.-W.; Boulton, A.J.; Bus, S.A. Diabetic foot ulcers: A review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef] [PubMed]
- FDA-NIH. Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource; Food and Drug Administration, National Institutes of Health: Silver Spring, MD, USA, 2016.
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.G.; Hummel, M.; Schenker, M.; Bonifacio, E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: The 2-year analysis of the German BABYDIAB Study. Diabetes 1999, 48, 460–468. [Google Scholar] [CrossRef]
- Ilonen, J.; Hammais, A.; Laine, A.-P.; Lempainen, J.; Vaarala, O.; Veijola, R.; Simell, O.; Knip, M. Patterns of β-cell autoantibody appearance and genetic associations during the first years of life. Diabetes 2013, 62, 3636–3640. [Google Scholar] [CrossRef] [PubMed]
- Krischer, J.P.; Lynch, K.F.; Schatz, D.A.; Ilonen, J.; Lernmark, Å.; Hagopian, W.A.; Rewers, M.J.; She, J.-X.; Simell, O.G.; Toppari, J.; et al. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: The TEDDY study. Diabetologia 2015, 58, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.G.; Rewers, M.; Simell, O.; Simell, T.; Lempainen, J.; Steck, A.; Winkler, C.; Ilonen, J.; Veijola, R.; Knip, M.; et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013, 309, 2473–2479. [Google Scholar] [CrossRef]
- Erlich, H.; Valdes, A.M.; Noble, J.; Carlson, J.A.; Varney, M.; Concannon, P.; Mychaleckyj, J.C.; Todd, J.A.; Bonella, P.; Fear, A.L.; et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: Analysis of the type 1 diabetes genetics consortium families. Diabetes 2008, 57, 1084–1092. [Google Scholar] [CrossRef]
- Oliveira, S.M.R.; Rebocho, A.; Ahmadpour, E.; Nissapatorn, V.; De Lourdes Pereira, M. Type 1 diabetes mellitus: A review on advances and challenges in creating insulin producing devices. Micromachines 2023, 14, 151. [Google Scholar] [CrossRef]
- Sims, E.K.; Besser, R.E.; Dayan, C.; Rasmussen, C.G.; Greenbaum, C.; Griffin, K.J.; Hagopian, W.; Knip, M.; Long, A.E.; Martin, F.; et al. Screening for type 1 diabetes in the general population: A status report and perspective. Diabetes 2022, 71, 610–623. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2016, 38 (Suppl. S1), S8–S16. [Google Scholar]
- Akram, M. A focused review of the role of ketone bodies in health and disease. J. Med. Food 2013, 16, 965–967. [Google Scholar] [CrossRef] [PubMed]
- Saydah, S.H.; Imperatore, G.; Henkin, L.; D’agostino, R.; Divers, J.; Mayer-Davis, E.J.; Dabelea, D.; Klingensmith, G.; Pihoker, C.; Lawrence, J.M. Trends and characteristics of self-reported case presentation of diabetes diagnosis among youth from 2002 to 2010: Findings from the SEARCH for diabetes in youth study. Diabetes Care 2015, 38, e84–e85. [Google Scholar] [CrossRef][Green Version]
- Care, D. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2015, 39, S13–S22. [Google Scholar] [CrossRef]
- Little, R.R.; Rohlfing, C.L. The long and winding road to optimal HbA1c measurement. Clin. Chim. Acta 2013, 418, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Shields, B.M.; McDonald, T.J.; Oram, R.; Hill, A.; Hudson, M.; Leete, P.; Pearson, E.R.; Richardson, S.J.; Morgan, N.G.; Hattersley, A.T.; et al. C-Peptide decline in type 1 diabetes has two phases: An initial exponential fall and a subsequent stable phase. Diabetes Care 2018, 41, 1486–1492. [Google Scholar] [CrossRef]
- Iqbal, S.; Abu Jayyab, A.; Alrashdi, A.M.; Reverté-Villarroya, S. The predictive ability of c-peptide in distinguishing type 1 diabetes from type 2 diabetes: A systematic review and meta-analysis. Endocr. Pract. 2023, 29, 379–387. [Google Scholar] [CrossRef]
- American Diabetes Association. Professional Practice Committee: Standards of Medical Care in Diabetes—2022; American Diabetes Association: Arlington, VA, USA, 2022; p. S3. [Google Scholar]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Prillaman, M. Why BMI is flawed—And how to redefine obesity. Nature 2023, 622, 232–233. [Google Scholar] [CrossRef]
- Jayedi, A.; Soltani, S.; Zargar, M.S.; Khan, T.A.; Shab-Bidar, S. Central fatness and risk of all cause mortality: Systematic review and dose-response meta-analysis of 72 prospective cohort studies. BMJ 2020, 370, m3324. [Google Scholar] [CrossRef]
- Balasubramanyam, A. Defining and Classifying New Subgroups of Diabetes. Annu. Rev. Med. 2021, 72, 63–74. [Google Scholar] [CrossRef]
- Xu, S.; Wu, Y.; Li, J.; Pan, X.; Zhang, X.; Liu, Y.; Zhang, F.; Tong, N. Evaluation of the value of diabetes risk scores in screening for undiagnosed diabetes and prediabetes: A community-based study in southwestern China. Postgrad. Med. 2020, 132, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.; Manco, M.; Satman, I.; Chan, J.; Schmidt, M.I.; Sesti, G.; Fiorentino, T.V.; Abdul-Ghani, M.; Jagannathan, R.; Aravindakshan, P.K.T.; et al. International Diabetes Federation Position Statement on the 1-h post-load plasma glucose for the diagnosis of intermediate hyperglycaemia and type 2 diabetes. Diabetes Res. Clin. Pract. 2024, 209, 111589. [Google Scholar] [CrossRef]
- World Health Organization. Classification of Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Rojas, J.; Bermudez, V.; Palmar, J.; Martínez, M.S.; Olivar, L.C.; Nava, M.; Tomey, D.; Rojas, M.; Salazar, J.; Garicano, C.; et al. Pancreatic beta cell death: Novel potential mechanisms in diabetes therapy. J. Diabetes Res. 2018, 2018, 9601801. [Google Scholar] [CrossRef]
- Cernea, S.; Dobreanu, M. Diabetes and beta cell function: From mechanisms to evaluation and clinical implications. Biochem. Medica 2013, 23, 266–280. [Google Scholar] [CrossRef]
- Metcalf, G.A.D. MicroRNAs: Circulating biomarkers for the early detection of imperceptible cancers via biosensor and machine-learning advances. Oncogene 2024, 43, 2135–2142. [Google Scholar] [CrossRef]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as biomarkers in disease: Latest findings regarding their role in diagnosis and prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sun, C.; Zhao, Y.; Wang, Q.; Guo, J.; Ye, B.; Yu, G. Overview of MicroRNAs as diagnostic and prognostic biomarkers for high-incidence cancers in 2021. Int. J. Mol. Sci. 2022, 23, 11389. [Google Scholar] [CrossRef]
- Guay, C.; Regazzi, R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat. Rev. Endocrinol. 2013, 9, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Hardikar, A.; Joglekar, M.; Wong, W.; Kunte, P.; Kulkarni, R.; Ahmed, I.; Farr, R.J.; Pham, N.H.T.; Coles, M.; Maynard, C.L.; et al. Applicability of a microRNA-based dynamic risk score for type 1 diabetes. Res. Sq. 2024, preprint. [Google Scholar] [CrossRef]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef]
- Wong, W.; Farr, R.; Joglekar, M.; Januszewski, A.; Hardikar, A. Probe-based real-time PCR approaches for quantitative measurement of microRNAs. J. Vis. Exp. JoVE 2015, 98, 52586. [Google Scholar]
- Fyvie, M.J.; Gillespie, K.M. The importance of biomarker development for monitoring type 1 diabetes progression rate and therapeutic responsiveness. Front. Immunol. 2023, 14, 1158278. [Google Scholar] [CrossRef]
- Watkins, R.A.; Evans-Molina, C.; Blum, J.S.; DiMeglio, L.A. Established and emerging biomarkers for the prediction of type 1 diabetes: A systematic review. Transl. Res. 2014, 164, 110–121. [Google Scholar] [CrossRef]
- Joglekar, M.V.; Kaur, S.; Pociot, F.; Hardikar, A. Prediction of progression to type 1 diabetes with dynamic biomarkers and risk scores. Lancet Diabetes Endocrinol. 2024, 12, 483–492. [Google Scholar] [CrossRef]
- Bonifacio, E. Predicting Type 1 Diabetes Using Biomarkers. Diabetes Care 2015, 38, 989–996. [Google Scholar] [CrossRef]
- Yi, L.; Swensen, A.C.; Qian, W.-J. Serum biomarkers for diagnosis and prediction of type 1 diabetes. Transl. Res. 2018, 201, 13–25. [Google Scholar] [CrossRef]
- Brenu, E.W.; Harris, M.; Hamilton-Williams, E.E. Circulating biomarkers during progression to type 1 diabetes: A systematic review. Front. Endocrinol. 2023, 14, 1117076. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Leung, S.-W. MicroRNA biomarkers of type 2 diabetes: Evidence synthesis from meta-analyses and pathway modelling. Diabetologia 2023, 66, 288–299. [Google Scholar] [CrossRef]
- Alicka, M.; Major, P.; Wysocki, M.; Marycz, K. Adipose-derived mesenchymal stem cells isolated from patients with type 2 diabetes show reduced “stemness” through an altered secretome profile, impaired anti-oxidative protection, and mitochondrial dynamics deterioration. J. Clin. Med. 2019, 8, 765. [Google Scholar] [CrossRef]
- Lopez, Y.O.N.; Retnakaran, R.; Zinman, B.; Pratley, R.E.; Seyhan, A.A. Predicting and understanding the response to short-term intensive insulin therapy in people with early type 2 diabetes. Mol. Metab. 2019, 20, 63–78. [Google Scholar] [CrossRef]
- Han, L.-L.; Wang, S.-H.; Yao, M.-Y.; Zhou, H. Urinary exosomal microRNA-145-5p and microRNA-27a-3p act as noninvasive diagnostic biomarkers for diabetic kidney disease. World J. Diabetes 2024, 15, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cui, C.; Xu, H. Downregulation of miR-145-5p elevates retinal ganglion cell survival to delay diabetic retinopathy progress by targeting FGF5. Biosci. Biotechnol. Biochem. 2019, 83, 1655–1662. [Google Scholar] [CrossRef]
- Barutta, F.; Bellini, S.; Guarrera, S.; Matullo, G.; Schalkwijk, C.; Stehouwer, C.D.; Chaturvedi, N.; Soedamah-Muthu, S.S.; Durazzo, M.; Gruden, G. Association of serum MicroRNA-145-5p levels with microvascular complications of type 1 Diabetes: The EURODIAB prospective complications study. Diabetes Res. Clin. Pract. 2022, 190, 109987. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tao, B.; Fan, S.; Cui, S.; Pu, Y.; Qiu, L.; Xia, H.; Xu, L. Over-expression of microRNA-145 drives alterations in β-adrenergic signaling and attenuates cardiac remodeling in heart failure post myocardial infarction. Aging 2020, 12, 11603–11622. [Google Scholar] [CrossRef]
- Liang, L.; Stone, R.C.; Stojadinovic, O.; Ramirez, H.; Pastar, I.; Maione, A.G.; Smith, A.; Yanez, V.; Veves, A.; Kirsner, R.S.; et al. Integrative analysis of miRNA and mRNA paired expression profiling of primary fibroblast derived from diabetic foot ulcers reveals multiple impaired cellular functions. Wound Repair Regen. 2016, 24, 943–953. [Google Scholar] [CrossRef]
- Wang, C.; Huang, L.; Li, J.; Liu, D.; Wu, B. MicroRNA miR-145-5p inhibits cutaneous wound healing by targeting PDGFD in diabetic foot ulcer. Biochem. Genet. 2024, 62, 2437–2454. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Kotlabova, K.; Krofta, L. Cardiovascular disease-associated MicroRNAs as novel biomarkers of first-trimester screening for gestational diabetes mellitus in the absence of other pregnancy-related complications. Int. J. Mol. Sci. 2022, 23, 10635. [Google Scholar] [CrossRef] [PubMed]
- Åkerman, L.; Casas, R.; Ludvigsson, J.; Tavira, B.; Skoglund, C.; van Wijnen, A. Serum miRNA levels are related to glucose homeostasis and islet autoantibodies in children with high risk for type 1 diabetes. PLoS ONE 2018, 13, e0191067. [Google Scholar] [CrossRef]
- Weale, C.J.; Matshazi, D.M.; Davids, S.F.G.; Raghubeer, S.; Erasmus, R.T.; Kengne, A.P.; Davison, G.M.; Matsha, T.E. MicroRNAs-1299, -126-3p and -30e-3p as potential diagnostic biomarkers for prediabetes. Diagnostics 2021, 11, 949. [Google Scholar] [CrossRef]
- Weale, C.J.; Matshazi, D.M.; Davids, S.F.G.; Raghubeer, S.; Erasmus, R.T.; Kengne, A.P.; Davison, G.M.; Matsha, T.E. Expression profiles of circulating microRNAs in South African type 2 diabetic individuals on treatment. Front. Genet. 2021, 12, 702410. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Liu, J. Silencing circ_0001879 inhibits the proliferation and migration of human retinal microvascular endothelial cells under high-glucose conditions via modulating miR-30-3p. Gene 2020, 760, 144992. [Google Scholar] [CrossRef]
- Samidurai, A.; Olex, A.L.; Ockaili, R.; Kraskauskas, D.; Roh, S.K.; Kukreja, R.C.; Das, A. Integrated Analysis of lncRNA–miRNA–mRNA Regulatory Network in Rapamycin-Induced Cardioprotection against Ischemia/Reperfusion Injury in Diabetic Rabbits. Cells 2023, 12, 2820. [Google Scholar] [CrossRef] [PubMed]
- Desjarlais, M.; Rivera, J.C.; Lahaie, I.; Cagnone, G.; Wirt, M.; Omri, S.; Chemtob, S.; Chen, J. MicroRNA expression profile in retina and choroid in oxygen-induced retinopathy model. PLoS ONE 2019, 14, e0218282. [Google Scholar] [CrossRef]
- Motshwari, D.D.; George, C.; Matshazi, D.M.; Weale, C.J.; Davids, S.F.G.; Zemlin, A.E.; Erasmus, R.T.; Kengne, A.P.; Matsha, T.E. Expression of whole blood miR-126-3p, -30a-5p, -1299, -182-5p and -30e-3p in chronic kidney disease in a South African community-based sample. Sci. Rep. 2022, 12, 4107. [Google Scholar] [CrossRef]
- Grieco, G.E.; Besharat, Z.M.; Licata, G.; Fignani, D.; Brusco, N.; Nigi, L.; Formichi, C.; Po, A.; Sabato, C.; Dardano, A.; et al. Circulating microRNAs as clinically useful biomarkers for Type 2 Diabetes Mellitus: miRNomics from bench to bedside. Transl. Res. 2022, 247, 137–157. [Google Scholar] [CrossRef]
- Stępień, E.Ł.; Durak-Kozica, M.; Kamińska, A.; Targosz-Korecka, M.; Libera, M.; Tylko, G.; Opalińska, A.; Kapusta, M.; Solnica, B.; Georgescu, A.; et al. Circulating ectosomes: Determination of angiogenic microRNAs in type 2 diabetes. Theranostics 2018, 8, 3874–3890. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Duan, L.; Guo, T.; Gao, Y.; Tian, L.; Liu, J.; Wang, S.; Yang, J. Downregulation of miR-30c promotes renal fibrosis by target CTGF in diabetic nephropathy. J. Diabetes Its Complicat. 2016, 30, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Ghai, V.; Wu, X.; Bheda-Malge, A.; Argyropoulos, C.P.; Bernardo, J.F.; Orchard, T.; Galas, D.; Wang, K. Genome-wide profiling of urinary extracellular vesicle microRNAs associated with diabetic nephropathy in type 1 diabetes. Kidney Int. Rep. 2018, 3, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Assmann, T.S.; Recamonde-Mendoza, M.; De Souza, B.M.; Crispim, D. MicroRNA expression profiles and type 1 diabetes mellitus: Systematic review and bioinformatic analysis. Endocr. Connect. 2017, 6, 773–790. [Google Scholar] [CrossRef]
- Nielsen, L.B.; Wang, C.; Sørensen, K.; Bang-Berthelsen, C.H.; Hansen, L.; Andersen, M.-L.M.; Hougaard, P.; Juul, A.; Zhang, C.-Y.; Pociot, F.; et al. Circulating levels of MicroRNA from children with newly diagnosed type 1 diabetes and healthy controls: Evidence that miR-25 associates to residual beta-cell function and glycaemic control during disease progression. Exp. Diabetes Res. 2012, 2012, 896362. [Google Scholar] [CrossRef]
- Demirsoy, H.; Ertural, D.Y.; Balci, Ş.; Çınkır, Ü.; Sezer, K.; Tamer, L.; Aras, N. Profiles of circulating miRNAs following metformin treatment in patients with type 2 diabetes. J. Med. Biochem. 2018, 37, 499. [Google Scholar] [CrossRef]
- Guay, C.; Kruit, J.K.; Rome, S.; Menoud, V.; Mulder, N.L.; Jurdzinski, A.; Mancarella, F.; Sebastiani, G.; Donda, A.; Gonzalez, B.J.; et al. Lymphocyte-derived exosomal MicroRNAs promote pancreatic β cell death and may contribute to type 1 diabetes development. Cell Metab. 2019, 29, 348–361. [Google Scholar] [CrossRef]
- Mostahfezian, M.; Azhir, Z.; Dehghanian, F.; Hojati, Z. Expression Pattern of microRNAs, miR-21, miR-155 and miR-338 in patients with type 1 diabetes. Arch. Med Res. 2019, 50, 79–85. [Google Scholar] [CrossRef]
- Catanzaro, G.; Conte, F.; Trocchianesi, S.; Splendiani, E.; Bimonte, V.M.; Mocini, E.; Filardi, T.; Po, A.; Besharat, Z.M.; Gentile, M.C.; et al. Network analysis identifies circulating miR-155 as predictive biomarker of type 2 diabetes mellitus development in obese patients: A pilot study. Sci. Rep. 2023, 13, 19496. [Google Scholar] [CrossRef]
- Nemecz, M.; Stefan, D.S.; Comarița, I.K.; Constantin, A.; Tanko, G.; Guja, C.; Georgescu, A. Microvesicle-associated and circulating microRNAs in diabetic dyslipidemia: miR-218, miR-132, miR-143, and miR-21, miR-122, miR-155 have biomarker potential. Cardiovasc. Diabetol. 2023, 22, 260. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, Y.; Tang, Y.; Zhao, X.; Xie, D.; Chen, M. Increased expression of miR-155 in peripheral blood and wound margin tissue of type 2 diabetes mellitus patients associated with diabetic foot ulcer. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 3415–3428. [Google Scholar] [CrossRef]
- Gholaminejad, A.; Tehrani, H.A.; Fesharaki, M.G. Identification of candidate microRNA biomarkers in diabetic nephropathy: A meta-analysis of profiling studies. J. Nephrol. 2018, 31, 813–831. [Google Scholar] [CrossRef]
- Santovito, D.; Toto, L.; De Nardis, V.; Marcantonio, P.; D’aLoisio, R.; Mastropasqua, A.; De Cesare, D.; Bucci, M.; Paganelli, C.; Natarelli, L.; et al. Plasma microRNA signature associated with retinopathy in patients with type 2 diabetes. Sci. Rep. 2021, 11, 4136. [Google Scholar] [CrossRef]
- Bi, Y.; Yang, S.; Zhang, Z. 127-OR: Identification of Exosomal miRNA as a Novel Causal Biomarker for Cognitive Impairment in Type 2 Diabetes Mellitus—Triangulating Evidence from Mendelian Randomization Analyses and Multicenter Cohort. Diabetes 2024, 73 (Suppl. S1), 127-OR. [Google Scholar] [CrossRef]
- Lin, G.; Liu, X.; Hasan, M. Key extracellular proteins and TF-miRNA co-regulatory network in diabetic foot ulcer: Bioinformatics and experimental insights. PLoS ONE 2024, 19, e0307205. [Google Scholar] [CrossRef] [PubMed]
- Pheiffer, C.; Dias, S.; Rheeder, P.; Adam, S. Decreased expression of circulating miR-20a-5p in South African women with gestational diabetes mellitus. Mol. Diagn. Ther. 2018, 22, 345–352. [Google Scholar] [CrossRef]
- Jin, Z.-Q. MicroRNA targets and biomarker validation for diabetes-associated cardiac fibrosis. Pharmacol. Res. 2021, 174, 105941. [Google Scholar] [CrossRef]
- Liu, X.; Guo, B.; Zhang, W.; Ma, B.; Li, Y. MiR-20a-5p overexpression prevented diabetic cardiomyopathy via inhibition of cardiomyocyte apoptosis, hypertrophy, fibrosis and JNK/NF-κB signalling pathway. J. Biochem. 2021, 170, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Zhang, T.; Lou, G.; Xu, W.; Dong, F.; Chen, G.; Liu, Y. Plasma miR-17, miR-20a, miR-20b and miR-122 as potential biomarkers for diagnosis of NAFLD in type 2 diabetes mellitus patients. Life Sci. 2018, 208, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Niu, Q.; Liang, K.; Li, X.; Jiang, J.; Bian, C. Effect of LncPVT1/miR-20a-5p on lipid metabolism and insulin resistance in NAFLD. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 4599–4608. [Google Scholar] [CrossRef]
- Sebastiani, G.; Grieco, F.A.; Spagnuolo, I.; Galleri, L.; Cataldo, D.; Dotta, F. Increased expression of microRNA miR-326 in type 1 diabetic patients with ongoing islet autoimmunity. Diabetes/Metab. Res. Rev. 2011, 27, 862–866. [Google Scholar] [CrossRef]
- Erener, S.; Marwaha, A.; Tan, R.; Panagiotopoulos, C.; Kieffer, T.J. Profiling of circulating microRNAs in children with recent onset of type 1 diabetes. J. Clin. Investig. 2017, 2, e89656. [Google Scholar] [CrossRef]
- Blanco, J.A.; Lambert, C.; Fernandez-Sanjurjo, M.; Morales-Sanchez, P.; Pujante, P.; Pinto-Hernández, P.; Iglesias-Gutiérrez, E.; Torre, E.M.; Delgado, E. miR-24-3p and body mass index as type 2 diabetes risk factors in spanish women 15 years after gestational diabetes mellitus diagnosis. Int. J. Mol. Sci. 2023, 24, 1152. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, P.; Pan, M.; Liu, Z.; An, G.; Han, J.; Dai, F.; Du, L.; Jin, X. Relationship between elevated microRNAs and growth factors levels in the vitreous of patients with proliferative diabetic retinopathy. J. Diabetes Its Complicat. 2021, 35, 108021. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhou, P.; Liu, Z.; Dai, F.; Pan, M.; An, G.; Han, J.; Du, L.; Jin, X. The aflibercept-induced MicroRNA profile in the vitreous of proliferative diabetic retinopathy patients detected by next-generation sequencing. Front. Pharmacol. 2021, 12, 781276. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Shen, F.; Ling, H.; Jin, P.; Zhou, D.; Li, Q. CircARHGAP12 triggers mesenchymal stromal cell autophagy to facilitate its effect on repairing diabetic wounds by sponging miR-301b-3p/ATG16L1 and miR-301b-3p/ULK2. J. Investig. Dermatol. 2022, 142, 1976–1989.e4. [Google Scholar] [CrossRef]
- Sánchez-Ceinos, J.; Rangel-Zuñiga, O.A.; Clemente-Postigo, M.; Podadera-Herreros, A.; Camargo, A.; Alcalá-Diaz, J.F.; Guzmán-Ruiz, R.; López-Miranda, J.; Malagón, M.M. miR-223-3p as a potential biomarker and player for adipose tissue dysfunction preceding type 2 diabetes onset. Mol. Ther. Nucleic Acids 2021, 23, 1035–1052. [Google Scholar] [CrossRef]
- Da’aS, S.I.; Ahmed, I.; Hasan, W.H.; Abdelrahman, D.A.; Aliyev, E.; Nisar, S.; Bhat, A.A.; Joglekar, M.V.; Hardikar, A.A.; Fakhro, K.A.; et al. The link between glycemic control measures and eye microvascular complications in a clinical cohort of type 2 diabetes with microRNA-223-3p signature. J. Transl. Med. 2023, 21, 171. [Google Scholar] [CrossRef]
- Abdeltawab, A.; Zaki, M.; Abdeldayem, Y.; Mohamed, A.; Zaied, S.M. Circulating micro RNA-223 and angiopoietin-like protein 8 as biomarkers of gestational diabetes mellitus. Br. J. Biomed. Sci. 2021, 78, 12–17. [Google Scholar] [CrossRef]
- Zang, J.; Maxwell, A.P.; Simpson, D.A.; McKay, G.J. Differential expression of urinary exosomal MicroRNAs miR-21-5p and miR-30b-5p in individuals with diabetic kidney disease. Sci. Rep. 2019, 9, 10900. [Google Scholar] [CrossRef]
- Pordzik, J.; Jakubik, D.; Jarosz-Popek, J.; Wicik, Z.; Eyileten, C.; De Rosa, S.; Indolfi, C.; Siller-Matula, J.M.; Czajka, P.; Postula, M. Significance of circulating microRNAs in diabetes mellitus type 2 and platelet reactivity: Bioinformatic analysis and review. Cardiovasc. Diabetol. 2019, 18, 113. [Google Scholar] [CrossRef] [PubMed]
- Weale, C.J.; Matshazi, D.M.; Davids, S.F.; Raghubeer, S.; Erasmus, R.T.; Kengne, A.P.; Davison, G.M.; Matsha, T.E. Circulating miR-30a-5p and miR-182-5p in prediabetes and screen-detected diabetes mellitus. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 5037–5047. [Google Scholar] [CrossRef]
- Prabu, P.; Rome, S.; Sathishkumar, C.; Gastebois, C.; Meugnier, E.; Mohan, V.; Balasubramanyam, M. MicroRNAs from urinary extracellular vesicles are non-invasive early biomarkers of diabetic nephropathy in type 2 diabetes patients with the ‘Asian Indian phenotype’. Diabetes Metab. 2019, 45, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Xu, X.; Chi, X.; Wang, M. Relationship of lncRNA FTX and miR-186-5p levels with diabetic peripheral neuropathy in type 2 diabetes and its bioinformatics analysis. Ir. J. Med Sci. 2024, 193, 2293–2299. [Google Scholar] [CrossRef]
- Jiang, J.; Mo, H.; Liu, C.; Wu, B.; Wu, Z.; Li, X.; Li, T.; He, S.; Li, S.; You, Q.; et al. Inhibition of miR-186-5p contributes to high glucose-induced injury in AC16 cardiomyocytes. Exp. Ther. Med. 2018, 15, 627–632. [Google Scholar] [CrossRef]
- Wang, K.-J.; Zhao, X.; Liu, Y.-Z.; Zeng, Q.-T.; Mao, X.-B.; Li, S.-N.; Zhang, M.; Jiang, C.; Zhou, Y.; Qian, C.; et al. Circulating MiR-19b-3p, MiR-134-5p and MiR-186-5p are promising novel biomarkers for early diagnosis of acute myocardial infarction. Cell. Physiol. Biochem. 2016, 38, 1015–1029. [Google Scholar] [CrossRef]
- Yuan, Y.; Guan, Y.; Shao, C.; Wang, H.; Zhang, S. Circ_0001953 contribute to retinal vascular endothelial cell injury induced by high glucose through regulating miR 186. Trop. J. Pharm. Res. 2023, 22, 563–569. [Google Scholar] [CrossRef]
- Olivieri, F.; Bonafè, M.; Spazzafumo, L.; Gobbi, M.; Prattichizzo, F.; Recchioni, R.; Marcheselli, F.; La Sala, L.; Galeazzi, R.; Rippo, M.R.; et al. Age- and glycemia-related miR-126-3p levels in plasma and endothelial cells. Aging 2014, 6, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lv, C.; Li, L.; Chen, S.; Liu, S.; Wang, C.; Su, B. Plasma miR-126 is a potential biomarker for early prediction of type 2 diabetes mellitus in susceptible individuals. BioMed Res. Int. 2013, 2013, 761617. [Google Scholar] [CrossRef]
- Ghaneh, T.; Zeinali, F.; Babini, H.; Astaraki, S.; Hassan-Zadeh, V. An increase in the expression of circulating miR30d-5p and miR126-3p is associated with intermediate hyperglycaemia in Iranian population. Arch. Physiol. Biochem. 2023, 129, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.-L.; An, M.-X.; Liu, Y.-L.; Xu, H.-C.; Lu, Z.-Q. MicroRNA-126: A promising novel biomarker in peripheral blood for diabetic retinopathy. Int. J. Ophthalmol. 2017, 10, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Barutta, F.; Bruno, G.; Matullo, G.; Chaturvedi, N.; Grimaldi, S.; Schalkwijk, C.; Stehouwer, C.D.; Fuller, J.H.; Gruden, G. MicroRNA-126 and micro-/macrovascular complications of type 1 diabetes in the EURODIAB Prospective Complications Study. Acta Diabetol. 2017, 54, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, G.; Grieco, G.E.; Bruttini, M.; Auddino, S.; Mori, A.; Toniolli, M.; Fignani, D.; Licata, G.; Aiello, E.; Nigi, L.; et al. A set of circulating microRNAs belonging to the 14q32 chromosome locus identifies two subgroups of individuals with recent-onset type 1 diabetes. Cell Rep. Med. 2024, 5, 101591. [Google Scholar] [CrossRef]
- Strycharz, J.; Wróblewski, A.; Zieleniak, A.; Świderska, E.; Matyjas, T.; Rucińska, M.; Pomorski, L.; Czarny, P.; Szemraj, J.; Drzewoski, J.; et al. Visceral adipose tissue of prediabetic and diabetic females shares a set of similarly upregulated microRNAs functionally annotated to inflammation, oxidative stress and insulin signaling. Antioxidants 2021, 10, 101. [Google Scholar] [CrossRef]
- Lovis, P.; Roggli, E.; Laybutt, D.R.; Gattesco, S.; Yang, J.-Y.; Widmann, C.; Abderrahmani, A.; Regazzi, R. Alterations in MicroRNA expression contribute to fatty acid–induced pancreatic β-cell dysfunction. Diabetes 2008, 57, 2728–2736. [Google Scholar] [CrossRef]
- Rong, Y.; Bao, W.; Shan, Z.; Liu, J.; Yu, X.; Xia, S.; Gao, H.; Wang, X.; Yao, P.; Hu, F.B.; et al. Increased MicroRNA-146a levels in plasma of patients with newly diagnosed type 2 diabetes mellitus. PLoS ONE 2013, 8, e73272. [Google Scholar] [CrossRef]
- Kong, L.; Zhu, J.; Han, W.; Jiang, X.; Xu, M.; Zhao, Y.; Dong, Q.; Pang, Z.; Guan, Q.; Gao, L.; et al. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: A clinical study. Acta Diabetol. 2011, 48, 61–69. [Google Scholar] [CrossRef]
- Assmann, T.S.; Recamonde-Mendoza, M.; Puñales, M.; Tschiedel, B.; Canani, L.H.; Crispim, D. MicroRNA expression profile in plasma from type 1 diabetic patients: Case-control study and bioinformatic analysis. Diabetes Res. Clin. Pract. 2018, 141, 35–46. [Google Scholar] [CrossRef]
- Xue, L.; Xiong, C.; Li, J.; Ren, Y.; Zhang, L.; Jiao, K.; Chen, C.; Ding, P. miR-200-3p suppresses cell proliferation and reduces apoptosis in diabetic retinopathy via blocking the TGF-β2/Smad pathway. Biosci. Rep. 2020, 40, BSR20201545. [Google Scholar] [CrossRef] [PubMed]
- Civantos, E.; Bosch, E.; Bustillo, E.R.; Zhenyukh, O.; Egido, J.; Lorenzo, O.; Mas, S. Sitagliptin ameliorates oxidative stress in experimental diabetic nephropathy by diminishing the miR-200a/Keap-1/Nrf2 antioxidant pathway. Diabetes Metab. Syndr. Obes. Targets Ther. 2017, 10, 207–222. [Google Scholar] [CrossRef]
- Gholaminejad, A.; Tehrani, H.A.; Fesharaki, M.G. Identification of candidate microRNA biomarkers in renal fibrosis: A meta-analysis of profiling studies. Biomarkers 2018, 23, 713–724. [Google Scholar] [CrossRef] [PubMed]
- You, P.; Chen, H.; Han, W.; Deng, J. miR-200a-3p overexpression alleviates diabetic cardiomyopathy injury in mice by regulating autophagy through the FOXO3/Mst1/Sirt3/AMPK axis. PeerJ 2023, 11, e15840. [Google Scholar] [CrossRef]
- Kim, K.W.; Ho, A.; Alshabee-Akil, A.; Hardikar, A.A.; Kay, T.W.; Rawlinson, W.D.; Craig, M.E. Coxsackievirus B5 infection induces dysregulation of microRNAs predicted to target known type 1 diabetes risk genes in human pancreatic islets. Diabetes 2016, 65, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, Z.; Chen, D.; Zhang, Z.; Lin, X.; Shen, Z.; Lin, Q.; Fan, K.; Wang, Q.; Zhang, W.; et al. SIRT1 and miR-34a-5p expression in PBMCs as potential biomarkers for patients with type 2 diabetes with cognitive impairments. J. Clin. Endocrinol. Metab. 2024, 109, 815–826. [Google Scholar] [CrossRef]
- Milcu, A.I.; Anghel, F.M.; Romanescu, M.; Chis, A.R.; Anghel, A.; Boruga, O. Plasma miR-19b, miR-34a, and miR-146a expression in patients with type 2 diabetes mellitus and cataract: A pilot study. Biomol. Biomed. 2024, 24, 537–544. [Google Scholar] [CrossRef]
- Klöting, N.; Berthold, S.; Kovacs, P.; Schön, M.R.; Fasshauer, M.; Ruschke, K.; Stumvoll, M.; Blüher, M. MicroRNA expression in human omental and subcutaneous adipose tissue. PLoS ONE 2009, 4, e4699. [Google Scholar] [CrossRef]
- Liang, X.; Xu, W. miR-181a-5p regulates the proliferation and apoptosis of glomerular mesangial cells by targeting KLF6. Exp. Ther. Med. 2020, 20, 1121–1128. [Google Scholar] [CrossRef]
- Li, H.; Zhang, P.; Li, F.; Yuan, G.; Wang, X.; Zhang, A.; Li, F. Plasma miR-22-5p, miR-132-5p, and miR-150-3p Are associated with acute myocardial infarction. BioMed Res. Int. 2019, 2019, 5012648. [Google Scholar] [CrossRef]
- Giannella, A.; Castelblanco, E.; Zambon, C.F.; Basso, D.; Hernandez, M.; Ortega, E.; Alonso, N.; Mauricio, D.; Avogaro, A.; Ceolotto, G.; et al. Circulating small noncoding RNA profiling as a potential biomarker of atherosclerotic plaque composition in type 1 diabetes. Diabetes Care 2023, 46, 551–560. [Google Scholar] [CrossRef]
- Yu, G.; Tam, H.C.H.; Huang, C.; Shi, M.; Lim, C.K.P.; Chan, J.C.N.; Ma, R.C.W. Lessons and Applications of Omics Research in Diabetes Epidemiology. Curr. Diabetes Rep. 2024, 24, 27–44. [Google Scholar] [CrossRef]
- Laakso, M. Biomarkers for type 2 diabetes. Mol. Metab. 2019, 27, S139–S146. [Google Scholar] [CrossRef]
- Mandal, S. New molecular biomarkers in precise diagnosis and therapy of Type 2 diabetes. Health Technol. 2019, 10, 601–608. [Google Scholar] [CrossRef]
- Ortiz-Martínez, M.; González-González, M.; Martagón, A.J.; Hlavinka, V.; Willson, R.C.; Rito-Palomares, M. Recent developments in biomarkers for diagnosis and screening of type 2 diabetes mellitus. Curr. Diabetes Rep. 2022, 22, 95–115. [Google Scholar] [CrossRef]
- Dorcely, B.; Katz, K.; Jagannathan, R.; Chiang, S.S.; Oluwadare, B.; Goldberg, I.J.; Bergman, M. Novel biomarkers for prediabetes, diabetes, and associated complications. Diabetes Metab. Syndr. Obes. Targets Ther. 2017, 10, 345–361. [Google Scholar] [CrossRef]
- Bhatia, P.; Raina, S.; Chugh, J.; Sharma, S. miRNAs: Early prognostic biomarkers for Type 2 diabetes mellitus? Biomark. Med. 2015, 9, 1025–1040. [Google Scholar] [CrossRef]
- Le, T.N.; Bright, R.; Truong, V.; Li, J.; Juneja, R.; Vasilev, K. Key biomarkers in type 2 diabetes patients: A systematic review. Diabetes Obes. Metab. 2025, 27, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Mezza, T.; Sinturel, F.; Li, L.; Di Giuseppe, G.; Quero, G.; Jornayvaz, F.R.; Guessous, I.; Dibner, C.; Schrauwen, P.; et al. Circulating 1,5-anhydroglucitol as a biomarker of ß-cell mass independent of a diabetes phenotype in human subjects. J. Clin. Endocrinol. Metab. 2022, 107, 2833–2843. [Google Scholar] [CrossRef]
- Selvin, E.; Rawlings, A.M.; Grams, M.; Klein, R.; Sharrett, A.R.; Steffes, M.; Coresh, J. Prognostic utility of fructosamine and glycated albumin for incident diabetes and microvascular complications. Lancet. Diabetes Endocrinol. 2014, 2, 279. [Google Scholar] [CrossRef]
- Wahid, S.T.; Sultan, J.; Handley, G.; Saeed, B.O.; Weaver, J.U.; Robinson, A.C.J. Serum fructosamine as a marker of 5-year risk of developing diabetes mellitus in patients exhibiting stress hyperglycaemia. Diabet. Med. 2002, 19, 543–548. [Google Scholar] [CrossRef]
- Koga, M. Glycated albumin; clinical usefulness. Clin. Chim. Acta 2014, 433, 96–104. [Google Scholar] [CrossRef]
- Weyer, C.; Hanson, R.L.; Tataranni, P.; Bogardus, C.; Pratley, R. A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: Evidence for a pathogenic role of relative hyperinsulinemia. Diabetes 2000, 49, 2094–2101. [Google Scholar] [CrossRef]
- Sokooti, S.; Kieneker, L.M.; de Borst, M.H.; Kobold, A.M.; Kootstra-Ros, J.E.; Gloerich, J.; van Gool, A.J.; Heerspink, H.J.L.; Gansevoort, R.T.; Dullaart, R.P.; et al. Plasma C-peptide and risk of developing type 2 diabetes in the general population. J. Clin. Med. 2020, 9, 3001. [Google Scholar] [CrossRef]
- González-González, J.G.; Violante-Cumpa, J.R.; Zambrano-Lucio, M.; Burciaga-Jimenez, E.; Castillo-Morales, P.L.; Garcia-Campa, M.; Solis, R.C.; González-Colmenero, A.D.; Rodríguez-Gutiérrez, R. HOMA-IR as a predictor of health outcomes in patients with metabolic risk factors: A systematic review and meta-analysis. High Blood Press. Cardiovasc. Prev. 2022, 29, 547–564. [Google Scholar] [CrossRef]
- Wareham, N.J.; Byrne, C.D.; Williams, R.; Day, N.; Hales, C.N. Fasting proinsulin concentrations predict the development of type 2 diabetes. Diabetes Care 1999, 22, 262–270. [Google Scholar] [CrossRef]
- Vangipurapu, J.; Stančáková, A.; Kuulasmaa, T.; Kuusisto, J.; Laakso, M.; Kulkarni, R. Both fasting and glucose-stimulated proinsulin levels predict hyperglycemia and incident type 2 diabetes: A population-based study of 9,396 finnish men. PLoS ONE 2015, 10, e0124028. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef]
- Sun, L.; Liang, L.; Gao, X.; Zhang, H.; Yao, P.; Hu, Y.; Ma, Y.; Wang, F.; Jin, Q.; Li, H.; et al. Early prediction of developing type 2 diabetes by plasma acylcarnitines: A population-based study. Diabetes Care 2016, 39, 1563–1570. [Google Scholar] [CrossRef]
- Jäger, S.; Cuadrat, R.; Wittenbecher, C.; Floegel, A.; Hoffmann, P.; Prehn, C.; Adamski, J.; Pischon, T.; Schulze, M.B. Mendelian randomization study on amino acid metabolism suggests tyrosine as causal trait for type 2 diabetes. Nutrients 2020, 12, 3890. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Sun, W.-W.; Chen, J.-C.; Zhang, H.-L.; Liu, J.; Lin, Y.; Lin, P.-C.; Wu, B.-X.; An, Y.-P.; Huang, L.; et al. Phenylalanine impairs insulin signaling and inhibits glucose uptake through modification of IRβ. Nat. Commun. 2022, 13, 4291. [Google Scholar] [CrossRef]
- Wittemans, L.B.L.; Lotta, L.A.; Oliver-Williams, C.; Stewart, I.D.; Surendran, P.; Karthikeyan, S.; Day, F.R.; Koulman, A.; Imamura, F.; Zeng, L.; et al. Assessing the causal association of glycine with risk of cardio-metabolic diseases. Nat. Commun. 2019, 10, 1060. [Google Scholar] [CrossRef]
- Chauhan, G.; Tabassum, R.; Mahajan, A.; Dwivedi, O.P.; Mahendran, Y.; Kaur, I.; Nigam, S.; Dubey, H.; Varma, B.; Madhu, S.V.; et al. Common variants of FTO and the risk of obesity and type 2 diabetes in Indians. J. Hum. Genet. 2011, 56, 720–726. [Google Scholar] [CrossRef]
- Sarhangi, N.; Sharifi, F.; Hashemian, L.; Doabsari, M.H.; Heshmatzad, K.; Rahbaran, M.; Jamaldini, S.H.; Meybodi, H.R.A.; Hasanzad, M. PPARG (Pro12Ala) genetic variant and risk of T2DM: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 12764. [Google Scholar] [CrossRef]
- Moazzam-Jazi, M.; Najd-Hassan-Bonab, L.; Masjoudi, S.; Tohidi, M.; Hedayati, M.; Azizi, F.; Daneshpour, M.S. Risk of type 2 diabetes and KCNJ11 gene polymorphisms: A nested case–control study and meta-analysis. Sci. Rep. 2022, 12, 20709. [Google Scholar] [CrossRef]
- del Bosque-Plata, L.; Martínez-Martínez, E.; Espinoza-Camacho, M.Á.; Gragnoli, C. The role of TCF7L2 in type 2 diabetes. Diabetes 2021, 70, 1220–1228. [Google Scholar] [CrossRef]
- Flannick, J.; Thorleifsson, G.; Beer, N.L.; Jacobs, S.B.R.; Grarup, N.; Burtt, N.P.; Mahajan, A.; Fuchsberger, C.; Atzmon, G.; Benediktsson, R.; et al. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat. Genet. 2014, 46, 357–363. [Google Scholar] [CrossRef]
- Wei, F.; Cai, C.; Feng, S.; Lv, J.; Li, S.; Chang, B.; Zhang, H.; Shi, W.; Han, H.; Ling, C.; et al. TOX and CDKN2A/B gene polymorphisms are associated with type 2 diabetes in Han Chinese. Sci. Rep. 2015, 5, 11900. [Google Scholar] [CrossRef] [PubMed]
- van Vliet-Ostaptchouk, J.V.; Onland-Moret, N.C.; van Haeften, T.W.; Franke, L.; Elbers, C.C.; Shiri-Sverdlov, R.; van der Schouw, Y.T.; Hofker, M.H.; Wijmenga, C. HHEX gene polymorphisms are associated with type 2 diabetes in the Dutch Breda cohort. Eur. J. Hum. Genet. 2008, 16, 652–656. [Google Scholar] [CrossRef]
- Huang, Q.; Yin, J.-Y.; Dai, X.-P.; Pei, Q.; Dong, M.; Zhou, Z.-G.; Huang, X.; Yu, M.; Zhou, H.-H.; Liu, Z.-Q. IGF2BP2 variations influence repaglinide response and risk of type 2 diabetes in Chinese population. Acta Pharmacol. Sin. 2010, 31, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morán, M.; Guerrero-Romero, F. increased levels of c-reactive protein in noncontrolled type II diabetic subjects. J. Diabetes Its Complicat. 1999, 13, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001, 286, 327–334. [Google Scholar] [CrossRef]
- Fève, B.; Bastard, J.-P. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009, 5, 305–311. [Google Scholar] [CrossRef]
- Moller, D. Potential Role of TNF-α in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol. Metab. 2000, 11, 212–217. [Google Scholar] [CrossRef]
- Masters, S.L.; Dunne, A.; Subramanian, S.L.; Hull, R.L.; Tannahill, G.M.; A Sharp, F.; Becker, C.; Franchi, L.; Yoshihara, E.; Chen, Z.; et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 2010, 11, 897–904. [Google Scholar] [CrossRef]
- Panee, J. Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes. Cytokine 2012, 60, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.-H.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef]
- Graessler, J.; Qin, Y.; Zhong, H.; Zhang, J.; Licinio, J.; Wong, M.-L.; Xu, A.; Chavakis, T.; Bornstein, A.B.; Ehrhart-Bornstein, M.; et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: Correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2012, 13, 514–522. [Google Scholar] [CrossRef]
- Mei, Z.; Wang, F.; Bhosle, A.; Dong, D.; Mehta, R.; Ghazi, A.; Zhang, Y.; Liu, Y.; Rinott, E.; Ma, S.; et al. Strain-specific gut microbial signatures in type 2 diabetes identified in a cross-cohort analysis of 8,117 metagenomes. Nat. Med. 2024, 30, 2265–2276. [Google Scholar] [CrossRef] [PubMed]
- Sanna, S.; Van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vila, A.V.; Võsa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.M.A.E.; Oosting, M.; et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef]
- Khan, S.; Maremanda, K.P.; Jena, G. Butyrate, a short-chain fatty acid and histone deacetylases inhibitor: Nutritional, physiological, and pharmacological aspects in diabetes. In Handbook of Nutrition, Diet, and Epigenetics; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 793–807. [Google Scholar]
- Stefan, N.; Fritsche, A.; Weikert, C.; Boeing, H.; Joost, H.-G.; Häring, H.-U.; Schulze, M.B. Plasma fetuin-a levels and the risk of type 2 diabetes. Diabetes 2008, 57, 2762–2767. [Google Scholar] [CrossRef]
- Ong, K.-L.; O’COnnell, R.; Januszewski, A.S.; Jenkins, A.J.; Xu, A.; Sullivan, D.R.; Barter, P.J.; Scott, R.S.; Taskinen, M.-R.; Waldman, B.; et al. Baseline circulating FGF21 concentrations and increase after fenofibrate treatment predict more rapid glycemic progression in type 2 diabetes: Results from the field study. Clin. Chem. 2017, 63, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.C.; Lee, C.H.; Fong, C.H.; Xu, A.; Tso, A.W.; Cheung, B.M.; Lam, K.S. Serum fibroblast growth factor 21 is a superior biomarker to other adipokines in predicting incident diabetes. Clin. Endocrinol. 2017, 86, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Ding, E.L.; Song, Y.; Manson, J.E.; Hunter, D.J.; Lee, C.C.; Rifai, N.; Buring, J.E.; Gaziano, J.M.; Liu, S. Sex hormone–binding globulin and risk of type 2 diabetes in women and men. N. Engl. J. Med. 2009, 361, 1152–1163. [Google Scholar] [CrossRef]
- Kwak, S.H.; Park, K.S. Recent progress in genetic and epigenetic research on type 2 diabetes. Exp. Mol. Med. 2016, 48, e220. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The role of adipokines in health and disease. Biomedicines 2023, 11, 1290. [Google Scholar] [CrossRef]
- Kirichenko, T.V.; Markina, Y.V.; Bogatyreva, A.I.; Tolstik, T.V.; Varaeva, Y.R.; Starodubova, A.V. The role of adipokines in inflammatory mechanisms of obesity. Int. J. Mol. Sci. 2022, 23, 14982. [Google Scholar] [CrossRef]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.I.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef]
- Feng, R.; Li, Y.; Wang, C.; Luo, C.; Liu, L.; Chuo, F.; Li, Q.; Sun, C. Higher vaspin levels in subjects with obesity and type 2 diabetes mellitus: A meta-analysis. Diabetes Res. Clin. Pract. 2014, 106, 88–94. [Google Scholar] [CrossRef]
- Youn, B.-S.; KlötIng, N.; Kratzsch, J.; Lee, N.; Park, J.W.; Song, E.-S.; Ruschke, K.; Oberbach, A.; Fasshauer, M.; Stumvoll, M.; et al. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes 2008, 57, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Lesná, J.; Tichá, A.; Hyšpler, R.; Musil, F.; Bláha, V.; Sobotka, L.; Zadák, Z.; Šmahelová, A. Omentin-1 plasma levels and cholesterol metabolism in obese patients with diabetes mellitus type 1: Impact of weight reduction. Nutr. Diabetes 2015, 5, e183. [Google Scholar] [CrossRef] [PubMed]
- Hedjazifar, S.; Shahidi, R.K.; Hammarstedt, A.; Bonnet, L.; Church, C.; Boucher, J.; Blüher, M.; Smith, U. The novel adipokine gremlin 1 antagonizes insulin action and is increased in type 2 diabetes and NAFLD/NASH. Diabetes 2020, 69, 331–341. [Google Scholar] [CrossRef]
- Post, A.; Dam, W.; Sokooti, S.; Groothof, D.; Gloerich, J.; van Gool, A.J.; Kremer, D.; Gansevoort, R.T.; Van den Born, J.; Kema, I.P.; et al. Circulating FGF21 concentration, fasting plasma glucose, and the risk of type 2 diabetes: Results from the PREVEND study. J. Clin. Endocrinol. Metab. 2023, 108, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef]
- Matsubara, M.; Maruoka, S.; Katayose, S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur. J. Endocrinol. 2002, 147, 173–180. [Google Scholar] [CrossRef]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Chen, B.H.; Song, Y.; Ding, E.L.; Roberts, C.K.; Manson, J.E.; Rifai, N.; Buring, J.E.; Gaziano, J.M.; Liu, S. Circulating levels of resistin and risk of type 2 diabetes in men and women: Results from two prospective cohorts. Diabetes Care 2009, 32, 329–334. [Google Scholar] [CrossRef]
- Mukherji, A.B.; Idowu, V.; Zhao, L.; Leung, L.L.K.; Shen, S.; Palaniappan, L.; Morser, J. Chemerin Levels in Individuals with Type 2 Diabetes and a Normal Weight versus Individuals with Type 2 Diabetes and Obesity: An Observational, Cross-Sectional Study. Biomedicines 2024, 12, 983. [Google Scholar] [CrossRef]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Zabel, B.A.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007, 282, 28175–28188. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-P.; Chung, F.-M.; Chang, D.-M.; Tsai, J.C.-R.; Huang, H.-F.; Shin, S.-J.; Lee, Y.-J. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2006, 91, 295–299. [Google Scholar] [CrossRef]
- Graham, T.E.; Yang, Q.; Blüher, M.; Hammarstedt, A.; Ciaraldi, T.P.; Henry, R.R.; Wason, C.J.; Oberbach, A.; Jansson, P.-A.; Smith, U.; et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N. Engl. J. Med. 2006, 354, 2552–2563. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Murray, D.L.; Choy, L.N.; Spiegelman, B.M. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 4854–4858. [Google Scholar] [CrossRef]
- Qu, D.; Liu, J.; Lau, C.W.; Huang, Y. IL-6 in diabetes and cardiovascular complications. Br. J. Pharmacol. 2014, 171, 3595–3603. [Google Scholar] [CrossRef]
- Zampetaki, A.; Kiechl, S.; Drozdov, I.; Willeit, P.; Mayr, U.; Prokopi, M.; Mayr, A.; Weger, S.; Oberhollenzer, F.; Bonora, E.; et al. Plasma MicroRNA profiling reveals loss of endothelial MiR-126 and other MicroRNAs in type 2 diabetes. Circ. Res. 2010, 107, 810–817. [Google Scholar] [CrossRef]
- Thomson, J.M.; Parker, J.; Perou, C.M.; Hammond, S.M. A custom microarray platform for analysis of microRNA gene expression. Nat. Methods 2004, 1, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; McAlexander, M.A.; Queen, S.E.; Adams, R.J. Real-time quantitative PCR and droplet digital PCR for plant miRNAs in mammalian blood provide little evidence for general uptake of dietary miRNAs: Limited evidence for general uptake of dietary plant xenomiRs. RNA Biol. 2013, 10, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zhang, L.; Qiu, H.; Wu, Y.; Wang, Z.; Zai, Y.; Liu, L.; Qu, J.; Kang, K.; Gou, D. An improved method for detecting circulating microRNAs with S-Poly(T) Plus real-time PCR. Sci. Rep. 2015, 5, 15100. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, X.; Li, Z.; Jiao, X.; Wang, Y.; Zhang, Y. Highly sensitive determination of microRNA using target-primed and branched rolling-circle amplification. Angew. Chem. Int. Ed. Engl. 2009, 48, 3268–3272. [Google Scholar] [CrossRef]
- Yao, B.; Li, J.; Huang, H.; Sun, C.; Wang, Z.; Fan, Y.; Chang, Q.; Li, S.; Xi, J. Quantitative analysis of zeptomole microRNAs based on isothermal ramification amplification. RNA 2009, 15, 1787–1794. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yan, J.; Li, Z.; Liu, C.; Cheng, Y. Simple and sensitive detection of microRNAs with ligase chain reaction. Chem. Commun. 2010, 46, 2432–2434. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Tang, Y.-W. Basic concepts of microarrays and potential applications in clinical microbiology. Clin. Microbiol. Rev. 2009, 22, 611–633. [Google Scholar] [CrossRef]
- Cao, X.; Chen, C.; Zhu, Q. Biosensors based on functional nucleic acids and isothermal amplification techniques. Talanta 2023, 253, 123977. [Google Scholar] [CrossRef]
- Gibriel, A.A.; Adel, O. Advances in ligase chain reaction and ligation-based amplifications for genotyping assays: Detection and applications. Mutat. Res. Mol. Mech. Mutagen. 2017, 773, 66–90. [Google Scholar] [CrossRef]
- Eggins, B.R. Biosensors: An Introduction; Springer-Verlag: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Chambers, J.P.; Arulanandam, B.P.; Matta, L.L.; Weis, A.; Valdes, J.J. Biosensor recognition elements. Curr. Issues Mol. Biol. 2008, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.S.; Mayo, M.W.; Bruno, J.G.; Bronk, B.V.; A Batt, C.; Chambers, J.P. A review of molecular recognition technologies for detection of biological threat agents. Biosens. Bioelectron. 2000, 15, 549–578. [Google Scholar] [CrossRef]
- Malhotra, B.D.; Ali, M.A. Nanomaterials in Biosensors: Fundamentals and Applications; Springer-Verlag: Berlin/Heidelberg, Germany, 2017; pp. 1–74. [Google Scholar]
- Teniou, A.; Rhouati, A.; Marty, J.-L. Recent Advances in Biosensors for Diagnosis of Autoimmune Diseases. Sensors 2024, 24, 1510. [Google Scholar] [CrossRef]
- Baranwal, A.; Polash, S.A.; Aralappanavar, V.K.; Behera, B.K.; Bansal, V.; Shukla, R. Recent Progress and Prospect of Metal–Organic Framework-Based Nanozymes in Biomedical Application. Nanomaterials 2024, 14, 244. [Google Scholar] [CrossRef]
- Baranwal, A.; Chandra, P. Clinical implications and electrochemical biosensing of monoamine neurotransmitters in body fluids, in vitro, in vivo, and ex vivo models. Biosens. Bioelectron. 2018, 121, 137–152. [Google Scholar] [CrossRef]
- Chalklen, T.; Jing, Q.; Kar-Narayan, S. Biosensors based on mechanical and electrical detection techniques. Sensors 2020, 20, 5605. [Google Scholar] [CrossRef]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Updike, S.J.; Hicks, G.P. The enzyme electrode. Nature 1967, 214, 986–988. [Google Scholar] [CrossRef]
- Updike, S.J.; Hicks, G.P. Reagentless substrate analysis with immobilized enzymes. Science 1967, 158, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Guilbault, G.; Lubrano, G. An enzyme electrode for the amperometric determination of glucose. Anal. Chim. Acta 1973, 64, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef]
- Yoo, E.-H.; Lee, S.-Y. Glucose biosensors: An overview of use in clinical practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef]
- Cass, A.E.G.; Davis, G.; Francis, G.D.; Hill, H.A.O.; Aston, W.J.; Higgins, I.J.; Plotkin, E.V.; Scott, L.D.L.; Turner, A.P.F. Ferrocene-mediated enzyme electrode for amperometric determination of glucose. Anal. Chem. 1984, 56, 667–671. [Google Scholar] [CrossRef]
- Chaubey, A.; Malhotra, B. Mediated biosensors. Biosens. Bioelectron. 2002, 17, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Shichiri, M.; Yamasaki, Y.; Kawamori, R.; Hakui, N.; Abe, H. Wearable artificial endocrine pancreas with needle-type glucose sensor. Lancet 1982, 320, 1129–1131. [Google Scholar] [CrossRef]
- Frew, J.E.; Hill, H.A.O. Electrochemical biosensors. Anal. Chem. 1987, 59, 933A–944A. [Google Scholar] [CrossRef]
- Matthews, D.; Bown, E.; Watson, A.; Holman, R.; Steemson, J.; Hughes, S.; Scott, D. Pen-sized digital 30-second blood glucose meter. Lancet 1987, 329, 778–779. [Google Scholar] [CrossRef]
- Khan, G.F.; Ohwa, M.; Wernet, W. Design of a stable charge transfer complex electrode for a third-generation amperometric glucose sensor. Anal. Chem. 1996, 68, 2939–2945. [Google Scholar] [CrossRef]
- Palmisano, F.; Zambonin, P.G.; Centonze, D.; Quinto, M. A Disposable, reagentless, third-generation glucose biosensor based on overoxidized poly(pyrrole)/tetrathiafulvalene−tetracyanoquinodimethane composite. Anal. Chem. 2002, 74, 5913–5918. [Google Scholar] [CrossRef] [PubMed]
- Mastrototaro, J.J. The MiniMed continuous glucose monitoring system. Diabetes Technol. Ther. 2000, 2 (Suppl. S1), 13–18. [Google Scholar] [CrossRef]
- Cox, M. An overview of continuous glucose monitoring systems. J. Pediatr. Health Care 2009, 23, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Csoeregi, E.; Schmidtke, D.W.; Heller, A. design and optimization of a selective subcutaneously implantable glucose electrode based on “wired” glucose oxidase. Anal. Chem. 1995, 67, 1240–1244. [Google Scholar] [CrossRef] [PubMed]
- Schmidtke, D.W.; Freeland, A.C.; Heller, A.; Bonnecaze, R.T. Measurement and modeling of the transient difference between blood and subcutaneous glucose concentrations in the rat after injection of insulin. Proc. Natl. Acad. Sci. USA 1998, 95, 294–299. [Google Scholar] [CrossRef]
- Klonoff, D.C. Noninvasive blood glucose monitoring. Diabetes Care 1997, 20, 433–437. [Google Scholar] [CrossRef]
- Goetz, M.; Cote, G.; Erckens, R.; March, W.; Motamedi, M. Application of a multivariate technique to Raman spectra for quantification of body chemicals. IEEE Trans. Biomed. Eng. 1995, 42, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, B.; March, W.F.; Adams, R.L. Noninvasive glucose monitoring of the aqueous humor of the eye: Part I. measurement of very small optical rotations. Diabetes Care 1982, 5, 254–258. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, H.; Ashton, H.S.; Spiers, S.; Shen, Y.; Freeborn, S.S.; Hannigan, J.; Lindberg, J.; Rae, P. Advances in photoacoustic noninvasive glucose testing. Clin. Chem. 1999, 45, 1587–1595. [Google Scholar] [CrossRef]
- Gabriely, I.; Wozniak, R.; Mevorach, M.; Kaplan, J.; Aharon, Y.; Shamoon, H. Transcutaneous glucose measurement using near-infrared spectroscopy during hypoglycemia. Diabetes Care 1999, 22, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Larin, K.V.; Eledrisi, M.S.; Motamedi, M.; Esenaliev, R.O. Noninvasive blood glucose monitoring with optical coherence tomography: A pilot study in human subjects. Diabetes Care 2002, 25, 2263–2267. [Google Scholar] [CrossRef]
- Tierney, M.; Tamada, J.; Potts, R.; Jovanovic, L.; Garg, S. Clinical evaluation of the GlucoWatch® biographer: A continual, non-invasive glucose monitor for patients with diabetes. Biosens. Bioelectron. 2001, 16, 621–629. [Google Scholar] [CrossRef]
- Corabian, P.; Chojecki, D. Exploratory Brief on Glucose Monitoring Technologies; Institute of Health Economics: Edmonton, AB, Canada, 2019. [Google Scholar]
- Kropff, J.; Choudhary, P.; Neupane, S.; Barnard, K.; Bain, S.C.; Kapitza, C.; Forst, T.; Link, M.; Dehennis, A.; DeVries, J.H. Accuracy and longevity of an implantable continuous glucose sensor in the PRECISE study: A 180-day, prospective, multicenter, pivotal trial. Diabetes Care 2017, 40, 63–68. [Google Scholar] [CrossRef]
- Boscari, F.; Vettoretti, M.; Cavallin, F.; Amato, A.M.L.; Uliana, A.; Vallone, V.; Avogaro, A.; Facchinetti, A.; Bruttomesso, D. Implantable and transcutaneous continuous glucose monitoring system: A randomized cross over trial comparing accuracy, efficacy and acceptance. J. Endocrinol. Investig. 2021, 45, 115–124. [Google Scholar] [CrossRef]
- Harb, F.; Azar, W.S.; Ghadieh, H.E.; Njeim, R.; Tawk, Y.; Costantine, J.; Kanj, R.; Eid, A.A. Future Developments in Invasive and Non-invasive Diabetes Monitoring. In Advanced Bioscience and Biosystems for Detection and Management of Diabetes; Springer: Berlin/Heidelberg, Germany, 2022; pp. 293–313. [Google Scholar]
- Irfani, M.Z.; Koesoema, A.P. Continuous and Non-Invasive Blood Glucose Measurements: A Narrative Review. In Proceedings of the 2023 10th International Conference on Information Technology, Computer, and Electrical Engineering (ICITACEE), Semarang, Indonesia, 31 August 2023–1 September 2023. [Google Scholar]
- Lee, I.; Probst, D.; Klonoff, D.; Sode, K. Continuous glucose monitoring systems—Current status and future perspectives of the flagship technologies in biosensor research. Biosens. Bioelectron. 2021, 181, 113054. [Google Scholar] [CrossRef]
- Research, G.V. Glucose Biosensors Market Size & Trends. In Glucose Biosensors Market Size, Share & Trends Analysis Report By Type (Electrochemical Biosensors, Optical Biosensors), by End Use (Hospitals, Homecare, Diagnostic Centers), by Region, and Segment Forecasts, 2025–2030; Grand View Research, Inc.: San Francisco, CA, USA, 2024; p. 190. [Google Scholar]
- Patil, R. Glucose Biosensors Market Size, Share, and Trends 2025 to 2034; Shivarkar, A., Ed.; Precedence Research: Ottawa, ON, Canada, 2024. [Google Scholar]
- Benjamin, E.M. Self-monitoring of blood glucose: The basics. Clin. Diabetes 2002, 20, 45–47. [Google Scholar] [CrossRef]
- Huang, X.; Leduc, C.; Ravussin, Y.; Li, S.; Davis, E.; Song, B.; Wang, Q.; Accili, D.; Leibel, R.; Lin, Q. Continuous monitoring of glucose in subcutaneous tissue using microfabricated differential affinity sensors. J. Diabetes Sci. Technol. 2012, 6, 1436–1444. [Google Scholar] [CrossRef]
- Malik, S.; Singh, J.; Goyat, R.; Saharan, Y.; Chaudhry, V.; Umar, A.; Ibrahim, A.A.; Akbar, S.; Ameen, S.; Baskoutas, S. Nanomaterials-based biosensor and their applications: A review. Heliyon 2023, 9, e19929. [Google Scholar] [CrossRef]
- Sannino, D. Types and classification of nanomaterials. In Nanotechnology: Trends and Future Applications; Springer: Singapore, 2021; pp. 15–38. [Google Scholar]
- Graybill, R.M.; Bailey, R.C. Emerging biosensing approaches for microRNA analysis. Anal. Chem. 2016, 88, 431–450. [Google Scholar] [CrossRef]
- Johnson, B.N.; Mutharasan, R. Biosensor-based microRNA detection: Techniques, design, performance, and challenges. Analyst 2014, 139, 1576–1588. [Google Scholar] [CrossRef] [PubMed]
- Khandan-Nasab, N.; Askarian, S.; Mohammadinejad, A.; Aghaee-Bakhtiari, S.H.; Mohajeri, T.; Oskuee, R.K. Biosensors, microfluidics systems and lateral flow assays for circulating microRNA detection: A review. Anal. Biochem. 2021, 633, 114406. [Google Scholar] [CrossRef]
- Lin, X.; Wang, K.; Luo, C.; Yang, M.; Wu, J. MicroRNA biosensors for early detection of hepatocellular carcinoma. Chemosensors 2023, 11, 504. [Google Scholar] [CrossRef]
- Kilic, T.; Erdem, A.; Ozsoz, M.; Carrara, S. microRNA biosensors: Opportunities and challenges among conventional and commercially available techniques. Biosens. Bioelectron. 2018, 99, 525–546. [Google Scholar] [CrossRef]
- Liu, W.-J.; Wang, L.-J.; Zhang, C.-Y. Progress in quantum dot-based biosensors for microRNA assay: A review. Anal. Chim. Acta 2023, 1278, 341615. [Google Scholar] [CrossRef]
- Hanoglu, S.B.; Harmanci, D.; Ucar, N.; Evran, S.; Timur, S. Recent approaches in magnetic nanoparticle-based biosensors of miRNA detection. Magnetochemistry 2023, 9, 23. [Google Scholar] [CrossRef]
- Gorgani, L.; Mohammadi, M.; Darzi, G.N.; Raoof, J.B. Metal-organic framework (MOF)-based biosensors for miRNA detection. Talanta 2024, 273, 125854. [Google Scholar] [CrossRef]
- Esmaeilzadeh, A.A.; Yaseen, M.M.; Khudaynazarov, U.; Al-Gazally, M.E.; Opulencia, M.J.C.; Jalil, A.T.; Mohammed, R.N. Recent advances on the electrochemical and optical biosensing strategies for monitoring microRNA-21: A review. Anal. Methods 2022, 14, 4449–4459. [Google Scholar] [CrossRef]
- Lu, X.; Yao, C.; Sun, L.; Li, Z. Plasmon-enhanced biosensors for microRNA analysis and cancer diagnosis. Biosens. Bioelectron. 2022, 203, 114041. [Google Scholar] [CrossRef]
- Meng, X.; Pang, X.; Yang, J.; Zhang, X.; Dong, H. Recent advances in electrochemiluminescence biosensors for MicroRNA detection. Small 2024, 20, e2307701. [Google Scholar] [CrossRef]
- Tran, H.V.; Piro, B. Recent trends in application of nanomaterials for the development of electrochemical microRNA biosensors. Microchim. Acta 2021, 188, 128. [Google Scholar] [CrossRef]
- Negahdary, M.; Angnes, L. Application of electrochemical biosensors for the detection of microRNAs (miRNAs) related to cancer. Chem. Rev. 2022, 464, 214565. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Sun, M.-H.; Zhang, Q.; Li, P.-F.; Wang, K.; Li, X.-M. Advances in Point-of-Care Testing of microRNAs Based on Portable Instruments and Visual Detection. Biosensors 2023, 13, 747. [Google Scholar] [CrossRef]
- Song, S.; Guo, Y.; Mao, D.; Gao, H.; Gao, Y.-P.; Kang, W. An ultrasensitive electrochemical/colorimetric dual-mode self-powered biosensing platform for lung cancer marker detection by multiple-signal amplification strategy. Anal. Chim. Acta 2024, 1316, 342827. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-Y.; Xie, W.-Z.; Tan, X.; Huang, K.-J.; Xu, J. Superior graphdiyne self-powered biosensing platform with highly sensitivity and reliability for dual-mode detection of MicroRNA by integrating T7 Exonuclease and 3D DNA walker induced rolling circle amplification. Anal. Chim. Acta 2023, 1239, 340696. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, J.; Lin, Y.; Wu, Y.; Luo, H.; Yan, J.; Huang, K.-J.; Tan, X. Smart enzyme-free amplification dual-mode self-powered platform designed on two-dimensional networked graphdiyne and DNA nanorods for ultra-sensitive detection of breast cancer biomarkers. Anal. Chim. Acta 2023, 1280, 341876. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Z.; Wang, L.-L.; Liu, Y.; Zhang, Y.-Q.; Li, M.-L.; Chen, C.-X.; Zhu, J.-W.; Yang, F.; Hu, Y.-H. Dual “on-off” signal conversion strategy based on surface plasmon coupling and resonance energy transfer for visual electrochemiluminescence ratiometric analysis of MiRNA-141. Biosens. Bioelectron. 2024, 253, 116162. [Google Scholar] [CrossRef] [PubMed]
- Zoughi, S.; Faridbod, F.; Moradi, S. Rapid enzyme-free detection of miRNA-21 in human ovarian cancerous cells using a fluorescent nanobiosensor designed based on hairpin DNA-templated silver nanoclusters. Anal. Chim. Acta 2024, 1320, 342968. [Google Scholar] [CrossRef]
- Dargah, M.M.; Youseftabar-Miri, L.; Divsar, F.; Hosseinjani-Pirdehi, H.; Mahani, M.; Bakhtiari, S.; Montazar, L. Triplex hairpin oligosensor for ultrasensitive determination of miRNA-155 as a cancer marker using Si quantum dots and Au nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 322, 124750. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Q.; Diao, Z.; Huo, D.; Hou, C. Label-free fluorescent biosensor based on AuNPs etching releasing signal for miRNA-155 detection. Talanta 2024, 278, 126481. [Google Scholar] [CrossRef]
- Afzalinia, A.; Mirzaee, M. Ultrasensitive fluorescent miRNA biosensor based on a “sandwich” oligonucleotide hybridization and fluorescence resonance energy transfer process using an Ln(III)-MOF and Ag nanoparticles for early cancer diagnosis: Application of central composite design. ACS Appl. Mater. Interfaces 2020, 12, 16076–16087. [Google Scholar] [CrossRef]
- Qin, Z.; Fu, J.; Wang, J.; Deng, S.; Xiong, F.; Gao, Q.; Ye, J.; Zhang, Y.; Li, S. An intelligent fluorescence sensing platform based on entropy-driven toehold-mediated strand displacement cycle reaction for point-of-care testing of miRNA. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 325, 125177. [Google Scholar] [CrossRef]
- Li, Y.; Tu, C.; Chen, Q.; Lin, Y.; Li, B.; Lyu, H. Enhanced red emission of upconversion nanoparticles via Li+ and Tm3+ codoping and active core-shell construction for sensitive detection of miRNAs. Anal. Chim. Acta 2025, 1335, 343429. [Google Scholar] [CrossRef]
- Jia, Z.; Tu, K.; Xu, Q.; Gao, W.; Liu, C.; Fang, B.; Zhang, M. A novel disease-associated nucleic acid sensing platform based on split DNA-scaffolded sliver nanocluster. Anal. Chim. Acta 2021, 1175, 338734. [Google Scholar] [CrossRef]
- Li, P.; Ye, Y.; Li, Y.; Xie, Z.; Ye, L.; Huang, J. A MoS2 nanosheet-based CRISPR/Cas12a biosensor for efficient miRNA quantification for acute myocardial infarction. Biosens. Bioelectron. 2024, 251, 116129. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Jiang, S.; Zhang, C.-Y. SiRNA-directed self-assembled quantum dot biosensor for simultaneous detection of multiple microRNAs at the single-particle level. Biosens. Bioelectron. 2020, 157, 112177. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Q.; Qi, L.; Fang, Q.; Shang, X.; Zhang, X.; Du, Y. Fluorophore and nanozyme-functionalized DNA walking: A dual-mode DNA logic biocomputing platform for microRNA sensing in clinical samples. Biosens. Bioelectron. 2024, 252, 116137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Jia, Y.; Wang, A.; Yang, J.; Yang, L. Dual-mode DNA walker-based optical fiber biosensor for ultrasensitive detection of microRNAs. Biosens. Bioelectron. 2023, 239, 115613. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, N.; Abarghoei, S.; Dadmehr, M.; Hosseini, M.; Sabahi, H.; Ganjali, M.R. Paper based colorimetric detection of miRNA-21 using Ag/Pt nanoclusters. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117529. [Google Scholar] [CrossRef]
- Zeng, Y.; Yue, H.; Cao, B.; Li, Y.; Yang, M.; Mao, C. Target-triggered formation of artificial enzymes on filamentous phage for ultrasensitive direct detection of circulating miRNA biomarkers in clinical samples. Angew. Chem. Int. Ed. Engl. 2022, 61, e202210121. [Google Scholar] [CrossRef]
- Aamri, M.; Mohammadi, H.; Amine, A. A highly sensitive colorimetric DNA sensor for MicroRNA-155 detection: Leveraging the peroxidase-like activity of copper nanoparticles in a double amplification strategy. Microchim. Acta 2024, 191, 32. [Google Scholar] [CrossRef]
- Shahsavar, K.; Shokri, E.; Hosseini, M. Sensitive colorimetric detection of miRNA-155 via G-quadruplex DNAzyme decorated spherical nucleic acid. Microchim. Acta 2022, 189, 357. [Google Scholar] [CrossRef]
- Cai, J.; Ding, L.; Gong, P.; Huang, J. A colorimetric detection of microRNA-148a in gastric cancer by gold nanoparticle–RNA conjugates. Nanotechnology 2019, 31, 095501. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhang, M.; Fu, Y.; Zhang, Q.; Si, Y.; Zhang, Y.; Fang, Y.; Zhang, D. Emerging electrochemical biosensors for lung cancer-associated protein biomarker and miRNA detection. Int. J. Biol. Macromol. 2024, 280, 135972. [Google Scholar] [CrossRef]
- Pothipor, C.; Aroonyadet, N.; Bamrungsap, S.; Jakmunee, J.; Ounnunkad, K. A highly sensitive electrochemical microRNA-21 biosensor based on intercalating methylene blue signal amplification and a highly dispersed gold nanoparticles/graphene/polypyrrole composite. Analyst 2021, 146, 2679–2688. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Nikokar, I.; Zokaei, M.; Bozorgzadeh, E. Design, development and evaluation of microRNA-199a-5p detecting electrochemical nanobiosensor with diagnostic application in Triple Negative Breast Cancer. Talanta 2018, 189, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, S.; Fatima, I.; Kiani, M.H.; Mohammadzadeh, V.; Arshad, R.; Bilal, M.; Rahdar, A.; Díez-Pascual, A.M.; Behzadmehr, R. Fluorescent-based nanosensors for selective detection of a wide range of biological macromolecules: A comprehensive review. Int. J. Biol. Macromol. 2022, 206, 115–147. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, J.; Ju, H.; Lu, H.; Wang, S.; Jin, S.; Hao, K.; Du, H.; Zhang, X. Highly sensitive multiple microRNA detection based on fluorescence quenching of graphene oxide and isothermal strand-displacement polymerase reaction. Anal. Chem. 2012, 84, 4587–4593. [Google Scholar] [CrossRef]
- Mohammadi, S.; Salimi, A. Fluorometric determination of microRNA-155 in cancer cells based on carbon dots and MnO2 nanosheets as a donor-acceptor pair. Microchim. Acta 2018, 185, 372. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, B.; Ma, Z.; Qiu, Z.; Hu, H.; Jiang, Y.; Gao, D. A microfluidic-based chemiluminescence biosensor for sensitive multiplex detection of exosomal microRNAs based on hybridization chain reaction. Talanta 2025, 281, 126838. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Chen, M.; Jia, X.; Dong, Y. A hot-spot-active magnetic graphene oxide substrate for microRNA detection based on cascaded chemiluminescence resonance energy transfer. Nanoscale 2015, 7, 3745–3753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-R.; Xu, J.-J.; Chen, H.-Y. Electrochemiluminescence ratiometry: A new approach to DNA biosensing. Anal. Chem. 2013, 85, 5321–5325. [Google Scholar] [CrossRef]
- Coutinho, C.; Somoza, Á. MicroRNA sensors based on gold nanoparticles. Anal. Bioanal. Chem. 2019, 411, 1807–1824. [Google Scholar] [CrossRef]
- Gao, X.; Xu, L.-P.; Wu, T.; Wen, Y.; Ma, X.; Zhang, X. An enzyme-amplified lateral flow strip biosensor for visual detection of microRNA-224. Talanta 2016, 146, 648–654. [Google Scholar] [CrossRef]
- Peng, W.; Zhao, Q.; Piao, J.; Zhao, M.; Huang, Y.; Zhang, B.; Gao, W.; Zhou, D.; Shu, G.; Gong, X.; et al. Ultra-sensitive detection of microRNA-21 based on duplex-specific nuclease-assisted target recycling and horseradish peroxidase cascading signal amplification. Sens. Actuators B Chem. 2018, 263, 289–297. [Google Scholar] [CrossRef]
- Pitou, M.; Papi, R.M.; Tzavellas, A.-N.; Choli-Papadopoulou, T. ssDNA-Modified Gold Nanoparticles as a Tool to Detect miRNA Biomarkers in Osteoarthritis. ACS Omega 2023, 8, 7529–7535. [Google Scholar] [CrossRef]
- Shi, H.-Y.; Yang, L.; Zhou, X.-Y.; Bai, J.; Gao, J.; Jia, H.-X.; Li, Q.-G. A gold nanoparticle-based colorimetric strategy coupled to duplex-specific nuclease signal amplification for the determination of microRNA. Microchim. Acta 2017, 184, 525–531. [Google Scholar] [CrossRef]
- Baranwal, A.; Shukla, R.; Bansal, V. Nanozyme-enhanced paper-based biosensor technologies. TrAC Trends Anal. Chem. 2024, 172, 117573. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Zhao, H.; Qu, Y.; Yuan, F.; Quan, X. A visible and label-free colorimetric sensor for miRNA-21 detection based on peroxidase-like activity of graphene/gold-nanoparticle hybrids. Anal. Methods 2016, 8, 2005–2012. [Google Scholar] [CrossRef]
- Yan, C.; Guo, J.; Qu, X.; Sun, H.; Chai, H.; Miao, P. Distance-Based Visual miRNA Biosensor with Strand Displacement Amplification-Mediated DNA Hydrogel Assembly. ACS Mater. Lett. 2024, 6, 2111–2117. [Google Scholar] [CrossRef]
- Li, D.; Cheng, W.; Yan, Y.; Zhang, Y.; Yin, Y.; Ju, H.; Ding, S. A colorimetric biosensor for detection of attomolar microRNA with a functional nucleic acid-based amplification machine. Talanta 2016, 146, 470–476. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Liu, C.; Gou, S.; Hu, S.; Guo, W. A portable colorimetric point-of-care testing platform for MicroRNA detection based on programmable entropy-driven dynamic DNA network modulated DNA-gold nanoparticle hybrid hydrogel film. Biosens. Bioelectron. 2023, 225, 115073. [Google Scholar] [CrossRef]
- Piao, J.; Zhao, Q.; Zhou, D.; Peng, W.; Gao, W.; Chen, M.; Shu, G.; Gong, X.; Chang, J. Enzyme-free colorimetric detection of MicroRNA-21 using metal chelator as label for signal generation and amplification. Anal. Chim. Acta 2019, 1052, 145–152. [Google Scholar] [CrossRef]
- Moabelo, K.L.; Lerga, T.M.; Jauset-Rubio, M.; Sibuyi, N.R.S.; O’sullivan, C.K.; Meyer, M.; Madiehe, A.M. A label-free gold nanoparticles-based optical aptasensor for the detection of retinol binding protein 4. Biosensors 2022, 12, 1061. [Google Scholar] [CrossRef] [PubMed]
- Torabi, R.; Ghourchian, H. Ultrasensitive nano-aptasensor for monitoring retinol binding protein 4 as a biomarker for diabetes prognosis at early stages. Sci. Rep. 2020, 10, 594. [Google Scholar] [CrossRef]
- Giorgi-Coll, S.; Marín, M.J.; Sule, O.; Hutchinson, P.J.; Carpenter, K.L. Aptamer-modified gold nanoparticles for rapid aggregation-based detection of inflammation: An optical assay for interleukin-6. Microchim. Acta 2020, 187, 13. [Google Scholar] [CrossRef]
- Kim, W.; Bang, A.; Kim, S.; Lee, G.-J.; Kim, Y.-H.; Choi, S. Adiponectin-targeted SERS immunoassay biosensing platform for early detection of gestational diabetes mellitus. Biosens. Bioelectron. 2022, 213, 114488. [Google Scholar] [CrossRef]
- He, J.; Wu, S.; Chen, W.; Kim, A.; Yang, W.; Wang, C.; Gu, Z.; Shen, J.; Dai, S.; Chen, W.; et al. Calligraphy of Nanoplasmonic Bioink-Based Multiplex Immunosensor for Precision Immune Monitoring and Modulation. ACS Appl. Mater. Interfaces 2023, 15, 50047–50057. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Meng, Q.; Han, J.; Zhao, W.; Chen, J.; Su, W.; Song, M.; Shi, C.; Wang, L. Electric field-induced alignment of Ag/Au nanowires for ultrasensitive in situ detection of Interleukin-6. Biosens. Bioelectron. 2025, 271, 117033. [Google Scholar] [CrossRef]
- Sytu, M.R.C.; Stoner, A.; Hahm, J.-I. Strain-Modulated and Nanorod-Waveguided Fluorescence in Single Zinc Oxide Nanorod-Based Immunodetection. Biosensors 2024, 14, 85. [Google Scholar] [CrossRef]
- Erkmen, C.; Tığ, G.A.; Uslu, B. Nanomaterial-based sandwich-type electrochemical aptasensor platform for sensitive voltammetric determination of leptin. Microchim. Acta 2022, 189, 396. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Hu, Q.; Xie, R.; Zhou, W.; Liu, X.; Wang, Y. Smartphone surface plasmon resonance imaging for the simultaneous and sensitive detection of acute kidney injury biomarkers with noninvasive urinalysis. Lab A Chip 2022, 22, 4941–4949. [Google Scholar] [CrossRef] [PubMed]
- Erkmen, C.; Tiğ, G.A.; Uslu, B. First label-free impedimetric aptasensor based on Au NPs/TiO2 NPs for the determination of leptin. Sens. Actuators B Chem. 2022, 358, 131420. [Google Scholar] [CrossRef]
- Islam, T.; Ahsan, A.; Hassan, M.; Afrin, H.; Pena-Zacarias, J.; Aldalbahi, A.; Alvarado-Tenorio, B.; Noveron, J.C. Nurunnabi detection of leptin using electrocatalyst mediated impedimetric sensing. ACS Biomater. Sci. Eng. 2022, 9, 2170–2180. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Jiang, K.; Wu, J.; Mi, H.; Peng, Y.-K.; Go, Y.Y.; Park, H.J.; Lee, J.-H. Fast and sensitive immuno-PCR assisted by plasmonic magnetic nanoparticles. Appl. Mater. Today 2021, 23, 101054. [Google Scholar] [CrossRef]
- Li, A.; Mo, X.; Lu, Y.; Zhu, G.; Liu, C.; Yang, X.; Huang, Y.; Sheng, J.; Zhang, H.; Meng, D.; et al. Digital SERS immunoassay of Interleukin-6 based on Au@Ag-Au nanotags. Biosens. Bioelectron. 2025, 270, 116973. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, L.; Li, H.; Hu, J.; Zhang, J.; Zhou, H.; Zhang, Z.; Du, X. An oriented antibody immobilization based electrochemical platform for detection of leptin in human with different body mass index. Sens. Actuators B Chem. 2022, 353, 131074. [Google Scholar] [CrossRef]
- Cai, J.; Gou, X.; Sun, B.; Li, W.; Li, D.; Liu, J.; Hu, F.; Li, Y. Porous graphene-black phosphorus nanocomposite modified electrode for detection of leptin. Biosens. Bioelectron. 2019, 137, 88–95. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Li, Q.; Dai, H.; Xiang, T.; Yang, G.; Li, L. A reusable colorimetric assay based on mixed valence state Ce-MOF@Pt nanoparticles for highly sensitive detection of visfatin. Anal. Chim. Acta 2021, 1146, 24–32. [Google Scholar] [CrossRef]
- Ali, M.; Sajid, M.; Khalid, M.A.U.; Kim, S.W.; Lim, J.H.; Huh, D.; Choi, K.H. A fluorescent lateral flow biosensor for the quantitative detection of Vaspin using upconverting nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 226, 117610. [Google Scholar] [CrossRef]
- Hosseini, Z.; Shi, D.; Yuan, J. A flexible multiplexed electrochemical biosensing platform with graphene and gold nanoparticle modification for enhanced e-ELISA point-of-care biomarker detection. Microchem. J. 2025, 208, 112437. [Google Scholar] [CrossRef]
- Liu, X.; Tseng, C.-L.; Lin, L.-Y.; Lee, C.-A.; Li, J.; Feng, L.; Song, L.; Li, X.; He, J.-H.; Sakthivel, R.; et al. Template-free synthesis of mesoporous Ce3NbO7/CeO2 hollow nanospheres for label-free electrochemical immunosensing of leptin. Sens. Actuators B Chem. 2021, 341, 130005. [Google Scholar] [CrossRef]
- Tertis, M.; Leva, P.I.; Bogdan, D.; Suciu, M.; Graur, F.; Cristea, C. Impedimetric aptasensor for the label-free and selective detection of Interleukin-6 for colorectal cancer screening. Biosens. Bioelectron. 2019, 137, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Mahani, M.; Faghihi-Fard, M.; Divsar, F.; Torkzadeh-Mahani, M.; Khakbaz, F. Ultrasensitive FRET-based aptasensor for interleukin-6 as a biomarker for COVID-19 progression using nitrogen-doped carbon quantum dots and gold nanoparticles. Microchim. Acta 2022, 189, 472. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, C.; He, J.; Chen, P. Pd@ Pt Nanodendrites as peroxidase nanomimics for enhanced colorimetric ELISA of cytokines with femtomolar sensitivity. Chemosensors 2022, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Adrover-Jaume, C.; Alba-Patiño, A.; Clemente, A.; Santopolo, G.; Vaquer, A.; Russell, S.M.; Barón, E.; Del Mar Gonzalez, M.; Ferrer, J.M.; Berman-Riu, M.; et al. Paper biosensors for detecting elevated IL-6 levels in blood and respiratory samples from COVID-19 patients. Sens. Actuators B Chem. 2021, 330, 129333. [Google Scholar] [CrossRef]
- Liang, B.; Wang, S.; Zheng, J.; Li, B.; Cheng, N.; Gan, N. All-in-one microfluidic immunosensing device for rapid and end-to-end determination of salivary biomarkers of cardiovascular diseases. Biosens. Bioelectron. 2025, 271, 117077. [Google Scholar] [CrossRef] [PubMed]
- Helmerhorst, E.; Chandler, D.J.; Nussio, M.; Mamotte, C.D. Real-time and label-free bio-sensing of molecular interactions by surface plasmon resonance: A laboratory medicine perspective. Clin. Biochem. Rev. 2012, 33, 161–173. [Google Scholar] [PubMed]
- Kukkar, D.; Chhillar, M.; Kim, K.-H. Application of SERS-based nanobiosensors to metabolite biomarkers of CKD. Biosens. Bioelectron. 2023, 232, 115311. [Google Scholar] [CrossRef] [PubMed]
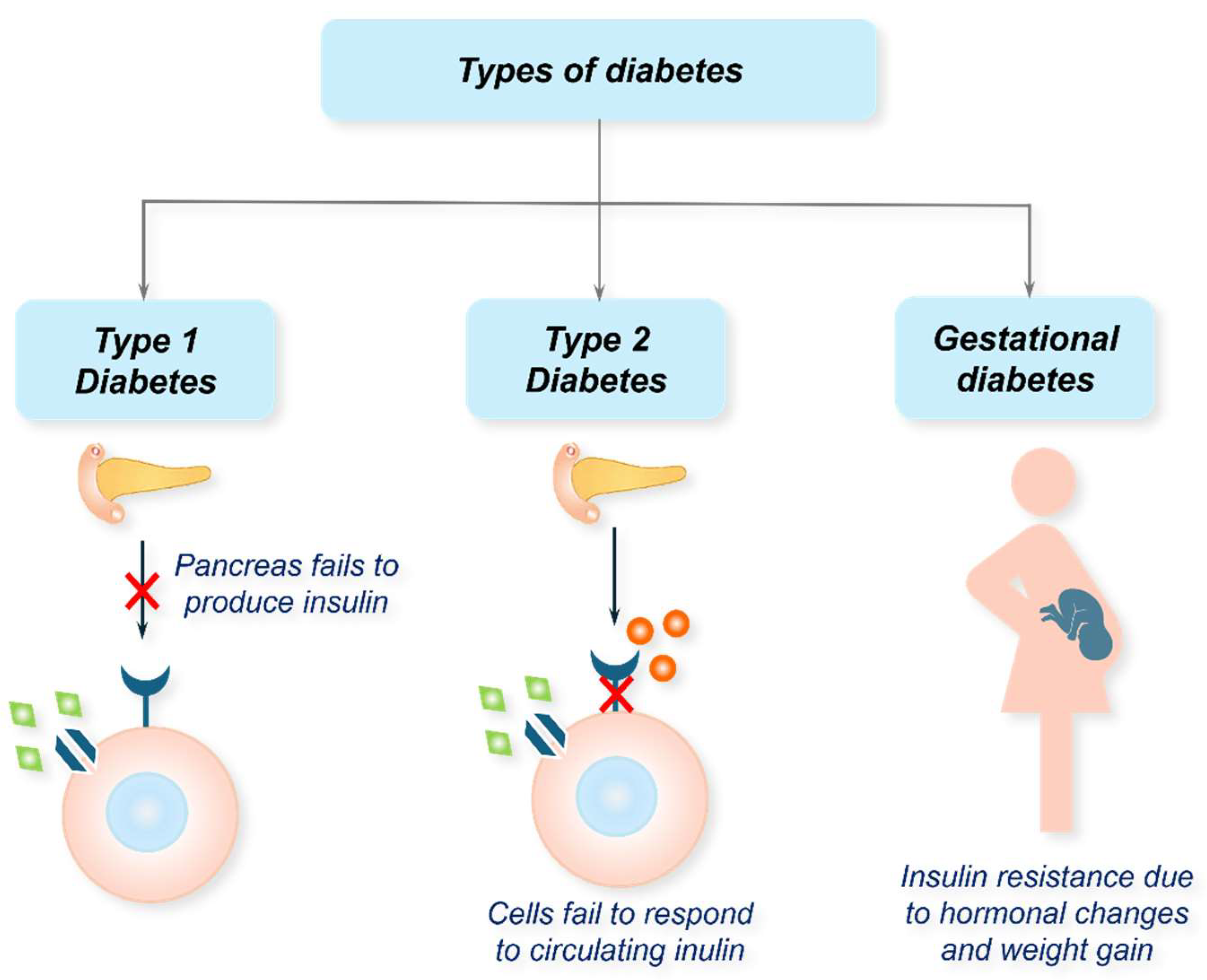
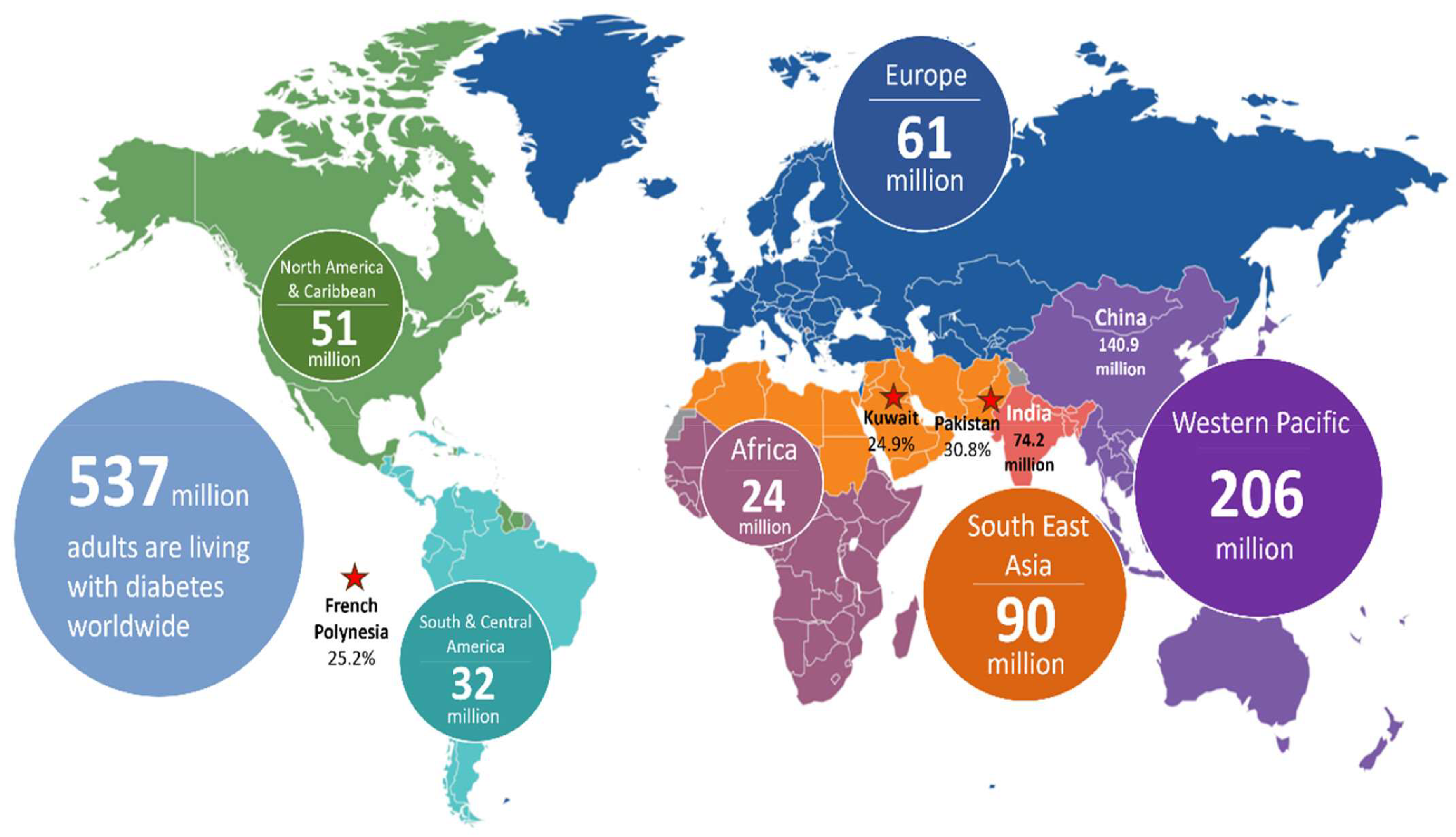
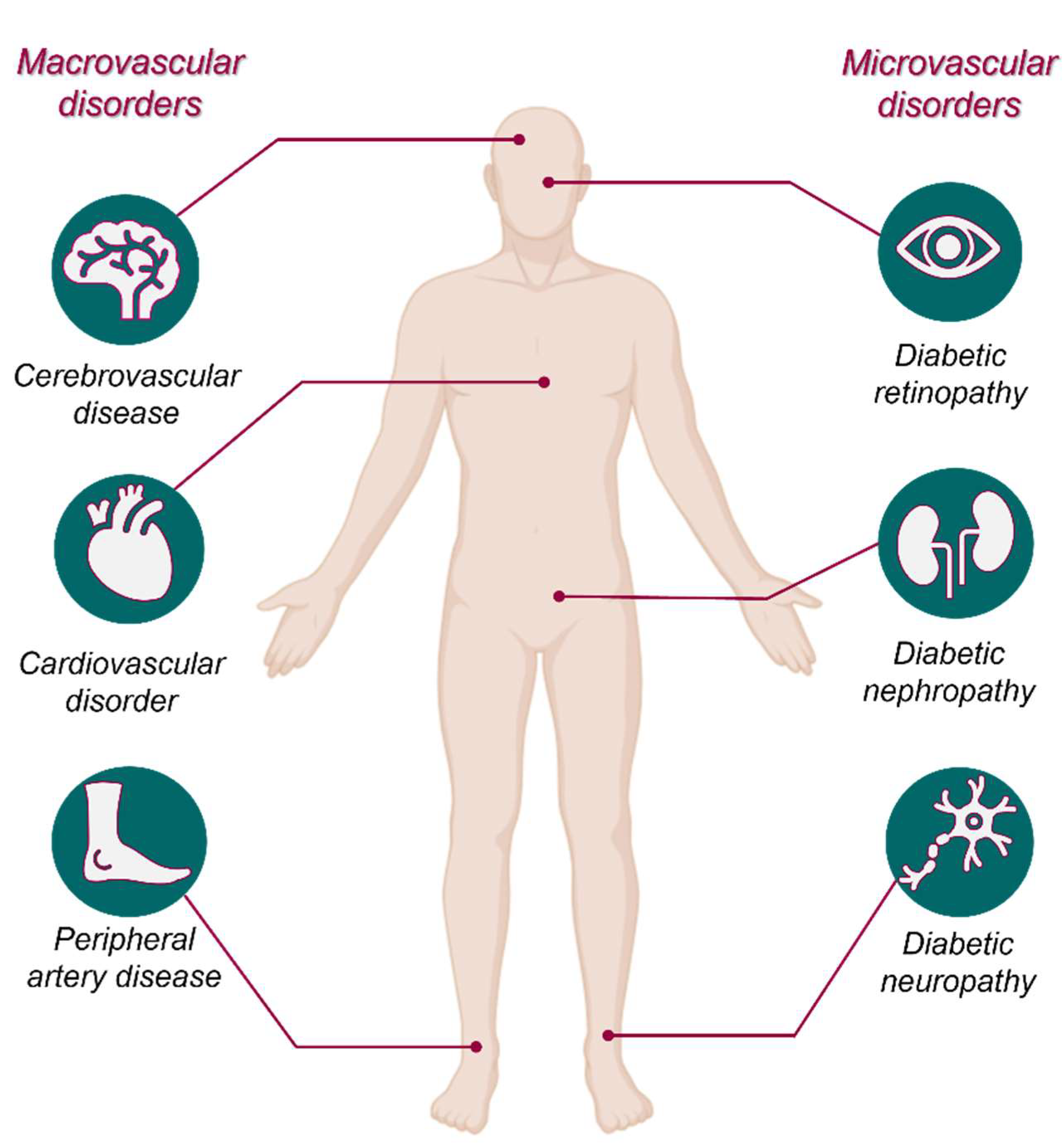
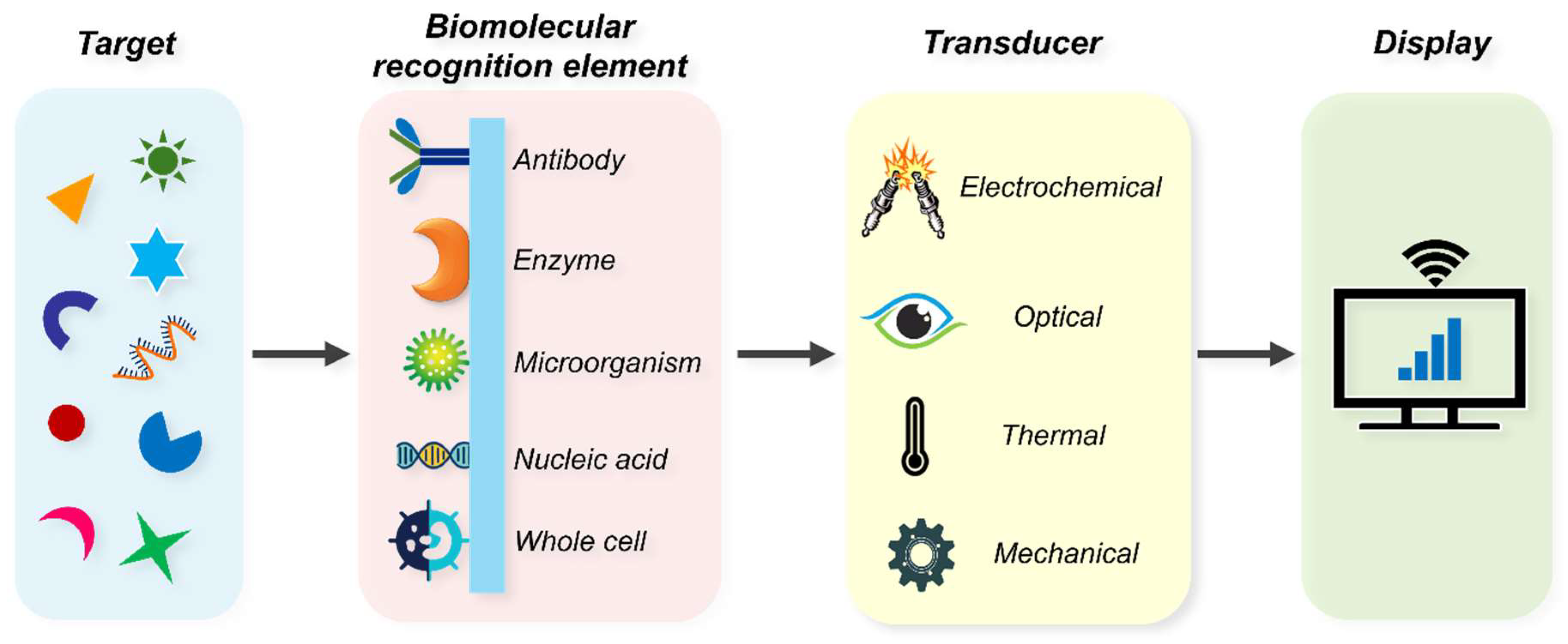
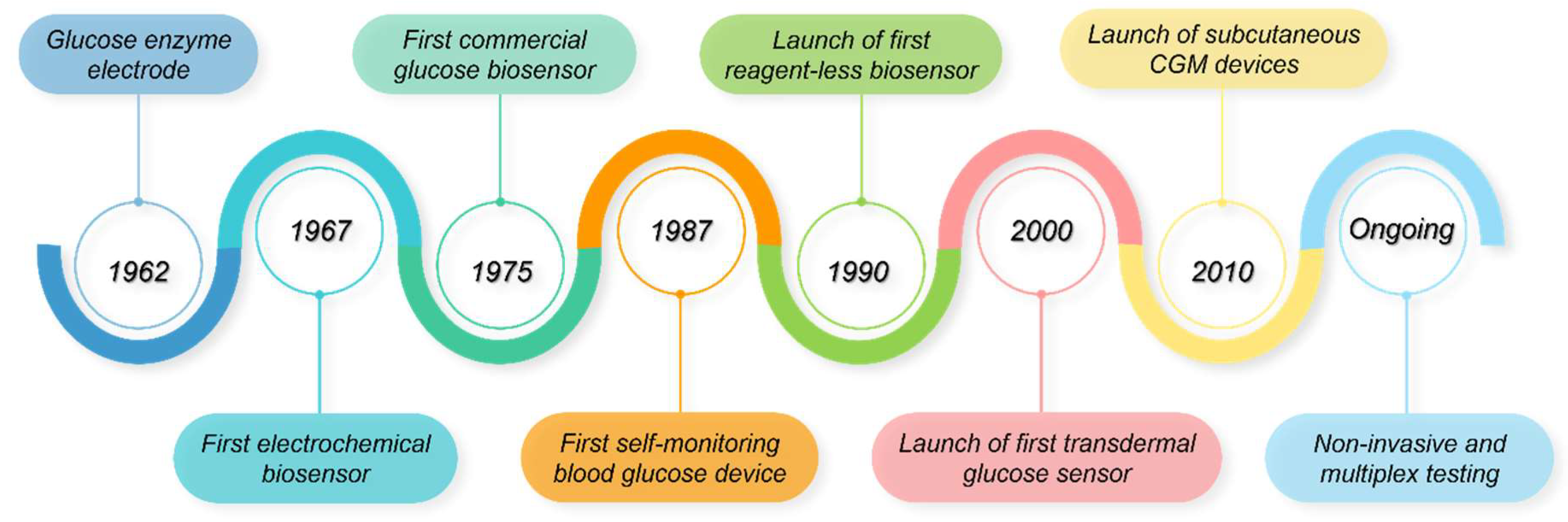

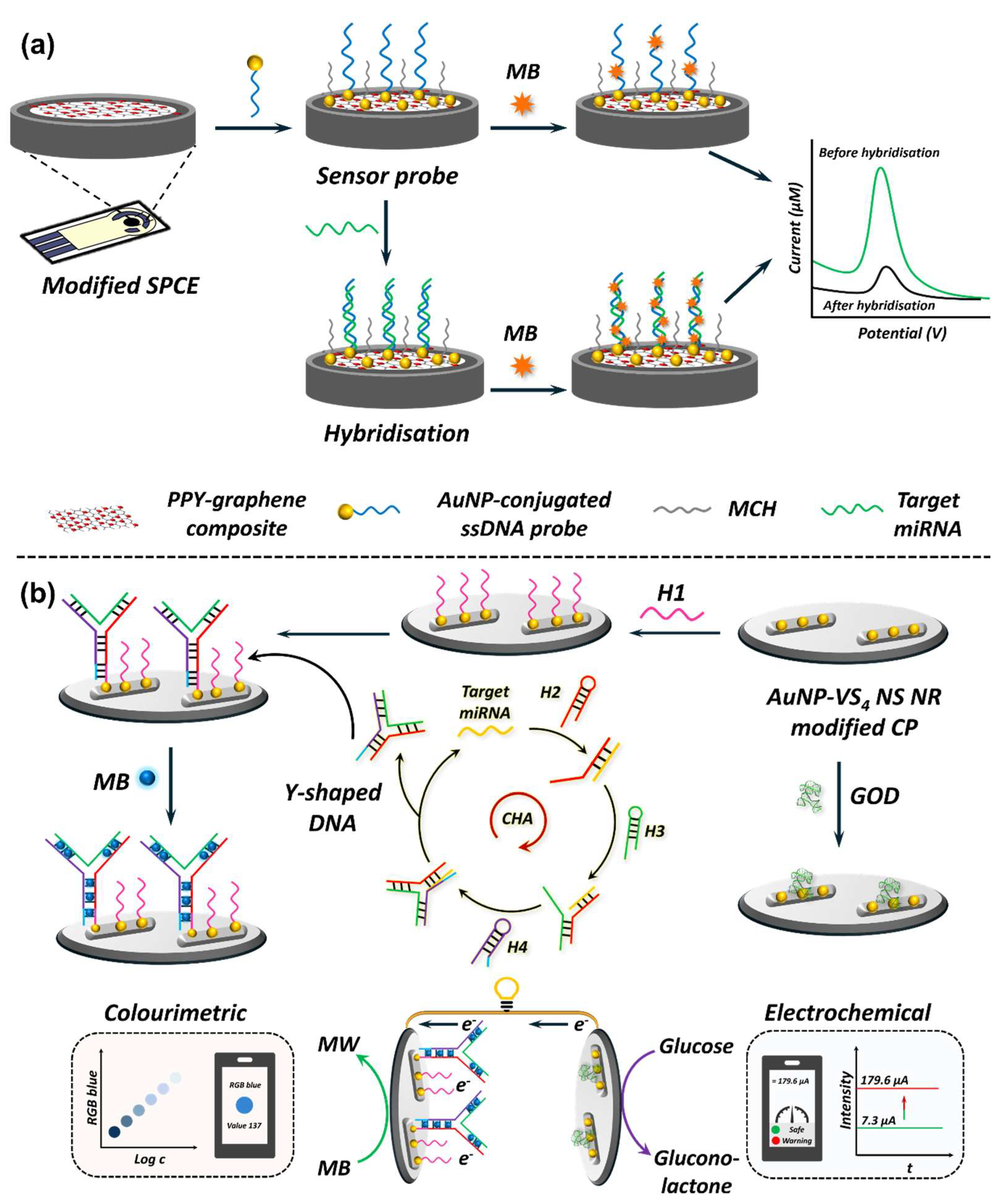
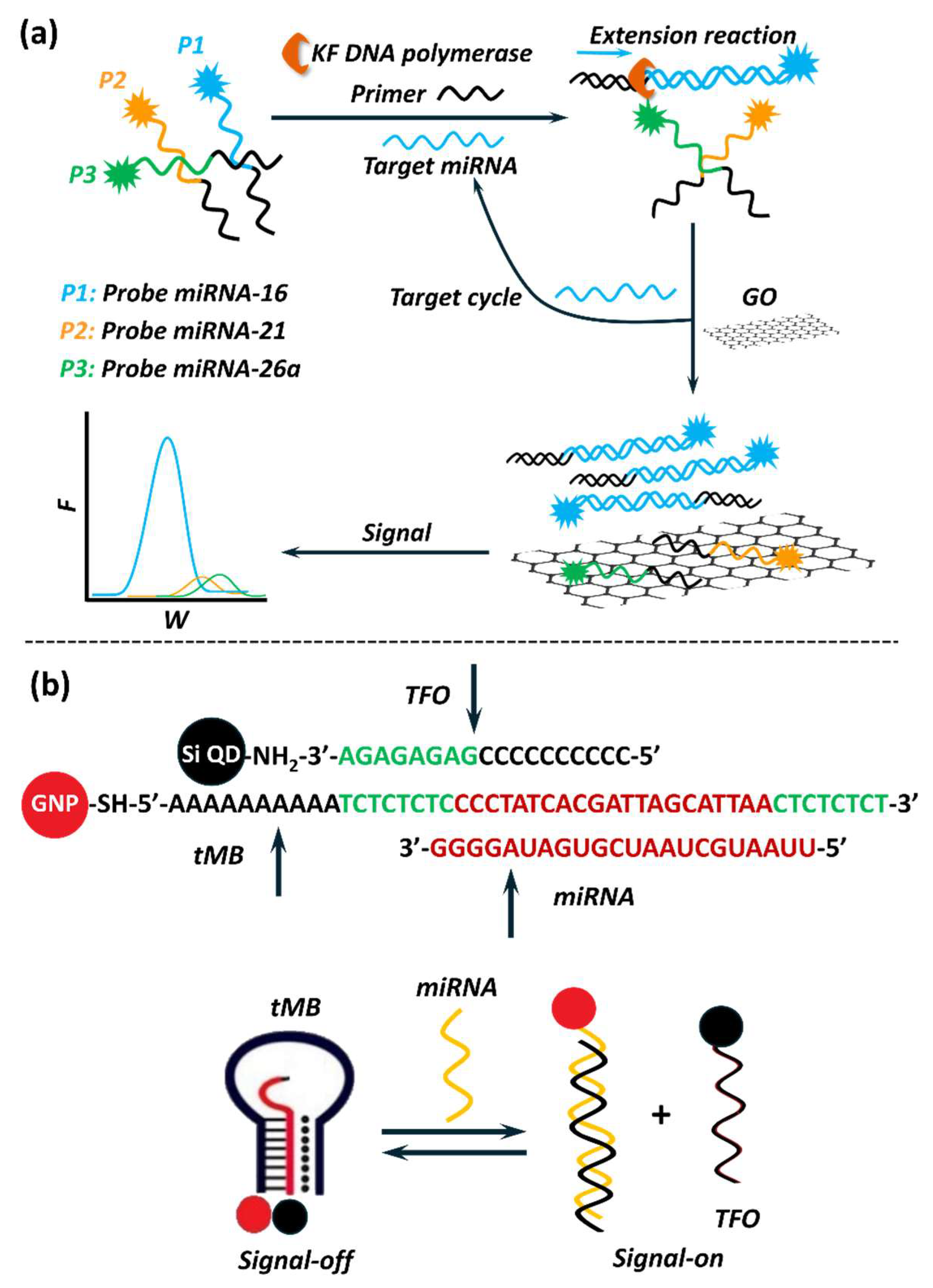
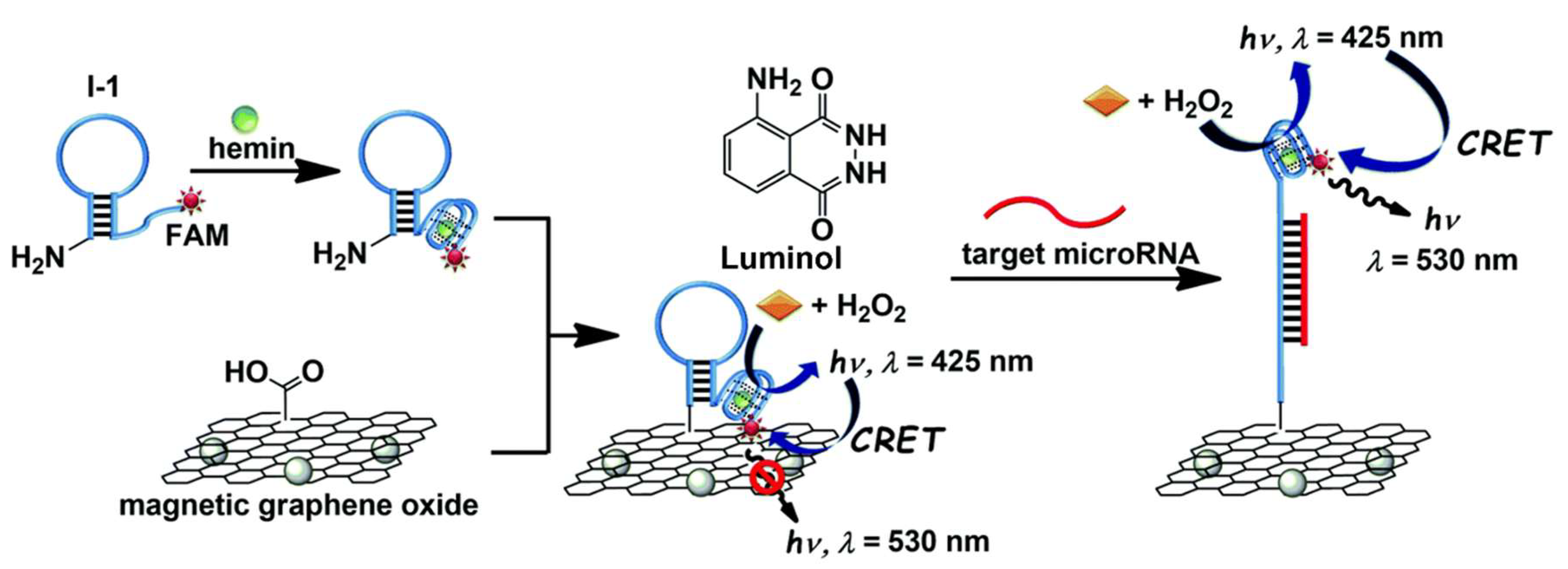
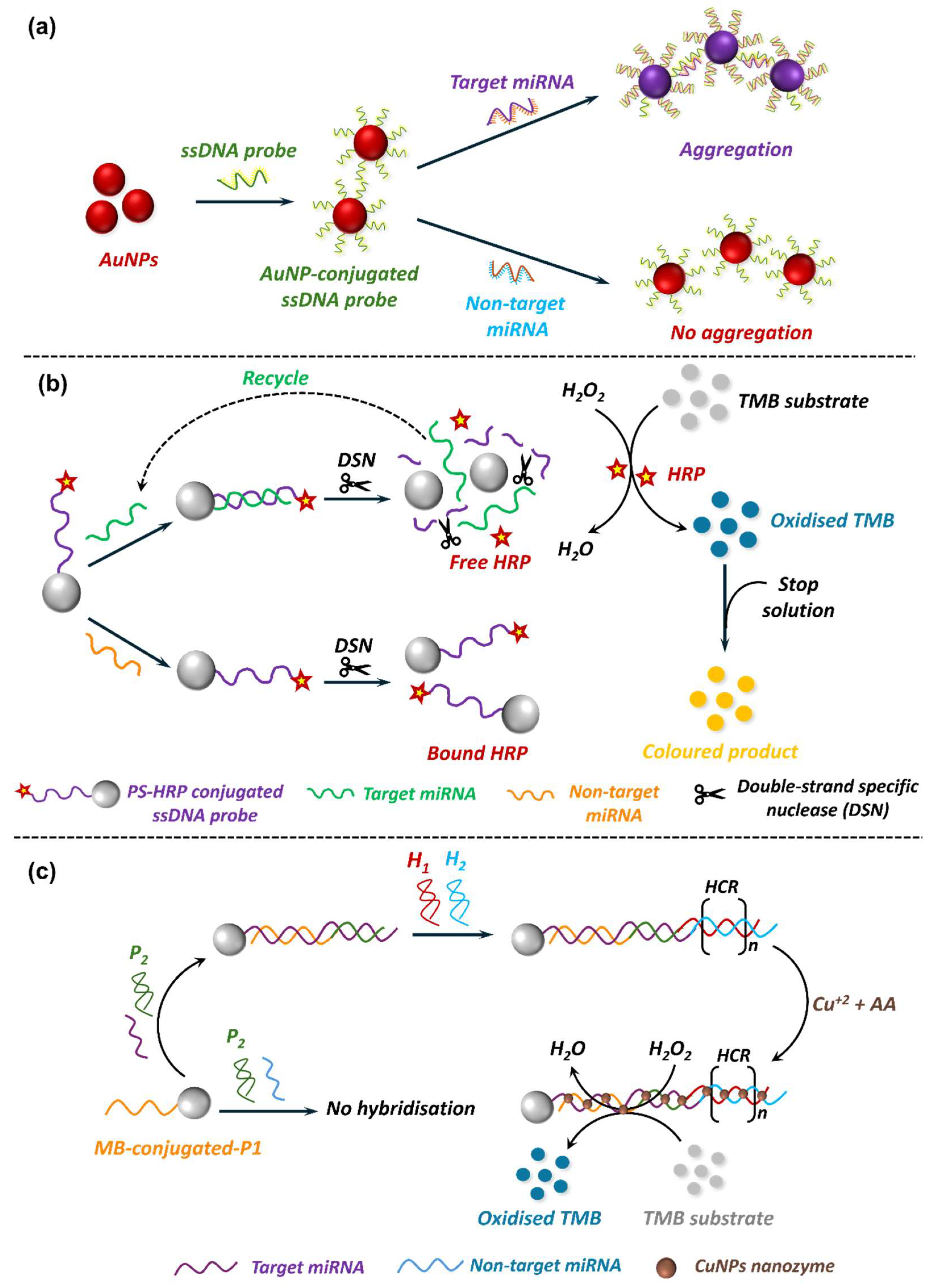
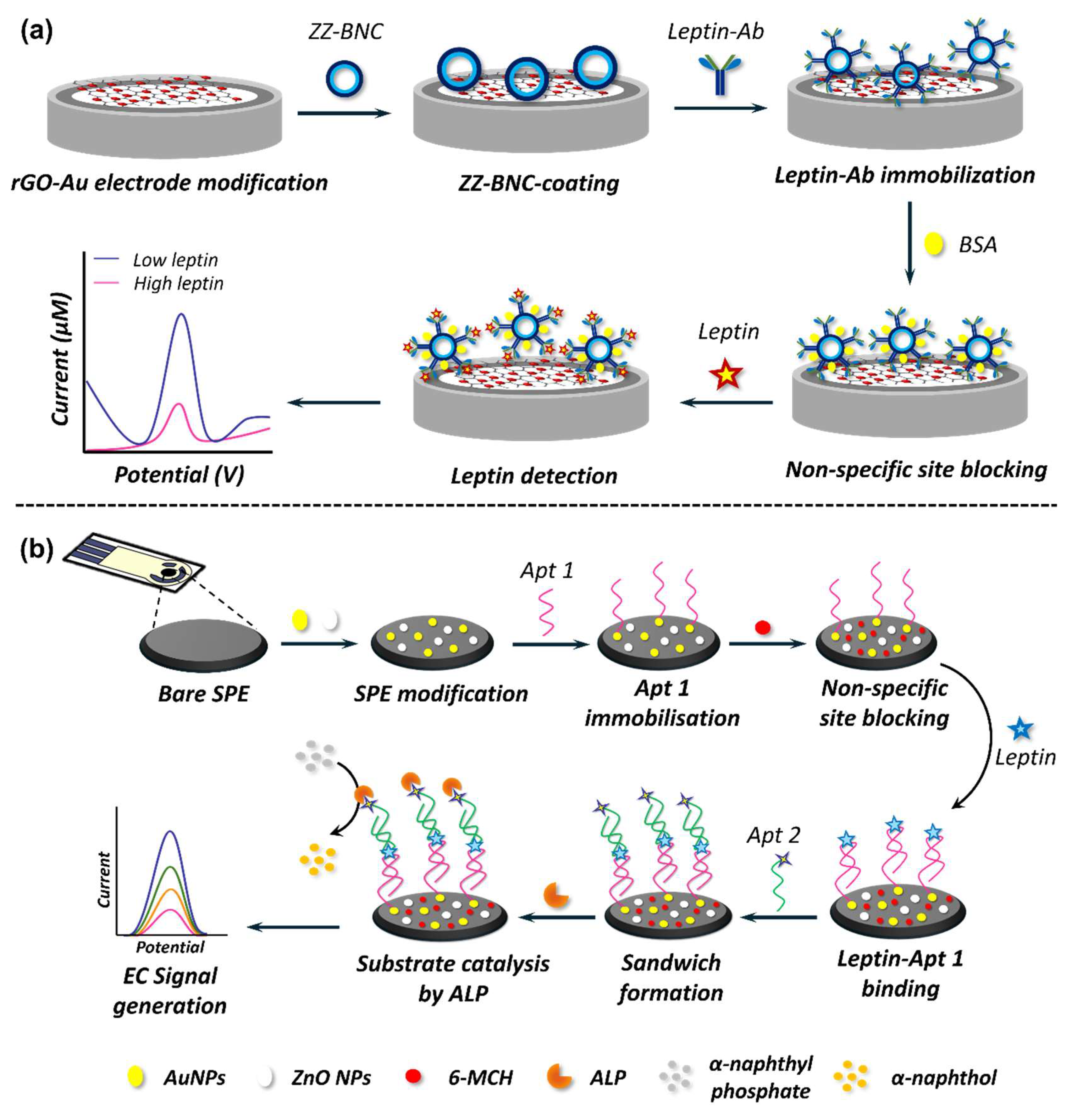
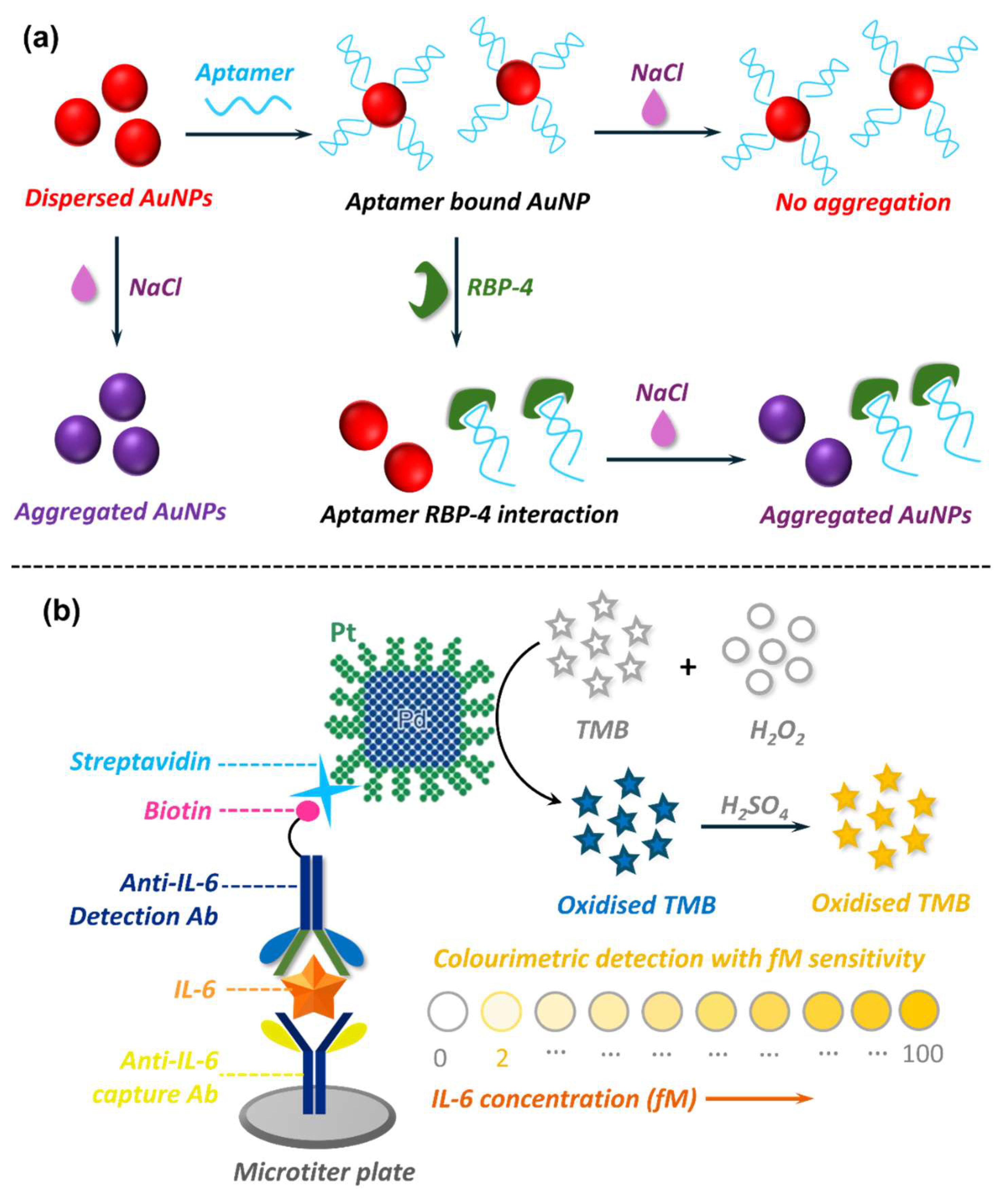
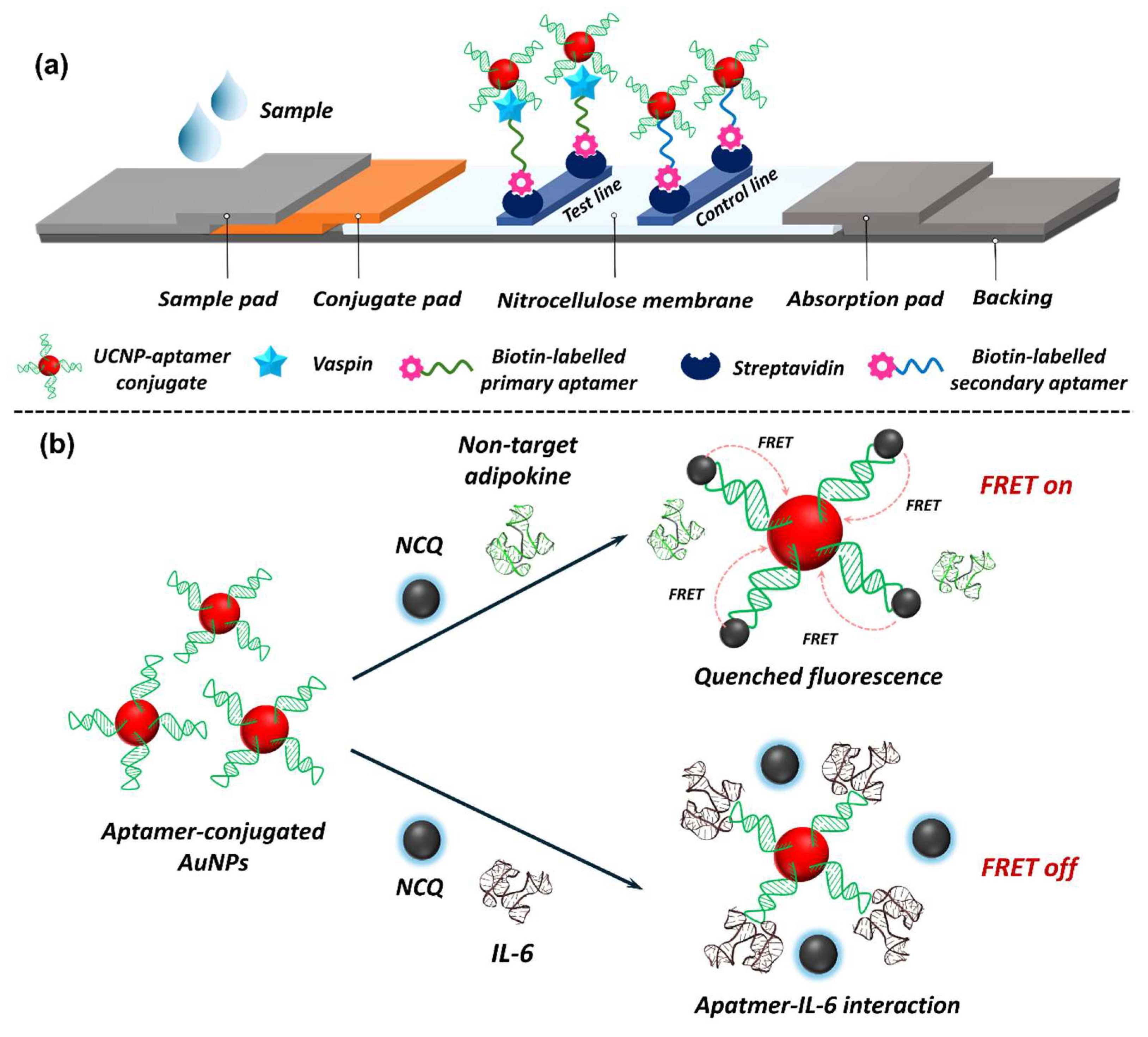
| Condition | ADA Criteria [37] | WHO Criteria [44] |
|---|---|---|
| Normal | FPG ≤ 100 mg/dL (≤5.5 mmol/L) | |
| 2 h PG during OGTT ≤ 140 mg/dL (≤7.7 mmol/L) | ||
| HbA1c ≤ 5.7% | ||
| Diabetes | FPG ≥ 126 mg/dL (≥7.0 mmol/L) | |
| RBG ≥ 200 mg/dL (≥11.1 mmol/L) | ||
| 2 h PG during OGTT ≥ 200 mg/dL (≥11.1 mmol/L) | ||
| HbA1c ≥ 6.5% | ||
| Pre-diabetes | FPG 101–124 mg/dL (5.6–6.9 mmol/L) | FPG 110–124 mg/dL (6.1–6.9 mmol/L) |
| HbA1c 5.7–6.4% | HbA1c 6.0–6.4% | |
| 2 h PG during OGTT 140–198 mg/dL (7.8–11.0 mmol/L) | ||
| Biomarker Type | Examples | Role in T2D | Reference |
|---|---|---|---|
| Glucose metabolism biomarkers | HbA1c, fasting glucose, glycated albumin, fructosamine, 1,5-AHG | Markers of impaired glucose metabolism and progression to T2D. | [140,145,146,147,148] |
| Insulin-Related biomarkers | Fasting insulin, C-peptide, HOMA-IR, proinsulin | Indicators of insulin resistance and beta-cell dysfunction. | [149,150,151,152,153] |
| Metabolites | BCAAs, acylcarnitines, tyrosine, phenylalanine, glycine | Indicators of altered metabolic pathways related to glucose and lipid metabolism. | [154,155,156,157,158] |
| Lipid biomarkers | Triglycerides, HDL, LDL, free fatty acids, lipoprotein(a) | Markers of dyslipidaemia and metabolic syndrome. | [159,160,161,162,163] |
| Genetic biomarkers | TCF7L2, FTO, PPARG, KCNJ11, SLC30A8, CDKN2A/B, TOX, HHEX, IGF2BP2 | Variants in genes associated with β-cell function, insulin sensitivity, and glucose metabolism. | [159,160,161,162,163,164,165,166] |
| Inflammatory biomarkers | CRP, IL-6, TNF-α, IL-1β, MCP-1 | Indicators of chronic inflammation linked to insulin resistance. | [167,168,169,170,171,172] |
| Gut Microbiota | Reduced Akkermansia muciniphila, increased Firmicutes/Bacteroidetes ratio, reduced butyrate levels | Patterns of gut microbiota composition linked to glucose metabolism and inflammation. | [173,174,175,176,177] |
| Proteomic biomarkers | Fetuin-A, ceruloplasmin, FGF21, SHBG | Indicators of protein-related pathways influencing glucose metabolism and insulin resistance. | [178,179,180,181] |
| Classification | Adipokine | Function | Association with T2D | Reference(s) |
|---|---|---|---|---|
| Anti-inflammatory | Adiponectin | Enhances insulin sensitivity, reduces inflammation, and promotes fatty acid oxidation. | Reduced levels are associated with insulin resistance and T2D. | [186,187] |
| Vaspin | Inhibits pro-inflammatory cytokines, enhances insulin sensitivity, and regulates metabolic homeostasis. | Elevated levels correlate with obesity and impaired insulin sensitivity | [188,189] | |
| Omentin-1 | Improves insulin sensitivity, modulates glucose metabolism, and suppresses inflammation. | Reduced levels correlate with insulin resistance and inflammation. | [190] | |
| Gremlin-1 | Improves insulin sensitivity, promotes metabolic homeostasis; emerging therapeutic target. | Increased levels correlate with obesity and insulin resistance. | [191] | |
| FGF-21 | Regulates glucose and lipid metabolism, reduces inflammation, and protects against metabolic stress. | Increased levels are linked to metabolic dysfunctions in T2D. | [192,193] | |
| Leptin | Regulates appetite and energy balance; elevated levels linked to chronic inflammation in obesity. | Increased levels are associated with obesity and leptin resistance. | [194] | |
| Pro-inflammatory | Resistin | Promotes insulin resistance, increases pro-inflammatory cytokine production, and links obesity to T2D. | Elevated levels are associated with an increased risk of T2D. | [195,196] |
| Chemerin | Increases inflammation, regulates adipocyte differentiation, and promotes insulin resistance. | Elevated levels are linked with metabolic syndrome and T2D. | [197,198] | |
| Visfatin | Enhances inflammatory responses and may modulate insulin secretion. | Elevated levels are observed in metabolic syndrome and T2D. | [199] | |
| RBP-4 | Induces insulin resistance, links adipose dysfunction to glucose intolerance, and promotes inflammation. | Elevated levels are strongly associated with insulin resistance. | [200] | |
| TNF-α | Promotes systemic inflammation and contributes to insulin resistance in obesity and T2D. | High levels are linked with systemic inflammation and insulin resistance | [201] | |
| IL-6 | Induces chronic low-grade inflammation, impairs insulin signalling and metabolic regulation. | Elevated levels (>3 pg/mL) correlate with systemic inflammation and T2D. | [202] |
| miRNA | Health Condition | Mode of Detection and Technique | Nanomaterial(s) | Analytical Performance | Remarks | Reference |
|---|---|---|---|---|---|---|
| miR-21 | Lung cancer | EC using OCV/ colourimetric dual-mode | VS4 NS-NR-AuNP hybrid nanocomposite | LR = 0.001–103 pg/mL LOD = 0.16 fg/mL Recovery = 84.1–100.6% | Required amplification = no Real sample validation = yes | [271] |
| miR-21 | Cancer | EC using OCV/ colourimetric dual-mode | Graphdiyne/AuNPs composites | LR = 0.5 fM–100 pM LOD = 0.15 fM (EC) 33 fM (Colourimetric) Recovery = 97.6–105.7% | Required amplification = yes Real sample validation = yes | [272] |
| miR-141 | Breast cancer | EC using OCV/ colourimetric dual-mode | Graphdiyne/AuNPs composites | LR = 0.0001–100 pM LOD = 21.9 aM Recovery = 99.0–106.3% | Required amplification = no Real sample validation = yes | [273] |
| miR-141 | Cancer | ECL/SPC dual-mode | S-BN QDs and WO3-x nanodots | LR = 10−17–10−10 M LOD = 10−17 M Recovery = 99.0–103% | Required amplification = no Real sample validation = yes | [274] |
| miR-21 | Ovarian cancer | Fluorescence | AgNCs | LR = 9 pM–1.55 nM LOD = 2 pM Recovery = 93–108% | Required amplification = no Real sample validation = yes | [275] |
| miR-155 | Cancer | Fluorescence | Si QDs and AuNPs | LR = 10–800 pM LOD = 10 pM Recovery = 99–100.2% | Required amplification = no Real sample validation = yes | [276] |
| miR-155 | Cancer | Fluorescence | MSN@R6G @AuNP | LR = 100 fM–100 nM LOD = 2.18 fM Recovery = 99–100.2% | Required amplification = no Real sample validation = yes | [277] |
| miR-155 | Cancer | Fluorescence | La(III)-MOF and AgNPs | LR = 2.7 fM–0.01 pM LOD = 5.2 fM Recovery = NR | Required amplification = no Clinical sample validation = yes | [278] |
| miR-125a-5p | Acute ischemic stroke | Fluorescence | AuNPs | LR = 0.01–10 µM LOD = NR Recovery = 100.6–107.4% | Required amplification = yes Real sample validation = yes | [279] |
| miR-222 | Cancer | Fluorescence | UCNPs | LR = 0.5–2.5 nM LOD = 0.077 nM Recovery = 97.6–102.1% | Required amplification = no Real sample validation = yes | [280] |
| miR-362 | Inflammatory bowel disease | Fluorescence | AgNCs | LR = 20–200 nM LOD = 6.5 nM Recovery = 74.7–92.8% | Required amplification = no Real sample validation = yes | [281] |
| miR-499 | Acute myocardial infarction | Fluorescence | MoS2 NS | LR = 0.1–13.33 nM LOD = 381.8 pM Recovery = 89.5–97.6% | Required amplification = yes Real sample validation = yes | [282] |
| miR-21 and miR-155 | Cancer | Fluorescence | QDs | LR = 0.1 pM–0.01 nM (both miRNA) LOD = 0.1 pM (both miRNA) Recovery = NR | Required amplification = no Clinical sample validation = yes No labelling required | [283] |
| miR-21 and miR-155 | Breast and lung cancer | Fluorescence/ photothermal dual-mode | CeO2@AuNP | LR = 10 pM–100 nM (miR-21) and 10 pM–200 nM (miR-155) LOD = 7.2 pM (miR-21) and 9.1 pM (miR-155) Recovery = NR | Required amplification = yes Clinical sample validation = yes Nanozyme-based detection | [284] |
| miR-21 and miR-155 | Cancer | Fluorescence (FL)/CL dual-mode | AuNPs | LR = 500 fM–10 nM (FL) 10 fM–10 nM (CL) for both miRNAs LOD = 107.2 fM (FL), 8.3 fM (CL) for miR-21 and 97.7 fM (FL), 11.2 fM (CL) for miR-155 Recovery = 93.4–121.8% (FL) 87.4–110.6% (CL) for miR-155 | Required amplification = yes Real sample validation = yes | [285] |
| miR-21 | Cancer | Colourimetric | Ag/PtNCs | LR = 10–1000 pM LOD = 4.1 pM Recovery = 93.8–106% | Required amplification = no Real sample validation = yes Nanozyme-based detection on µPAD | [286] |
| miR-21 | Cancer | Colourimetric | Fe3O4 NPs | LR = 5 fM–10 pM LOD = 5 fM Recovery = 97.5 –104% | Required amplification = yes Real sample validation = yes | [287] |
| miR-155 | Cancer | Colourimetric | CuNPs | LR = 80–500 aM LOD = 22 aM Recovery = 98.8–103.4% | Required amplification = yes Real sample validation = yes Nanozyme-based detection | [288] |
| miR-155 | Cancer | Colourimetric | AuNPs | LR = 1–100 nM LOD = 0.7 nM Recovery = 96.8–103% | Required amplification = no Real sample validation = yes | [289] |
| miR-148a | Gastric cancer | Colourimetric | AuNPs | LR = NR LOD = 1.9 nM Recovery = NR | Required amplification = no Real sample validation = no | [290] |
| Adipokine | Health Condition(s) | Mode of Detection and Technique | Nanomaterial | Analytical Performance | Remarks | Reference |
|---|---|---|---|---|---|---|
| Leptin | Obesity | EC using EIS | FeNi NPs | LR = 0.5 pg/mL–80 ng/mL LOD = 157.4 fg/mL Recovery = NR | Antibody-based detection. Real sample validation matrix was mouse serum. | [322] |
| Leptin | Neuro-degenerative disease and brain trauma | EC using DPV | ZnO NPs and AuNPs | LR = 0.01–100 pg/mL LOD = 0.035 pg/mL Recovery = 96.3–103.4% (serum) 97.8–108.8% (plasma) | Aptamer pair-based detection. Real sample validation matrix was serum and plasma. | [319] |
| Leptin | Traumatic brain injury | EC using EIS | TiO2 NPs and AuNPs | LR = 1–100 pg/mL and 100–1000 pg/mL LOD = 0.31 pg/mL Recovery = 96.4–107.8% | Aptamer-based detection. Real sample validation matrix was serum and plasma. | [321] |
| Leptin | Obesity-related conditions | EC using DPV | rGO and AuNPs | LR = 0.001–1000 pg/mL LOD = 0.87 fg/mL Recovery = NR | Antibody-based detection. Real sample validation matrix was mice and human serum | [325] |
| Leptin | Non-alcoholic fatty liver | EC using SWV | Porous graphene functionalised black phosphorous and AuNPs | LR = 0.15 pg/mL–2.5 ng/mL LOD = 0.036 fg/mL Recovery = 98–100.8% | Antibody-based detection. Real sample validation matrix was serum. | [326] |
| Leptin | Obesity | EC using DPV | Ce3NbO7/CeO2 hollow nanospheres | LR = 0.5 pg/mL– 12 ng/mL LOD = 0.14 fg/mL Recovery = 86.05–94.7% | Antibody-based detection. Real sample validation matrix was plasma | [330] |
| TNF-α and IL-6 | Inflammation and immune response | EC using chronoamperometry (CA) | rGO and AuNPs | LR = 0.1–500 pg/mL LOD = 0.69 pg/mL Recovery = NR | Antibody-based detection. Real samples were not tested. | [329] |
| IL-6 | Colorectal cancer | EC using EIS | AuNPs | LR = 5 pg/mL–10 ng/mL LOD = 1.6 pg/mL Recovery = 103.9–109.6% | Aptamer-based detection. Real sample validation matrix was serum. | [331] |
| TNF-α | Inflammatory and autoimmune diseases | Fluorescence | ZnO NRs | LR = 0.1–100 fg/mL LOD = NR Recovery = NR | Antibody-based detection. Real samples were not tested. | [318] |
| Vaspin | Obesity and T2D | Fluorescence | UCNPs | LR = 0.1–55 ng/mL LOD = 39 pg/mL Recovery = 96.9–101% | Aptamer pair-based detection on an LFD. Real sample validation matrix was serum. | [328] |
| IL-6 | Infectious disease | Fluorescence | CDs and AuNPs | LR = 1.5–5.9 pg/mL LOD = 0.82 pg/mL Recovery = 95.7–102.9% | Aptamer-based detection. Real sample validation matrix was serum. | [332] |
| IL-6 | Inflammatory diseases | Colourimetric | Pd@Pt NDs | LR = 0.05–2 pg/mL LOD = 0.04 pg/mL Recovery = 93.7–105.5% | Nanozyme-based detection using antibodies. Real sample validation matrix was serum. | [333] |
| Visfatin | Metabolic disorder | Colourimetric | PtNP-modified Ce-MOF | LR = 1–100 ng/mL LOD = 0.11 pg/mL Recovery = 96.7–98.6% | Nanozyme-based detection using aptamer. Real sample validation matrix was serum. | [327] |
| RBP-4 | Obesity and T2D | Colourimetric | AuNPs | LR = 0–125 nM LOD = 90.76 nM Recovery = NR | Salt-induced NP aggregation mediated aptamer-based detection. Real sample validation was not performed. | [312] |
| IL-6 | Inflammation | Colourimetric | AuNPs | LR = NR LOD = 1.95 µg/mL Recovery = NR | Salt-induced NP aggregation-based detection by using aptamer-pair. Real samples were not tested. | [314] |
| IL-6 | Infectious disease | Colourimetric | AuNPs | LR = 1 fg/mL–100 pg/mL LOD = 1 fg/mL Recovery = NR | Antibody-mediated detection on paper-based analytical device. Real sample validation matrix was human blood and bronchial aspirate. | [334] |
| IL-6 | Cardiovascular disorders | Colourimetric | Magnetic beads | LR = 1.6–100 ng/mL LOD = 0.4 ng/mL Recovery = 99–114% | Antibody-based microfluidic detection. Real sample validation matrix was saliva. | [335] |
| TNF-α and IL-6 | Immuno-modulation therapy | SPR | AuNRs | LR = NR LOD = 69.15 pg/mL (TNF-α) 0.93 pg/mL (IL-6) Recovery = NR | Multiplex detection using nano-plasmonic ink and antibodies. Practical validation was performed in an immunomodulated macrophage sample. | [316] |
| RBP-4 | Acute kidney injury | SPR | MNPs | LR = NR LOD = 84 pg/mL Recovery = 96.7–98.6% | Smartphone-assisted antibody-mediated detection. Real sample validation matrix was urine. | [320] |
| IL-6 | Cancer, cardiovascular disorders, diabetes, rheumatoid arthritis | SERS | Au@Ag-Au core–shell | LR = 100 fg/mL– 1 ng/mL LOD = 12.4 fg/mL Recovery = 92.4–105.3% | Antibody-based detection. Real sample validation matrix was spiked serum saliva and clinical serum samples. | [324] |
| Adiponectin | GDM | SERS | AuNTs | LR = 0.1 ng/mL–1 µg/mL LOD = 30 fg/mL Recovery = 93.1–106.02% | Antibody-mediated detection. Real sample validation matrix was serum from pregnant women | [315] |
| RBP-4 | Obesity and T2D | CL | AuNPs | LR = 0.001–2 ng/mL LOD = 1 pg/mL Recovery = NR | Aptamer-based detection. Practical application was validated using patient samples. | [313] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranwal, A.; Bansal, V.; Shukla, R. Emerging Biomarkers and Nanobiosensing Strategies in Diabetes. Biosensors 2025, 15, 639. https://doi.org/10.3390/bios15100639
Baranwal A, Bansal V, Shukla R. Emerging Biomarkers and Nanobiosensing Strategies in Diabetes. Biosensors. 2025; 15(10):639. https://doi.org/10.3390/bios15100639
Chicago/Turabian StyleBaranwal, Anupriya, Vipul Bansal, and Ravi Shukla. 2025. "Emerging Biomarkers and Nanobiosensing Strategies in Diabetes" Biosensors 15, no. 10: 639. https://doi.org/10.3390/bios15100639
APA StyleBaranwal, A., Bansal, V., & Shukla, R. (2025). Emerging Biomarkers and Nanobiosensing Strategies in Diabetes. Biosensors, 15(10), 639. https://doi.org/10.3390/bios15100639





