Recent Progress in Flexible Microelectrode Arrays for Combined Electrophysiological and Electrochemical Sensing
Abstract
1. Introduction
2. Tonic and Phasic Electrochemical Detection of Neurotransmitters Using Flexible MEAs
3. Conductive Polymers and Composite Coatings for Electrophysiological Recording and Neurotransmitter Detection
4. Enzymatic and Aptamer-Based Detection
5. Integration of Carbon Material in Flexible MEAs
6. Acquiring Electrochemical and Electrophysiological Measurements from a Single Device
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MEAs | Microelectrode arrays |
| DA | Dopamine |
| 5-HT | Serotonin |
| AD | Adenosine |
| ACh | Acetylcholine |
| MT | Melatonin |
| GLU | Glutamate |
| GABA | γ-aminobutyric acid |
| H2O2 | Hydrogen Peroxide |
| XNA | Xeno Nucleic Acid |
| FSCV | Fast Scan Cyclic Voltammetry |
| SWV | Square Wave Voltammetry |
| CFEs | Carbon Fiber Microelectrodes |
| GN | Guanosine |
| M-ENK | Methionine-enkephalin |
| CPA | Constant potential amperometry |
| m-PD | m-phenylenediamine |
| AA | ascorbic acid |
| DOPAC | 3,4-dihydroxyphenylacetic acid |
| CNT | carbon nanotubes |
| GC | Glassy carbon |
| FSCAV | fast-scan controlled-adsorption voltammetry |
| GCF | GC fiber-like |
| PPy | Polypyrrole |
| GO | graphene oxide |
| SWCNTs | single-wall carbon nanotube |
| NSR | Signal-to-noise-ratio |
| WT | wild type |
| MU | Δ19 Clock mutant |
| rGO | Reduced graphene oxide |
| PtNPs | platinum nanoparticles |
| nanoPt | Nano Platinum |
| TBI | traumatic brain injury |
| FETs | field-effect transistors |
| MB | methylene blue |
| PSB | poly(sulfobetaine methacrylate) |
| BDD | polycrystalline diamond |
| MW-PACVD | microwave plasma-assisted chemical vapor deposition |
| RIE | reactive ion etcher |
| LPCVD | low-stress low-pressure chemical vapor deposition |
| LIG | Laser-induced graphene |
| NAc | Nucleus Accumbens |
| BLA | Basolateral Amygdala |
| LFPs | Local Field Potentials |
| CNS | Central Nervous System |
| FSA | Fast Sampling Amperometry |
| PEDOT/CNT | poly(3,4-ethylenedioxythiophene) (PEDOT)/carbon nanotubes (CNT)-coated (PEDOT/CNT) |
References
- Pereda, A.E. Electrical synapses and their functional interactions with chemical synapses. Nat. Rev. Neurosci. 2014, 15, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Alcami, P.; Pereda, A.E. Beyond plasticity: The dynamic impact of electrical synapses on neural circuits. Nat. Rev. Neurosci. 2019, 20, 253–271. [Google Scholar] [CrossRef]
- Miller, A.C.; Voelker, L.H.; Shah, A.N.; Moens, C.B. Neurobeachin is required postsynaptically for electrical and chemical synapse formation. Curr. Biol. 2015, 25, 16–28. [Google Scholar] [CrossRef]
- Lerner, T.N.; Ye, L.; Deisseroth, K. Communication in neural circuits: Tools, opportunities, and challenges. Cell 2016, 164, 1136–1150. [Google Scholar] [CrossRef] [PubMed]
- Jorgenson, L.A.; Newsome, W.T.; Anderson, D.J.; Bargmann, C.I.; Brown, E.N.; Deisseroth, K.; Donoghue, J.P.; Hudson, K.L.; Ling, G.S.; MacLeish, P.R. The BRAIN Initiative: Developing technology to catalyse neuroscience discovery. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140164. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C.T. Now is the critical time for engineered neuroplasticity. Neurotherapeutics 2018, 15, 628–634. [Google Scholar] [CrossRef]
- Nicolelis, M.A.; Lebedev, M.A. Principles of neural ensemble physiology underlying the operation of brain–machine interfaces. Nat. Rev. Neurosci. 2009, 10, 530–540. [Google Scholar] [CrossRef]
- Parastarfeizabadi, M.; Kouzani, A.Z. Advances in closed-loop deep brain stimulation devices. J. Neuroeng. Rehabil. 2017, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Lujan, J.L.; Trevathan, J.K.; Ross, E.K.; Bartoletta, J.J.; Park, H.O.; Paek, S.B.; Nicolai, E.N.; Lee, J.H.; Min, H.-K. WINCS Harmoni: Closed-loop dynamic neurochemical control of therapeutic interventions. Sci. Rep. 2017, 7, 46675. [Google Scholar] [CrossRef]
- Little, S.; Pogosyan, A.; Neal, S.; Zavala, B.; Zrinzo, L.; Hariz, M.; Foltynie, T.; Limousin, P.; Ashkan, K.; FitzGerald, J. Adaptive deep brain stimulation in advanced Parkinson disease. Ann. Neurol. 2013, 74, 449–457. [Google Scholar] [CrossRef]
- Chapman, C.A.; Goshi, N.; Seker, E. Multifunctional Neural Interfaces for Closed-Loop Control of Neural Activity. Adv. Funct. Mater. 2018, 28, 1703523. [Google Scholar] [CrossRef]
- Atcherley, C.W.; Laude, N.D.; Parent, K.L.; Heien, M.L. Fast-scan controlled-adsorption voltammetry for the quantification of absolute concentrations and adsorption dynamics. Langmuir 2013, 29, 14885–14892. [Google Scholar] [CrossRef]
- Rutherford, E.C.; Pomerleau, F.; Huettl, P.; Strömberg, I.; Gerhardt, G.A. Chronic second-by-second measures of l-glutamate in the central nervous system of freely moving rats. J. Neurochem. 2007, 102, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Edell, D.J.; Toi, V.V.; McNeil, V.M.; Clark, L. Factors influencing the biocompatibility of insertable silicon microshafts in cerebral cortex. IEEE Trans. Biomed. Eng. 1992, 39, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Szarowski, D.; Andersen, M.; Retterer, S.; Spence, A.; Isaacson, M.; Craighead, H.G.; Turner, J.; Shain, W. Brain responses to micro-machined silicon devices. Brain Res. 2003, 983, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, R.C.; Wightman, R.M.; Kristensen, E.W. Diffusional distortion in the monitoring of dynamic events. Anal. Chem. 1988, 60, 652–656. [Google Scholar] [CrossRef]
- Kawagoe, K.; Garris, P.; Wiedemann, D.; Wightman, R. Regulation of transient dopamine concentration gradients in the microenvironment surrounding nerve terminals in the rat striatum. Neuroscience 1992, 51, 55–64. [Google Scholar] [CrossRef]
- Schwerdt, H.N.; Kim, M.; Karasan, E.; Amemori, S.; Homma, D.; Shimazu, H.; Yoshida, T.; Langer, R.; Graybiel, A.M.; Cima, M.J. Subcellular electrode arrays for multisite recording of dopamine in vivo. In Proceedings of the 2017 IEEE 30th International Conference on Micro Electro Mechanical Systems (MEMS), Las Vegas, NV, USA, 22–26 January 2017; pp. 549–552. [Google Scholar]
- Fattahi, P.; Yang, G.; Kim, G.; Abidian, M.R. A review of organic and inorganic biomaterials for neural interfaces. Adv. Mater. 2014, 26, 1846–1885. [Google Scholar] [CrossRef] [PubMed]
- Wellman, S.M.; Eles, J.R.; Ludwig, K.A.; Seymour, J.P.; Michelson, N.J.; McFadden, W.E.; Vazquez, A.L.; Kozai, T.D. A materials roadmap to functional neural interface design. Adv. Funct. Mater. 2018, 28, 1701269. [Google Scholar] [CrossRef] [PubMed]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 2005, 148, 1–18. [Google Scholar] [CrossRef]
- McConnell, G.C.; Rees, H.D.; Levey, A.I.; Gutekunst, C.-A.; Gross, R.E.; Bellamkonda, R.V. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J. Neural Eng. 2009, 6, 056003. [Google Scholar] [CrossRef] [PubMed]
- Agorelius, J.; Tsanakalis, F.; Friberg, A.; Thorbergsson, P.T.; Pettersson, L.M.E.; Schouenborg, J. An array of highly flexible electrodes with a tailored configuration locked by gelatin during implantation—Initial evaluation in cortex cerebri of awake rats. Front. Neurosci. 2015, 9, 331. [Google Scholar] [CrossRef]
- Marin, C.; Fernández, E. Biocompatibility of intracortical microelectrodes: Current status and future prospects. Front. Neuroeng. 2010, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Roitbak, T.; Syková, E. Diffusion barriers evoked in the rat cortex by reactive astrogliosis. Glia 1999, 28, 40–48. [Google Scholar] [CrossRef]
- Castagnola, E.; Zheng, X.S.; Cui, X.T. Flexible and soft materials and devices for neural interface. In Handbook of Neuroengineering; Springer: Berlin/Heidelberg, Germany, 2023; pp. 79–139. [Google Scholar]
- Luan, L.; Robinson, J.T.; Aazhang, B.; Chi, T.; Yang, K.; Li, X.; Rathore, H.; Singer, A.; Yellapantula, S.; Fan, Y. Recent advances in electrical neural interface engineering: Minimal invasiveness, longevity, and scalability. Neuron 2020, 108, 302–321. [Google Scholar] [CrossRef]
- Luan, L.; Wei, X.; Zhao, Z.; Siegel, J.J.; Potnis, O.; Tuppen, C.A.; Lin, S.; Kazmi, S.; Fowler, R.A.; Holloway, S. Ultraflexible nanoelectronic probes form reliable, glial scar–free neural integration. Sci. Adv. 2017, 3, e1601966. [Google Scholar] [CrossRef]
- Wei, X.; Luan, L.; Zhao, Z.; Li, X.; Zhu, H.; Potnis, O.; Xie, C. Nanofabricated ultraflexible electrode arrays for high-density intracortical recording. Adv. Sci. 2018, 5, 1700625. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhu, H.; Li, X.; Sun, L.; He, F.; Chung, J.E.; Liu, D.F.; Frank, L.; Luan, L.; Xie, C. Ultraflexible electrode arrays for months-long high-density electrophysiological mapping of thousands of neurons in rodents. Nat. Biomed. Eng. 2023, 7, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Hong, G.; Gao, T.; Lieber, C.M. Mesh nanoelectronics: Seamless integration of electronics with tissues. Acc. Chem. Res. 2018, 51, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Hong, G.; Lin, D.; Schuhmann, T.G., Jr.; Sullivan, A.T.; Viveros, R.D.; Park, H.-G.; Lieber, C.M. Nanoenabled direct contact interfacing of syringe-injectable mesh electronics. Nano Lett. 2019, 19, 5818–5826. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-M.; Chung, T.-W.; Wu, P.-W.; Chen, P.-C. A cost-effective fabrication of iridium oxide films as biocompatible electrostimulation electrodes for neural interface applications. J. Alloys Compd. 2017, 692, 339–345. [Google Scholar] [CrossRef]
- Meyer, R.D.; Cogan, S.F.; Nguyen, T.H.; Rauh, R.D. Electrodeposited iridium oxide for neural stimulation and recording electrodes. IEEE Trans. Neural Syst. Rehabil. Eng. 2001, 9, 2–11. [Google Scholar] [CrossRef]
- Negi, S.; Bhandari, R.; Rieth, L.; Solzbacher, F. In vitro comparison of sputtered iridium oxide and platinum-coated neural implantable microelectrode arrays. Biomed. Mater. 2010, 5, 015007. [Google Scholar] [CrossRef]
- Cogan, S.F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef]
- Boehler, C.; Vieira, D.M.; Egert, U.; Asplund, M. NanoPt—A nanostructured electrode coating for neural recording and microstimulation. ACS Appl. Mater. Interfaces 2020, 12, 14855–14865. [Google Scholar] [CrossRef] [PubMed]
- Boehler, C.; Oberueber, F.; Stieglitz, T.; Asplund, M. Nanostructured platinum as an electrochemically and mechanically stable electrode coating. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju Island, Republic of Korea, 11–15 July 2017; pp. 1058–1061. [Google Scholar]
- Wu, B.; Castagnola, E.; McClung, C.A.; Cui, X.T. PEDOT/CNT Flexible MEAs Reveal New Insights into the Clock Gene’s Role in Dopamine Dynamics. Adv. Sci. 2024, 11, 2308212. [Google Scholar] [CrossRef]
- Cui, X.; Martin, D.C. Electrochemical deposition and characterization of poly (3, 4-ethylenedioxythiophene) on neural microelectrode arrays. Sens. Actuators B Chem. 2003, 89, 92–102. [Google Scholar] [CrossRef]
- Khodagholy, D.; Gelinas, J.N.; Thesen, T.; Doyle, W.; Devinsky, O.; Malliaras, G.G.; Buzsáki, G. NeuroGrid: Recording action potentials from the surface of the brain. Nat. Neurosci. 2015, 18, 310–315. [Google Scholar] [CrossRef]
- Asplund, M.; Nyberg, T.; Inganäs, O. Electroactive polymers for neural interfaces. Polym. Chem. 2010, 1, 1374–1391. [Google Scholar] [CrossRef]
- Castagnola, E.; Robbins, E.M.; Wu, B.; Pwint, M.Y.; Garg, R.; Cohen-Karni, T.; Cui, X.T. Flexible glassy carbon multielectrode array for in vivo multisite detection of tonic and phasic dopamine concentrations. Biosensors 2022, 12, 540. [Google Scholar] [CrossRef]
- Wu, B.; Castagnola, E.; Cui, X.T. Zwitterionic polymer coated and aptamer functionalized flexible micro-electrode arrays for in vivo cocaine sensing and electrophysiology. Micromachines 2023, 14, 323. [Google Scholar] [CrossRef] [PubMed]
- Huffman, M.L.; Venton, B.J. Carbon-fiber microelectrodes for in vivo applications. Analyst 2009, 134, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Puthongkham, P.; Venton, B.J. Recent advances in fast-scan cyclic voltammetry. Analyst 2020, 145, 1087–1102. [Google Scholar] [CrossRef]
- Swamy, B.K.; Venton, B.J. Subsecond detection of physiological adenosine concentrations using fast-scan cyclic voltammetry. Anal. Chem. 2007, 79, 744–750. [Google Scholar] [CrossRef]
- Venton, B.J.; Cao, Q. Fundamentals of fast-scan cyclic voltammetry for dopamine detection. Analyst 2020, 145, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Hensley, A.L.; Colley, A.R.; Ross, A.E. Real-time detection of melatonin using fast-scan cyclic voltammetry. Anal. Chem. 2018, 90, 8642–8650. [Google Scholar] [CrossRef] [PubMed]
- Robbins, E.M.; Wong, B.; Pwint, M.Y.; Salavatian, S.; Mahajan, A.; Cui, X.T. Improving Sensitivity and Longevity of In Vivo Glutamate Sensors with Electrodeposited NanoPt. ACS Appl. Mater. Interfaces 2024, 16, 40570–40580. [Google Scholar] [CrossRef] [PubMed]
- Salavatian, S.; Robbins, E.M.; Kuwabara, Y.; Castagnola, E.; Cui, X.T.; Mahajan, A. Real-time in vivo thoracic spinal glutamate sensing during myocardial ischemia. Am. J. Physiol.-Heart Circ. Physiol. 2023, 325, H1304–H1317. [Google Scholar] [CrossRef] [PubMed]
- Billa, S.; Yanamadala, Y.; Hossain, I.; Siddiqui, S.; Moldovan, N.; Murray, T.A.; Arumugam, P.U. Brain-implantable multifunctional probe for simultaneous detection of glutamate and GABA neurotransmitters: Optimization and in vivo studies. Micromachines 2022, 13, 1008. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, Y.; Figueroa-Miranda, G.; Musall, S.; Li, H.; Martínez-Roque, M.A.; Hu, Q.; Feng, L.; Mayer, D.; Offenhäusser, A. Aptamer based biosensor platforms for neurotransmitters analysis. TrAC Trends Anal. Chem. 2023, 162, 117021. [Google Scholar] [CrossRef]
- Tan, C.; Robbins, E.M.; Wu, B.; Cui, X.T. Recent advances in in vivo neurochemical monitoring. Micromachines 2021, 12, 208. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, N.; Matarasso, A.; Heck, I.; Li, H.; Lu, W.; Phaup, J.G.; Schneider, M.J.; Wu, Y.; Weng, Z. Implantable aptamer-graphene microtransistors for real-time monitoring of neurochemical release in vivo. Nano Lett. 2022, 22, 3668–3677. [Google Scholar] [CrossRef] [PubMed]
- Downs, A.M.; Plaxco, K.W. Real-time, in vivo molecular monitoring using electrochemical aptamer based sensors: Opportunities and challenges. ACS Sens. 2022, 7, 2823–2832. [Google Scholar] [CrossRef]
- Grace, A.A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 2016, 17, 524–532. [Google Scholar] [CrossRef]
- Grace, A.A. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction 2000, 95, 119–128. [Google Scholar] [CrossRef]
- Zhang, L.; Doyon, W.M.; Clark, J.J.; Phillips, P.E.; Dani, J.A. Controls of tonic and phasic dopamine transmission in the dorsal and ventral striatum. Mol. Pharmacol. 2009, 76, 396–404. [Google Scholar] [CrossRef]
- Saylor, R.A.; Hersey, M.; West, A.; Buchanan, A.M.; Berger, S.N.; Nijhout, H.F.; Reed, M.C.; Best, J.; Hashemi, P. In vivo hippocampal serotonin dynamics in male and female mice: Determining effects of acute escitalopram using fast scan cyclic voltammetry. Front. Neurosci. 2019, 13, 362. [Google Scholar] [CrossRef] [PubMed]
- Mitch Taylor, I.; Jaquins-Gerstl, A.; Sesack, S.R.; Michael, A.C. Domain-dependent effects of DAT inhibition in the rat dorsal striatum. J. Neurochem. 2012, 122, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Taylor, I.M.; Nesbitt, K.M.; Walters, S.H.; Varner, E.L.; Shu, Z.; Bartlow, K.M.; Jaquins-Gerstl, A.S.; Michael, A.C. Kinetic diversity of dopamine transmission in the dorsal striatum. J. Neurochem. 2015, 133, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Rafi, H.; Zestos, A.G. Recent advances in FSCV detection of neurochemicals via waveform and carbon microelectrode modification. J. Electrochem. Soc. 2021, 168, 057520. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.G.; Sombers, L.A. Fast scan cyclic voltammetry: Chemical sensing in the brain and beyond. Anal. Chem. 2018, 90, 490. [Google Scholar] [CrossRef]
- Meunier, C.J.; Sombers, L.A. Fast-scan voltammetry for in vivo measurements of neurochemical dynamics. Brain Reward Syst. 2021, 165, 93–123. [Google Scholar]
- Keithley, R.B.; Takmakov, P.; Bucher, E.S.; Belle, A.M.; Owesson-White, C.A.; Park, J.; Wightman, R.M. Higher sensitivity dopamine measurements with faster-scan cyclic voltammetry. Anal. Chem. 2011, 83, 3563–3571. [Google Scholar] [CrossRef]
- Roberts, J.G.; Toups, J.V.; Eyualem, E.; McCarty, G.S.; Sombers, L.A. In situ electrode calibration strategy for voltammetric measurements in vivo. Anal. Chem. 2013, 85, 11568–11575. [Google Scholar] [CrossRef]
- Ganesana, M.; Lee, S.T.; Wang, Y.; Venton, B.J. Analytical techniques in neuroscience: Recent advances in imaging, separation, and electrochemical methods. Anal. Chem. 2017, 89, 314–341. [Google Scholar] [CrossRef] [PubMed]
- Hafizi, S.; Kruk, Z.L.; Stamford, J.A. Fast cyclic voltammetry: Improved sensitivity to dopamine with extended oxidation scan limits. J. Neurosci. Methods 1990, 33, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Heien, M.L.; Phillips, P.E.; Stuber, G.D.; Seipel, A.T.; Wightman, R.M. Overoxidation of carbon-fiber microelectrodes enhances dopamine adsorption and increases sensitivity. Analyst 2003, 128, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.P.; Dietz, S.M.; Wightman, R.M. Fast-scan cyclic voltammetry of 5-hydroxytryptamine. Anal. Chem. 1995, 67, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Dunham, K.E.; Venton, B.J. Improving serotonin fast-scan cyclic voltammetry detection: New waveforms to reduce electrode fouling. Analyst 2020, 145, 7437–7446. [Google Scholar] [CrossRef]
- Cooper, S.E.; Venton, B.J. Fast-scan cyclic voltammetry for the detection of tyramine and octopamine. Anal. Bioanal. Chem. 2009, 394, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.G.; Lugo-Morales, L.Z.; Loziuk, P.L.; Sombers, L.A. Real-time chemical measurements of dopamine release in the brain. Dopamine Methods Protoc. 2013, 964, 275–294. [Google Scholar]
- Cavelier, P.; Hamann, M.; Rossi, D.; Mobbs, P.; Attwell, D. Tonic excitation and inhibition of neurons: Ambient transmitter sources and computational consequences. Prog. Biophys. Mol. Biol. 2005, 87, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Lustig, C. Forebrain cholinergic signaling: Wired and phasic, not tonic, and causing behavior. J. Neurosci. 2020, 40, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Ruivo, L.M.T.-G.; Baker, K.L.; Conway, M.W.; Kinsley, P.J.; Gilmour, G.; Phillips, K.G.; Isaac, J.T.; Lowry, J.P.; Mellor, J.R. Coordinated acetylcholine release in prefrontal cortex and hippocampus is associated with arousal and reward on distinct timescales. Cell Rep. 2017, 18, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.A. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: A hypothesis for the etiology of schizophrenia. Neuroscience 1991, 41, 1–24. [Google Scholar] [CrossRef]
- Grace, A.A. The tonic/phasic model of dopamine system regulation: Its relevance for understanding how stimulant abuse can alter basal ganglia function. Drug Alcohol Depend. 1995, 37, 111–129. [Google Scholar] [CrossRef]
- Budygin, E.A.; Bass, C.E.; Grinevich, V.P.; Deal, A.L.; Bonin, K.D.; Weiner, J.L. Opposite consequences of tonic and phasic increases in accumbal dopamine on alcohol-seeking behavior. IScience 2020, 23, 100877. [Google Scholar] [CrossRef]
- Ledo, A.; Lourenco, C.F.; Laranjinha, J.; Gerhardt, G.A.; Barbosa, R.M. Concurrent measurements of neurochemical and electrophysiological activity with microelectrode arrays: New perspectives for constant potential amperometry. Curr. Opin. Electrochem. 2018, 12, 129–140. [Google Scholar] [CrossRef]
- Johnson, M.D.; Franklin, R.K.; Gibson, M.D.; Brown, R.B.; Kipke, D.R. Implantable microelectrode arrays for simultaneous electrophysiological and neurochemical recordings. J. Neurosci. Methods 2008, 174, 62–70. [Google Scholar] [CrossRef]
- Lundblad, M.; Price, D.A.; Burmeister, J.J.; Quintero, J.E.; Huettl, P.; Pomerleau, F.; Zahniser, N.R.; Gerhardt, G.A. Tonic and phasic amperometric monitoring of dopamine using microelectrode arrays in rat striatum. Appl. Sci. 2020, 10, 6449. [Google Scholar] [CrossRef]
- Quintero, J.E.; Pomerleau, F.; Huettl, P.; Johnson, K.W.; Offord, J.; Gerhardt, G.A. Methodology for rapid measures of glutamate release in rat brain slices using ceramic-based microelectrode arrays: Basic characterization and drug pharmacology. Brain Res. 2011, 1401, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rand, E.; Periyakaruppan, A.; Tanaka, Z.; Zhang, D.A.; Marsh, M.P.; Andrews, R.J.; Lee, K.H.; Chen, B.; Meyyappan, M.; Koehne, J.E. A carbon nanofiber based biosensor for simultaneous detection of dopamine and serotonin in the presence of ascorbicacid. Biosens. Bioelectron. 2013, 42, 434–438. [Google Scholar] [CrossRef]
- Tan, C.; Doughty, P.T.; Magee, K.; Murray, T.A.; Siddiqui, S.; Arumugam, P.U. Effect of process parameters on electrochemical performance of a glutamate microbiosensor. J. Electrochem. Soc. 2020, 167, 027528. [Google Scholar] [CrossRef]
- Burmeister, J.J.; Palmer, M.; Gerhardt, G.A. Ceramic-based multisite microelectrode array for rapid choline measures in brain tissue. Anal. Chim. Acta 2003, 481, 65–74. [Google Scholar] [CrossRef]
- Burmeister, J.J.; Pomerleau, F.; Huettl, P.; Gash, C.R.; Werner, C.E.; Bruno, J.P.; Gerhardt, G.A. Ceramic-based multisite microelectrode arrays for simultaneous measures of choline and acetylcholine in CNS. Biosens. Bioelectron. 2008, 23, 1382–1389. [Google Scholar] [CrossRef]
- Mattinson, C.E.; Burmeister, J.J.; Quintero, J.E.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A. Tonic and phasic release of glutamate and acetylcholine neurotransmission in sub-regions of the rat prefrontal cortex using enzyme-based microelectrode arrays. J. Neurosci. Methods 2011, 202, 199–208. [Google Scholar] [CrossRef][Green Version]
- Tseng, T.T.-C.; Monbouquette, H.G. Implantable microprobe with arrayed microsensors for combined amperometric monitoring of the neurotransmitters, glutamate and dopamine. J. Electroanal. Chem. 2012, 682, 141–146. [Google Scholar] [CrossRef]
- Atcherley, C.W. Voltammetric Measurements of Tonic and Phasic Neurotransmission. Ph.D. Thesis, The University of Arizona, Tucson, AZ, USA, 2014. [Google Scholar]
- Burrell, M.H.; Atcherley, C.W.; Heien, M.L.; Lipski, J. A novel electrochemical approach for prolonged measurement of absolute levels of extracellular dopamine in brain slices. ACS Chem. Neurosci. 2015, 6, 1802–1812. [Google Scholar] [CrossRef]
- Abdalla, A.; Atcherley, C.W.; Pathirathna, P.; Samaranayake, S.; Qiang, B.; Peña, E.; Morgan, S.L.; Heien, M.L.; Hashemi, P. In vivo ambient serotonin measurements at carbon-fiber microelectrodes. Anal. Chem. 2017, 89, 9703–9711. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Park, C.; Kim, D.H.; Shin, H.; Kang, Y.M.; DeWaele, M.; Lee, J.; Min, H.-K.; Blaha, C.D.; Bennet, K.E. Monitoring in vivo changes in tonic extracellular dopamine level by charge-balancing multiple waveform fast-scan cyclic voltammetry. Anal. Chem. 2016, 88, 10962–10970. [Google Scholar] [CrossRef]
- Rusheen, A.E.; Gee, T.A.; Jang, D.P.; Blaha, C.D.; Bennet, K.E.; Lee, K.H.; Heien, M.L.; Oh, Y. Evaluation of electrochemical methods for tonic dopamine detection in vivo. TrAC Trends Anal. Chem. 2020, 132, 116049. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Rodeberg, N.T.; Wightman, R.M. Measurement of basal neurotransmitter levels using convolution-based nonfaradaic current removal. Anal. Chem. 2018, 90, 7181–7189. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Oh, Y.; Shin, H.; Kim, J.; Kang, Y.; Sim, J.; Cho, H.U.; Lee, H.K.; Jung, S.J.; Blaha, C.D. Fast cyclic square-wave voltammetry to enhance neurotransmitter selectivity and sensitivity. Anal. Chem. 2018, 90, 13348–13355. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Oh, Y.; Park, C.; Kang, Y.; Cho, H.U.; Blaha, C.D.; Bennet, K.E.; Heien, M.L.; Kim, I.Y.; Lee, K.H. Sensitive and selective measurement of serotonin in vivo using fast cyclic square-wave voltammetry. Anal. Chem. 2019, 92, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Goyal, A.; Barnett, J.H.; Rusheen, A.E.; Yuen, J.; Jha, R.; Hwang, S.M.; Kang, Y.; Park, C.; Cho, H.-U. Tonic serotonin measurements in vivo using N-shaped multiple cyclic square wave voltammetry. Anal. Chem. 2021, 93, 16987–16994. [Google Scholar] [CrossRef]
- Oh, Y.; Heien, M.L.; Park, C.; Kang, Y.M.; Kim, J.; Boschen, S.L.; Shin, H.; Cho, H.U.; Blaha, C.D.; Bennet, K.E. Tracking tonic dopamine levels in vivo using multiple cyclic square wave voltammetry. Biosens. Bioelectron. 2018, 121, 174–182. [Google Scholar] [CrossRef]
- Kim, D.-K.; Tolliver, T.J.; Huang, S.-J.; Martin, B.J.; Andrews, A.M.; Wichems, C.; Holmes, A.; Lesch, K.-P.; Murphy, D.L. Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology 2005, 49, 798–810. [Google Scholar] [CrossRef] [PubMed]
- Wickens, J.; Alexander, M.; Miller, R. Two dynamic modes of striatal function under dopaminergic-cholinergic control: Simulation and analysis of a model. Synapse 1991, 8, 1–12. [Google Scholar] [CrossRef]
- Salinas, A.G.; Davis, M.I.; Lovinger, D.M.; Mateo, Y. Dopamine dynamics and cocaine sensitivity differ between striosome and matrix compartments of the striatum. Neuropharmacology 2016, 108, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Threlfell, S.; Cragg, S.J. Dopamine signaling in dorsal versus ventral striatum: The dynamic role of cholinergic interneurons. Front. Syst. Neurosci. 2011, 5, 11. [Google Scholar] [CrossRef]
- Collins, A.L.; Saunders, B.T. Heterogeneity in striatal dopamine circuits: Form and function in dynamic reward seeking. J. Neurosci. Res. 2020, 98, 1046–1069. [Google Scholar] [CrossRef] [PubMed]
- Castagnola, E.; Cao, Q.; Robbins, E.; Wu, B.; Pwint, M.Y.; Siwakoti, U.; Cui, X.T. Glassy Carbon Fiber-Like Multielectrode Arrays for Neurochemical Sensing and Electrophysiology Recording. Adv. Mater. Technol. 2024, 15, 2400863. [Google Scholar] [CrossRef]
- Castagnola, E.; Robbins, E.M.; Krahe, D.D.; Wu, B.; Pwint, M.Y.; Cao, Q.; Cui, X.T. Stable in-vivo electrochemical sensing of tonic serotonin levels using PEDOT/CNT-coated glassy carbon flexible microelectrode arrays. Biosens. Bioelectron. 2023, 230, 115242. [Google Scholar] [CrossRef] [PubMed]
- Pranti, A.S.; Schander, A.; Bödecker, A.; Lang, W. PEDOT: PSS coating on gold microelectrodes with excellent stability and high charge injection capacity for chronic neural interfaces. Sens. Actuators B Chem. 2018, 275, 382–393. [Google Scholar] [CrossRef]
- Ansaldo, A.; Castagnola, E.; Maggiolini, E.; Fadiga, L.; Ricci, D. Superior electrochemical performance of carbon nanotubes directly grown on sharp microelectrodes. ACS Nano 2011, 5, 2206–2214. [Google Scholar] [CrossRef] [PubMed]
- Baranauskas, G.; Maggiolini, E.; Castagnola, E.; Ansaldo, A.; Mazzoni, A.; Angotzi, G.N.; Vato, A.; Ricci, D.; Panzeri, S.; Fadiga, L. Carbon nanotube composite coating of neural microelectrodes preferentially improves the multiunit signal-to-noise ratio. J. Neural Eng. 2011, 8, 066013. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Martin, D.C. Fuzzy gold electrodes for lowering impedance and improving adhesion with electrodeposited conducting polymer films. Sens. Actuators A Phys. 2003, 103, 384–394. [Google Scholar] [CrossRef]
- Cui, X.; Lee, V.A.; Raphael, Y.; Wiler, J.A.; Hetke, J.F.; Anderson, D.J.; Martin, D.C. Surface modification of neural recording electrodes with conducting polymer/biomolecule blends. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2001, 56, 261–272. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.; Jiang, Q.; Xu, J. Effective approaches to improve the electrical conductivity of PEDOT: PSS: A review. Adv. Electron. Mater. 2015, 1, 1500017. [Google Scholar] [CrossRef]
- Boehler, C.; Kleber, C.; Martini, N.; Xie, Y.; Dryg, I.; Stieglitz, T.; Hofmann, U.; Asplund, M. Actively controlled release of Dexamethasone from neural microelectrodes in a chronic in vivo study. Biomaterials 2017, 129, 176–187. [Google Scholar] [CrossRef]
- Vázquez, M.; Bobacka, J.; Ivaska, A.; Lewenstam, A. Influence of oxygen and carbon dioxide on the electrochemical stability of poly (3, 4-ethylenedioxythiophene) used as ion-to-electron transducer in all-solid-state ion-selective electrodes. Sens. Actuators B Chem. 2002, 82, 7–13. [Google Scholar] [CrossRef]
- Cui, X.T.; Zhou, D.D. Poly (3, 4-ethylenedioxythiophene) for chronic neural stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Musk, E. An integrated brain-machine interface platform with thousands of channels. J. Med. Internet Res. 2019, 21, e16194. [Google Scholar] [CrossRef] [PubMed]
- Vara, H.; Collazos-Castro, J.E. Enhanced spinal cord microstimulation using conducting polymer-coated carbon microfibers. Acta Biomater. 2019, 90, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.S.; Tan, C.; Castagnola, E.; Cui, X.T. Electrode materials for chronic electrical microstimulation. Adv. Healthc. Mater. 2021, 10, 2100119. [Google Scholar] [CrossRef]
- Green, R.A.; Hassarati, R.T.; Bouchinet, L.; Lee, C.S.; Cheong, G.L.; Jin, F.Y.; Dodds, C.W.; Suaning, G.J.; Poole-Warren, L.A.; Lovell, N.H. Substrate dependent stability of conducting polymer coatings on medical electrodes. Biomaterials 2012, 33, 5875–5886. [Google Scholar] [CrossRef]
- Ouyang, L.; Wei, B.; Kuo, C.-C.; Pathak, S.; Farrell, B.; Martin, D.C. Enhanced PEDOT adhesion on solid substrates with electrografted P (EDOT-NH2). Sci. Adv. 2017, 3, e1600448. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Weaver, C.L.; Zhou, D.D.; Greenberg, R.; Cui, X.T. Highly stable carbon nanotube doped poly (3, 4-ethylenedioxythiophene) for chronic neural stimulation. Biomaterials 2011, 32, 5551–5557. [Google Scholar] [CrossRef] [PubMed]
- Charkhkar, H.; Knaack, G.L.; McHail, D.G.; Mandal, H.S.; Peixoto, N.; Rubinson, J.F.; Dumas, T.C.; Pancrazio, J.J. Chronic intracortical neural recordings using microelectrode arrays coated with PEDOT–TFB. Acta Biomater. 2016, 32, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Taylor, I.M.; Patel, N.A.; Freedman, N.C.; Castagnola, E.; Cui, X.T. Direct in vivo electrochemical detection of resting dopamine using poly (3, 4-ethylenedioxythiophene)/carbon nanotube functionalized microelectrodes. Anal. Chem. 2019, 91, 12917. [Google Scholar] [CrossRef]
- Taylor, I.M.; Robbins, E.M.; Catt, K.A.; Cody, P.A.; Happe, C.L.; Cui, X.T. Enhanced dopamine detection sensitivity by PEDOT/graphene oxide coating on in vivo carbon fiber electrodes. Biosens. Bioelectron. 2017, 89, 400–410. [Google Scholar] [CrossRef]
- Vreeland, R.F.; Atcherley, C.W.; Russell, W.S.; Xie, J.Y.; Lu, D.; Laude, N.D.; Porreca, F.; Heien, M.L. Biocompatible PEDOT: Nafion composite electrode coatings for selective detection of neurotransmitters in vivo. Anal. Chem. 2015, 87, 2600–2607. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.; Liu, F.; Hendrix, A.; Asrat, T.; Connaughton, V.; Zestos, A.G. Timed electrodeposition of PEDOT: Nafion onto carbon fiber-microelectrodes enhances dopamine detection in zebrafish retina. J. Electrochem. Soc. 2020, 167, 115501. [Google Scholar] [CrossRef]
- Demuru, S.; Deligianni, H. Surface PEDOT: Nafion coatings for enhanced dopamine, serotonin and adenosine sensing. J. Electrochem. Soc. 2017, 164, G129. [Google Scholar] [CrossRef]
- Kozai, T.D.; Catt, K.; Du, Z.; Na, K.; Srivannavit, O.; Razi-ul, M.H.; Seymour, J.; Wise, K.D.; Yoon, E.; Cui, X.T. Chronic in vivo evaluation of PEDOT/CNT for stable neural recordings. IEEE Trans. Biomed. Eng. 2015, 63, 111–119. [Google Scholar] [CrossRef]
- He, E.; Xu, S.; Dai, Y.; Wang, Y.; Xiao, G.; Xie, J.; Xu, S.; Fan, P.; Mo, F.; Wang, M. SWCNTs/PEDOT: PSS-modified microelectrode arrays for dual-mode detection of electrophysiological signals and dopamine concentration in the striatum under isoflurane anesthesia. ACS Sens. 2021, 6, 3377–3386. [Google Scholar] [CrossRef]
- Xiao, G.; Xu, S.; Song, Y.; Zhang, Y.; Li, Z.; Gao, F.; Xie, J.; Sha, L.; Xu, Q.; Shen, Y. In situ detection of neurotransmitters and epileptiform electrophysiology activity in awake mice brains using a nanocomposites modified microelectrode array. Sens. Actuators B Chem. 2019, 288, 601–610. [Google Scholar] [CrossRef]
- Fan, P.; Wang, Y.; Dai, Y.; Jing, L.; Liang, W.; Lu, B.; Yang, G.; Song, Y.; Wu, Y.; Cai, X. Flexible microelectrode array probe for simultaneous detection of neural discharge and dopamine in striatum of mice aversion system. Sens. Actuators B Chem. 2023, 390, 133990. [Google Scholar] [CrossRef]
- Wang, X.; Xu, M.; Yang, H.; Jiang, W.; Jiang, J.; Zou, D.; Zhu, Z.; Tao, C.; Ni, S.; Zhou, Z. Ultraflexible Neural Electrodes Enabled Synchronized Long-Term Dopamine Detection and Wideband Chronic Recording Deep in Brain. ACS Nano 2024, 18, 34272–34287. [Google Scholar] [CrossRef] [PubMed]
- Castagnola, E.; Carli, S.; Vomero, M.; Scarpellini, A.; Prato, M.; Goshi, N.; Fadiga, L.; Kassegne, S.; Ricci, D. Multilayer poly (3, 4-ethylenedioxythiophene)-dexamethasone and poly (3, 4-ethylenedioxythiophene)-polystyrene sulfonate-carbon nanotubes coatings on glassy carbon microelectrode arrays for controlled drug release. Biointerphases 2017, 12, 031002. [Google Scholar] [CrossRef]
- Vomero, M.; Castagnola, E.; Ciarpella, F.; Maggiolini, E.; Goshi, N.; Zucchini, E.; Carli, S.; Fadiga, L.; Kassegne, S.; Ricci, D. Highly stable glassy carbon interfaces for long-term neural stimulation and low-noise recording of brain activity. Sci. Rep. 2017, 7, 40332. [Google Scholar] [CrossRef] [PubMed]
- Nimbalkar, S.; Castagnola, E.; Balasubramani, A.; Scarpellini, A.; Samejima, S.; Khorasani, A.; Boissenin, A.; Thongpang, S.; Moritz, C.; Kassegne, S. Ultra-capacitive carbon neural probe allows simultaneous long-term electrical stimulations and high-resolution neurotransmitter detection. Sci. Rep. 2018, 8, 6958. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced carbon electrode materials for molecular electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef]
- Hanssen, B.L.; Siraj, S.; Wong, D.K. Recent strategies to minimise fouling in electrochemical detection systems. Rev. Anal. Chem. 2016, 35, 1–28. [Google Scholar] [CrossRef]
- Updike, S.J.; Shults, M.C.; Rhodes, R.K.; Gilligan, B.J.; Luebow, J.O.; Von Heimburg, D. Enzymatic glucose sensors: Improved long-term performance: In vitro: And: In vivo. Asaio J. 1994, 40, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, J.J.; Gerhardt, G.A. Self-referencing ceramic-based multisite microelectrodes for the detection and elimination of interferences from the measurement of L-glutamate and other analytes. Anal. Chem. 2001, 73, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, J.J.; Price, D.A.; Pomerleau, F.; Huettl, P.; Quintero, J.E.; Gerhardt, G.A. Challenges of simultaneous measurements of brain extracellular GABA and glutamate in vivo using enzyme-coated microelectrode arrays. J. Neurosci. Methods 2020, 329, 108435. [Google Scholar] [CrossRef] [PubMed]
- Clay, M.; Monbouquette, H.G. Simulated Performance of Electroenzymatic Glutamate Biosensors In Vivo Illuminates the Complex Connection to Calibration In Vitro. ACS Chem. Neurosci. 2021, 12, 4275–4285. [Google Scholar] [CrossRef]
- Qin, S.; Van der Zeyden, M.; Oldenziel, W.H.; Cremers, T.I.; Westerink, B.H. Microsensors for in vivo measurement of glutamate in brain tissue. Sensors 2008, 8, 6860–6884. [Google Scholar] [CrossRef] [PubMed]
- Disney, A.A.; McKinney, C.; Grissom, L.; Lu, X.; Reynolds, J.H. A multi-site array for combined local electrochemistry and electrophysiology in the non-human primate brain. J. Neurosci. Methods 2015, 255, 29–37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, X.; Liu, J. Biosensors and sensors for dopamine detection. View 2021, 2, 20200102. [Google Scholar] [CrossRef]
- Baluta, S.; Zając, D.; Szyszka, A.; Malecha, K.; Cabaj, J. Enzymatic platforms for sensitive neurotransmitter detection. Sensors 2020, 20, 423. [Google Scholar] [CrossRef]
- Scoggin, J.L.; Tan, C.; Nguyen, N.H.; Kansakar, U.; Madadi, M.; Siddiqui, S.; Arumugam, P.U.; DeCoster, M.A.; Murray, T.A. An enzyme-based electrochemical biosensor probe with sensitivity to detect astrocytic versus glioma uptake of glutamate in real time in vitro. Biosens. Bioelectron. 2019, 126, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Chae, U.; Woo, J.; Cho, Y.; Han, J.-K.; Yang, S.H.; Yang, E.; Shin, H.; Kim, H.; Yu, H.-Y.; Lee, C.J. A neural probe for concurrent real-time measurement of multiple neurochemicals with electrophysiology in multiple brain regions in vivo. Proc. Natl. Acad. Sci. USA 2023, 120, e2219231120. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Song, Y.; Zhang, S.; Yang, L.; Xu, S.; Zhang, Y.; Xu, H.; Gao, F.; Li, Z.; Cai, X. A high-sensitive nano-modified biosensor for dynamic monitoring of glutamate and neural spike covariation from rat cortex to hippocampal sub-regions. J. Neurosci. Methods 2017, 291, 122–130. [Google Scholar] [CrossRef]
- Wei, W.; Song, Y.; Wang, L.; Zhang, S.; Luo, J.; Xu, S.; Cai, X. An implantable microelectrode array for simultaneous L-glutamate and electrophysiological recordings in vivo. Microsyst. Nanoeng. 2015, 1, 1–6. [Google Scholar] [CrossRef]
- Ziegler, C.; Göpel, W. Biosensor development. Curr. Opin. Chem. Biol. 1998, 2, 585–591. [Google Scholar] [CrossRef]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V. Enzyme biosensors for biomedical applications: Strategies for safeguarding analytical performances in biological fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef] [PubMed]
- Reyes-De-Corcuera, J.I.; Olstad, H.E.; García-Torres, R. Stability and stabilization of enzyme biosensors: The key to successful application and commercialization. Annu. Rev. Food Sci. Technol. 2018, 9, 293–322. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bai, Y.; Han, G.; Li, M. Porous-reduced graphene oxide for fabricating an amperometric acetylcholinesterase biosensor. Sens. Actuators B Chem. 2013, 185, 706–712. [Google Scholar] [CrossRef]
- Sun, G.; Wei, X.; Zhang, D.; Huang, L.; Liu, H.; Fang, H. Immobilization of enzyme electrochemical biosensors and their application to food bioprocess monitoring. Biosensors 2023, 13, 886. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Verma, D.; Singh, P.C. Exploring the Potential of Enzyme-Immobilized MOFs: Biosensing, Biocatalysis, Targeted Drug Delivery and Cancer Therapy. J. Mater. Chem. B 2024, 12, 10198–10214. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Cho, H.-U.; Hwang, S.; Kwak, Y.; Kwon, H.; Heien, M.L.; Bennet, K.E.; Oh, Y.; Shin, H.; Lee, K.H. Understanding the different effects of fouling mechanisms on working and reference electrodes in fast-scan cyclic voltammetry for neurotransmitter detection. Analyst 2024, 149, 3008–3016. [Google Scholar] [CrossRef]
- Campuzano, S.; Pedrero, M.; Yáñez-Sedeño, P.; Pingarrón, J.M. Antifouling (bio) materials for electrochemical (bio) sensing. Int. J. Mol. Sci. 2019, 20, 423. [Google Scholar] [CrossRef] [PubMed]
- Taylor, I.M.; Du, Z.; Bigelow, E.T.; Eles, J.R.; Horner, A.R.; Catt, K.A.; Weber, S.G.; Jamieson, B.G.; Cui, X.T. Aptamer-functionalized neural recording electrodes for the direct measurement of cocaine in vivo. J. Mater. Chem. B 2017, 5, 2445–2458. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.S.; Gifford, R. Biosensors for real-time in vivo measurements. Biosens. Bioelectron. 2005, 20, 2388–2403. [Google Scholar] [CrossRef]
- Baker, B.R.; Lai, R.Y.; Wood, M.S.; Doctor, E.H.; Heeger, A.J.; Plaxco, K.W. An electronic, aptamer-based small-molecule sensor for the rapid, label-free detection of cocaine in adulterated samples and biological fluids. J. Am. Chem. Soc. 2006, 128, 3138–3139. [Google Scholar] [CrossRef]
- Zhao, C.; Cheung, K.M.; Huang, I.-W.; Yang, H.; Nakatsuka, N.; Liu, W.; Cao, Y.; Man, T.; Weiss, P.S.; Monbouquette, H.G. Implantable aptamer–field-effect transistor neuroprobes for in vivo neurotransmitter monitoring. Sci. Adv. 2021, 7, eabj7422. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, M.; He, J.; Zhang, Y.; Liang, Y.; Liu, H.; Zhang, Z. Aptamer-functionalized carbon nanotube field-effect transistor biosensors for Alzheimer’s disease serum biomarker detection. ACS Sens. 2022, 7, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Jiang, L.; van Geest, E.P.; Lima, L.M.; Schneider, G.F. Sensing at the surface of graphene field-effect transistors. Adv. Mater. 2017, 29, 1603610. [Google Scholar] [CrossRef] [PubMed]
- Sadighbayan, D.; Hasanzadeh, M.; Ghafar-Zadeh, E. Biosensing based on field-effect transistors (FET): Recent progress and challenges. TrAC Trends Anal. Chem. 2020, 133, 116067. [Google Scholar] [CrossRef]
- Green, N.S.; Norton, M.L. Interactions of DNA with graphene and sensing applications of graphene field-effect transistor devices: A review. Anal. Chim. Acta 2015, 853, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowski, R.; Jarczewska, M.; Górski, Ł.; Malinowska, E. From small molecules toward whole cells detection: Application of electrochemical aptasensors in modern medical diagnostics. Sensors 2021, 21, 724. [Google Scholar] [CrossRef]
- Oberhaus, F.V.; Frense, D.; Beckmann, D. Immobilization techniques for aptamers on gold electrodes for the electrochemical detection of proteins: A review. Biosensors 2020, 10, 45. [Google Scholar] [CrossRef]
- Grabowska, I.; Hepel, M.; Kurzątkowska-Adaszyńska, K. Advances in design strategies of multiplex electrochemical aptasensors. Sensors 2021, 22, 161. [Google Scholar] [CrossRef]
- Kang, D.; White, R.J.; Xia, F.; Zuo, X.; Vallée-Bélisle, A.; Plaxco, K.W. DNA biomolecular-electronic encoder and decoder devices constructed by multiplex biosensors. NPG Asia Mater. 2012, 4, e1. [Google Scholar] [CrossRef]
- Wen, Y.; Pei, H.; Wan, Y.; Su, Y.; Huang, Q.; Song, S.; Fan, C. DNA nanostructure-decorated surfaces for enhanced aptamer-target binding and electrochemical cocaine sensors. Anal. Chem. 2011, 83, 7418–7423. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, L.; Li, H.; Yuan, R.; Chai, Y. Electrochemical aptamer biosensor based on ATP-induced 2D DNA structure switching for rapid and ultrasensitive detection of ATP. Anal. Chem. 2022, 94, 6819–6826. [Google Scholar] [CrossRef]
- Jiang, Y.; Ma, W.; Ji, W.; Wei, H.; Mao, L. Aptamer superstructure-based electrochemical biosensor for sensitive detection of ATP in rat brain with in vivo microdialysis. Analyst 2019, 144, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, A.; Schundner, A.; Frick, M.; Kranz, C. Electrochemical detection of ATP release in-vitro and in-vivo. Curr. Opin. Electrochem. 2023, 39, 101282. [Google Scholar] [CrossRef]
- Azadbakht, A.; Roushani, M.; Abbasi, A.R.; Derikvand, Z. Design and characterization of electrochemical dopamine–aptamer as convenient and integrated sensing platform. Anal. Biochem. 2016, 507, 47–57. [Google Scholar] [CrossRef]
- Abu-Ali, H.; Ozkaya, C.; Davis, F.; Walch, N.; Nabok, A. Electrochemical aptasensor for detection of dopamine. Chemosensors 2020, 8, 28. [Google Scholar] [CrossRef]
- Cuhadar, S.N.; Durmaz, H.; Yildirim-Tirgil, N. Multi-detection of seratonin and dopamine based on an electrochemical aptasensor. Chem. Pap. 2024, 78, 7175–7185. [Google Scholar] [CrossRef]
- Li, J.; Si, Y.; Park, Y.E.; Choi, J.-S.; Jung, S.M.; Lee, J.E.; Lee, H.J. A serotonin voltammetric biosensor composed of carbon nanocomposites and DNA aptamer. Microchim. Acta 2021, 188, 146. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, X.; Su, L.; Qi, H.; Yue, X.; Qi, H. Label-free Electrochemical Aptasensor for the Determination of Serotonin. Electroanalysis 2022, 34, 1048–1053. [Google Scholar] [CrossRef]
- Golabchi, A.; Wu, B.; Cao, B.; Bettinger, C.J.; Cui, X.T. Zwitterionic polymer/polydopamine coating reduce acute inflammatory tissue responses to neural implants. Biomaterials 2019, 225, 119519. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, B.; Eles, J.R.; Vazquez, A.L.; Kozai, T.D.; Cui, X.T. Zwitterionic polymer coating suppresses microglial encapsulation to neural implants in vitro and in vivo. Adv. Biosyst. 2020, 4, 1900287. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Denno, M.E.; Pyakurel, P.; Venton, B.J. Recent trends in carbon nanomaterial-based electrochemical sensors for biomolecules: A review. Anal. Chim. Acta 2015, 887, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Castagnola, E.; Garg, R.; Rastogi, S.K.; Cohen-Karni, T.; Cui, X.T. 3D fuzzy graphene microelectrode array for dopamine sensing at sub-cellular spatial resolution. Biosens. Bioelectron. 2021, 191, 113440. [Google Scholar] [CrossRef] [PubMed]
- Castagnola, E.; Ansaldo, A.; Fadiga, L.; Ricci, D. Chemical vapour deposited carbon nanotube coated microelectrodes for intracortical neural recording. Physica Status Solidi (B) 2010, 247, 2703–2707. [Google Scholar] [CrossRef]
- Rodeberg, N.T.; Sandberg, S.G.; Johnson, J.A.; Phillips, P.E.; Wightman, R.M. Hitchhiker’s guide to voltammetry: Acute and chronic electrodes for in vivo fast-scan cyclic voltammetry. ACS Chem. Neurosci. 2017, 8, 221–234. [Google Scholar] [CrossRef]
- Meunier, C.J.; Denison, J.D.; McCarty, G.S.; Sombers, L.A. Interpreting dynamic interfacial changes at carbon fiber microelectrodes using electrochemical impedance spectroscopy. Langmuir 2020, 36, 4214–4223. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.Y.; Langhals, N.B.; Patel, P.R.; Deng, X.; Zhang, H.; Smith, K.L.; Lahann, J.; Kotov, N.A.; Kipke, D.R. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat. Mater. 2012, 11, 1065–1073. [Google Scholar] [CrossRef]
- Schwerdt, H.N.; Zhang, E.; Kim, M.J.; Yoshida, T.; Stanwicks, L.; Amemori, S.; Dagdeviren, H.E.; Langer, R.; Cima, M.J.; Graybiel, A.M. Cellular-scale probes enable stable chronic subsecond monitoring of dopamine neurochemicals in a rodent model. Commun. Biol. 2018, 1, 144. [Google Scholar] [CrossRef]
- Guitchounts, G.; Cox, D. 64-channel carbon fiber electrode arrays for chronic electrophysiology. Sci. Rep. 2020, 10, 3830. [Google Scholar] [CrossRef]
- Patel, P.R.; Popov, P.; Caldwell, C.M.; Welle, E.J.; Egert, D.; Pettibone, J.R.; Roossien, D.H.; Becker, J.B.; Berke, J.D.; Chestek, C.A. High density carbon fiber arrays for chronic electrophysiology, fast scan cyclic voltammetry, and correlative anatomy. J. Neural Eng. 2020, 17, 056029. [Google Scholar] [CrossRef]
- Schwerdt, H.N.; Gibson, D.J.; Amemori, K.; Stanwicks, L.L.; Yoshida, T.; Cima, M.J.; Graybiel, A.M. Chronic multi-modal monitoring of neural activity in rodents and primates. In The Integrated Sensors for Biological and Neural Sensing; SPIE digital library: Washington, DC, USA, 2021; pp. 11–17. [Google Scholar]
- Schwerdt, H.; Amemori, K.; Gibson, D.; Stanwicks, L.; Yoshida, T.; Bichot, N.; Amemori, S.; Desimone, R.; Langer, R.; Cima, M. Dopamine and beta-band oscillations differentially link to striatal value and motor control. Sci. Adv. 2020, 6, eabb9226. [Google Scholar] [CrossRef]
- Welle, E.J.; Patel, P.R.; Woods, J.E.; Petrossians, A.; Della Valle, E.; Vega-Medina, A.; Richie, J.M.; Cai, D.; Weiland, J.D.; Chestek, C.A. Ultra-small carbon fiber electrode recording site optimization and improved in vivo chronic recording yield. J. Neural Eng. 2020, 17, 026037. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.R.; Na, K.; Zhang, H.; Kozai, T.D.; Kotov, N.A.; Yoon, E.; Chestek, C.A. Insertion of linear 8.4 μm diameter 16 channel carbon fiber electrode arrays for single unit recordings. J. Neural Eng. 2015, 12, 046009. [Google Scholar] [CrossRef]
- Schwerdt, H.N.; Kim, M.J.; Amemori, S.; Homma, D.; Yoshida, T.; Shimazu, H.; Yerramreddy, H.; Karasan, E.; Langer, R.; Graybiel, A.M. Subcellular probes for neurochemical recording from multiple brain sites. Lab Chip 2017, 17, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Schwerdt, H.N.; Shimazu, H.; Amemori, K.-i.; Amemori, S.; Tierney, P.L.; Gibson, D.J.; Hong, S.; Yoshida, T.; Langer, R.; Cima, M.J. Long-term dopamine neurochemical monitoring in primates. Proc. Natl. Acad. Sci. USA 2017, 114, 13260–13265. [Google Scholar] [CrossRef]
- Patel, P.R.; Zhang, H.; Robbins, M.T.; Nofar, J.B.; Marshall, S.P.; Kobylarek, M.J.; Kozai, T.D.; Kotov, N.A.; Chestek, C.A. Chronic in vivo stability assessment of carbon fiber microelectrode arrays. J. Neural Eng. 2016, 13, 066002. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Rusinek, C.A.; Thompson, C.H.; Setien, M.; Guo, Y.; Rechenberg, R.; Gong, Y.; Weber, A.J.; Becker, M.F.; Purcell, E. Flexible, diamond-based microelectrodes fabricated using the diamond growth side for neural sensing. Microsyst. Nanoeng. 2020, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Vomero, M.; Van Niekerk, P.; Nguyen, V.; Gong, N.; Hirabayashi, M.; Cinopri, A.; Logan, K.; Moghadasi, A.; Varma, P.; Kassegne, S. A novel pattern transfer technique for mounting glassy carbon microelectrodes on polymeric flexible substrates. J. Micromech. Microeng. 2016, 26, 025018. [Google Scholar] [CrossRef]
- Castagnola, E.; Thongpang, S.; Hirabayashi, M.; Nava, G.; Nimbalkar, S.; Nguyen, T.; Lara, S.; Oyawale, A.; Bunnell, J.; Moritz, C. Glassy carbon microelectrode arrays enable voltage-peak separated simultaneous detection of dopamine and serotonin using fast scan cyclic voltammetry. Analyst 2021, 146, 3955–3970. [Google Scholar] [CrossRef]
- Kassegne, S.; Vomero, M.; Gavuglio, R.; Hirabayashi, M.; Özyilmaz, E.; Nguyen, S.; Rodriguez, J.; Özyilmaz, E.; van Niekerk, P.; Khosla, A. Electrical impedance, electrochemistry, mechanical stiffness, and hardness tunability in glassy carbon MEMS μECoG electrodes. Microelectron. Eng. 2015, 133, 36–44. [Google Scholar] [CrossRef]
- Vahidi, N.W.; Rudraraju, S.; Castagnola, E.; Cea, C.; Nimbalkar, S.; Hanna, R.; Arvizu, R.; Dayeh, S.A.; Gentner, T.Q.; Kassegne, S. Epi-Intra neural probes with glassy carbon microelectrodes help elucidate neural coding and stimulus encoding in 3D volume of tissue. J. Neural Eng. 2020, 17, 046005. [Google Scholar] [CrossRef] [PubMed]
- Goshi, N.; Castagnola, E.; Vomero, M.; Gueli, C.; Cea, C.; Zucchini, E.; Bjanes, D.; Maggiolini, E.; Moritz, C.; Kassegne, S. Glassy carbon MEMS for novel origami-styled 3D integrated intracortical and epicortical neural probes. J. Micromech. Microeng. 2018, 28, 065009. [Google Scholar] [CrossRef]
- Castagnola, E.; Vahidi, N.W.; Nimbalkar, S.; Rudraraju, S.; Thielk, M.; Zucchini, E.; Cea, C.; Carli, S.; Gentner, T.Q.; Ricci, D. In vivo dopamine detection and single unit recordings using intracortical glassy carbon microelectrode arrays. MRS Adv. 2018, 3, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Faul, E.-B.A.; Broussard, A.M.; Rivera, D.R.; Pwint, M.Y.; Wu, B.; Cao, Q.; Bailey, D.; Cui, X.T.; Castagnola, E. Batch Fabrication of Microelectrode Arrays with Glassy Carbon Microelectrodes and Interconnections for Neurochemical Sensing: Promises and Challenges. Micromachines 2024, 15, 277. [Google Scholar] [CrossRef]
- Nam, K.-H.; Abdulhafez, M.; Castagnola, E.; Tomaraei, G.N.; Cui, X.T.; Bedewy, M. Laser direct write of heteroatom-doped graphene on molecularly controlled polyimides for electrochemical biosensors with nanomolar sensitivity. Carbon 2022, 188, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; James, D.K.; Tour, J.M. Laser-induced graphene. Acc. Chem. Res. 2018, 51, 1609–1620. [Google Scholar] [CrossRef]
- Stanford, M.G.; Zhang, C.; Fowlkes, J.D.; Hoffman, A.; Ivanov, I.N.; Rack, P.D.; Tour, J.M. High-resolution laser-induced graphene. Flexible electronics beyond the visible limit. ACS Appl. Mater. Interfaces 2020, 12, 10902–10907. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef]
- Le, T.S.D.; Phan, H.P.; Kwon, S.; Park, S.; Jung, Y.; Min, J.; Chun, B.J.; Yoon, H.; Ko, S.H.; Kim, S.W. Recent advances in laser-induced graphene: Mechanism, fabrication, properties, and applications in flexible electronics. Adv. Funct. Mater. 2022, 32, 2205158. [Google Scholar] [CrossRef]
- Fu, X.-Y.; Jiang, H.-B.; Han, D.-D.; Zhang, Y.-L.; Zhang, P.-L. Direct laser writing of planar and stretchable supercapacitors based on a graphene oxide and manganese dioxide nanoparticle composite on a paper substrate. Appl. Phys. Lett. 2025, 126, 023906. [Google Scholar] [CrossRef]
- Abdulhafez, M.; Tomaraei, G.N.; Bedewy, M. Fluence-dependent morphological transitions in laser-induced graphene electrodes on polyimide substrates for flexible devices. ACS Appl. Nano Mater. 2021, 4, 2973–2986. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Yuan, L.; Zhang, B.; Bishop, E.S.; Wang, K.; Tang, J.; Zheng, Y.-Q.; Xu, W.; Niu, S. A tissue-like neurotransmitter sensor for the brain and gut. Nature 2022, 606, 94–101. [Google Scholar] [CrossRef]
- Lee, Y.; Low, M.J.; Yang, D.; Nam, H.K.; Le, T.-S.D.; Lee, S.E.; Han, H.; Kim, S.; Vu, Q.H.; Yoo, H. Ultra-thin light-weight laser-induced-graphene (LIG) diffractive optics. Light Sci. Appl. 2023, 12, 146. [Google Scholar] [CrossRef]
- Johnson, M.; Franklin, R.; Scott, K.; Brown, R.; Kipke, D. Neural probes for concurrent detection of neurochemical and electrophysiological signals in vivo. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 1–4 September 2005; pp. 7325–7328. [Google Scholar]
- Zhang, S.; Song, Y.; Wang, M.; Zhang, Z.; Fan, X.; Song, X.; Zhuang, P.; Yue, F.; Chan, P.; Cai, X. A silicon based implantable microelectrode array for electrophysiological and dopamine recording from cortex to striatum in the non-human primate brain. Biosens. Bioelectron. 2016, 85, 53–61. [Google Scholar] [CrossRef]
- Stamford, J.A.; Palij, P.; Davidson, C.; Jorm, C.M.; Millar, J. Simultaneous “real-time” electrochemical and electrophysiological recording in brain slices with a single carbon-fibre microelectrode. J. Neurosci. Methods 1993, 50, 279–290. [Google Scholar] [CrossRef]
- Cheer, J.F.; Heien, M.L.; Garris, P.A.; Carelli, R.M.; Wightman, R.M. Simultaneous dopamine and single-unit recordings reveal accumbens GABAergic responses: Implications for intracranial self-stimulation. Proc. Natl. Acad. Sci. USA 2005, 102, 19150–19155. [Google Scholar] [CrossRef]
- Walton, L.R.; Boustead, N.G.; Carroll, S.; Wightman, R.M. Effects of glutamate receptor activation on local oxygen changes. ACS Chem. Neurosci. 2017, 8, 1598–1608. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, C.N.; Johnson, J.A.; Verber, M.D.; Wightman, R.M. An implantable multimodal sensor for oxygen, neurotransmitters, and electrophysiology during spreading depolarization in the deep brain. Analyst 2017, 142, 2912–2920. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.L.; Venton, B.J.; Heien, M.L.; Wightman, R.M. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin. Chem. 2003, 49, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.M.; Hashemi, P. Fast-Scan Cyclic Voltammetry Analysis of Dynamic Serotonin Reponses to Acute Escitalopram. ACS Chem. Neurosci. 2013, 4, 715–720. [Google Scholar] [CrossRef]
- Swamy, B.K.; Venton, B.J. Carbon nanotube-modified microelectrodes for simultaneous detection of dopamine and serotonin in vivo. Analyst 2007, 132, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Buchanan, A.M.; Witt, C.E.; Hashemi, P. Frontiers in electrochemical sensors for neurotransmitter detection: Towards measuring neurotransmitters as chemical diagnostics for brain disorders. Anal. Methods 2019, 11, 2738–2755. [Google Scholar] [CrossRef]
- Bucher, E.S.; Wightman, R.M. Electrochemical analysis of neurotransmitters. Annu. Rev. Anal. Chem. 2015, 8, 239–261. [Google Scholar] [CrossRef]
- Owesson-White, C.; Belle, A.M.; Herr, N.R.; Peele, J.L.; Gowrishankar, P.; Carelli, R.M.; Wightman, R.M. Cue-evoked dopamine release rapidly modulates D2 neurons in the nucleus accumbens during motivated behavior. J. Neurosci. 2016, 36, 6011–6021. [Google Scholar] [CrossRef]
- Takmakov, P.; McKinney, C.J.; Carelli, R.M.; Wightman, R.M. Instrumentation for fast-scan cyclic voltammetry combined with electrophysiology for behavioral experiments in freely moving animals. Rev. Sci. Instrum. 2011, 82, 074302. [Google Scholar] [CrossRef]
- Bucher, E.S.; Brooks, K.; Verber, M.D.; Keithley, R.B.; Owesson-White, C.; Carroll, S.; Takmakov, P.; McKinney, C.J.; Wightman, R.M. Flexible software platform for fast-scan cyclic voltammetry data acquisition and analysis. Anal. Chem. 2013, 85, 10344–10353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lin, S.-C.; Nicolelis, M.A. Spatiotemporal coupling between hippocampal acetylcholine release and theta oscillations in vivo. J. Neurosci. 2010, 30, 13431–13440. [Google Scholar] [CrossRef]
- Ledo, A.; Lourenço, C.F.; Laranjinha, J.; Gerhardt, G.A.; Barbosa, R.M. Combined in vivo amperometric oximetry and electrophysiology in a single sensor: A tool for epilepsy research. Anal. Chem. 2017, 89, 12383–12390. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hajnal, B.; Entz, L.; Fabó, D.; Herrero, J.L.; Mehta, A.D.; Keller, C.J. Intracortical dynamics underlying repetitive stimulation predicts changes in network connectivity. J. Neurosci. 2019, 39, 6122–6135. [Google Scholar] [CrossRef] [PubMed]
- Beuter, A.; Lefaucheur, J.-P.; Modolo, J. Closed-loop cortical neuromodulation in Parkinson’s disease: An alternative to deep brain stimulation? Clin. Neurophysiol. 2014, 125, 874–885. [Google Scholar] [CrossRef]
- Grahn, P.J.; Mallory, G.W.; Khurram, O.U.; Berry, B.M.; Hachmann, J.T.; Bieber, A.J.; Bennet, K.E.; Min, H.-K.; Chang, S.-Y.; Lee, K.H. A neurochemical closed-loop controller for deep brain stimulation: Toward individualized smart neuromodulation therapies. Front. Neurosci. 2014, 8, 169. [Google Scholar] [CrossRef]
- Daly, J.J.; Wolpaw, J.R. Brain–computer interfaces in neurological rehabilitation. Lancet Neurol. 2008, 7, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, A.; Francisco, G.E.; L Contreras-Vidal, J. Applications of brain–machine interface systems in stroke recovery and rehabilitation. Curr. Phys. Med. Rehabil. Rep. 2014, 2, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Ourtine, G.; Micera, S.; DiGiovanna, J.; del R Millán, J. Brain–machine interface: Closer to therapeutic reality? Lancet 2013, 381, 515–517. [Google Scholar] [CrossRef]
- Aqrawe, Z.; Montgomery, J.; Travas-Sejdic, J.; Svirskis, D. Conducting polymers for neuronal microelectrode array recording and stimulation. Sens. Actuators B Chem. 2018, 257, 753–765. [Google Scholar] [CrossRef]
- Zhang, Q.; Beirne, S.; Shu, K.; Esrafilzadeh, D.; Huang, X.-F.; Wallace, G.G. Electrical stimulation with a conductive polymer promotes neurite outgrowth and synaptogenesis in primary cortical neurons in 3D. Sci. Rep. 2018, 8, 9855. [Google Scholar] [CrossRef]
- Thompson, B.C.; Richardson, R.T.; Moulton, S.E.; Evans, A.J.; O’Leary, S.; Clark, G.M.; Wallace, G.G. Conducting polymers, dual neurotrophins and pulsed electrical stimulation—Dramatic effects on neurite outgrowth. J. Control. Release 2010, 141, 161–167. [Google Scholar] [CrossRef]
- Wang, K.; Fishman, H.A.; Dai, H.; Harris, J.S. Neural stimulation with a carbon nanotube microelectrode array. Nano Lett. 2006, 6, 2043–2048. [Google Scholar] [CrossRef]
- Huan, Y.; Gill, J.P.; Fritzinger, J.B.; Patel, P.R.; Richie, J.M.; Della Valle, E.; Weiland, J.D.; Chestek, C.A.; Chiel, H.J. Carbon fiber electrodes for intracellular recording and stimulation. J. Neural Eng. 2021, 18, 066033. [Google Scholar] [CrossRef]
- Vitale, F.; Summerson, S.R.; Aazhang, B.; Kemere, C.; Pasquali, M. Neural stimulation and recording with bidirectional, soft carbon nanotube fiber microelectrodes. ACS Nano 2015, 9, 4465–4474. [Google Scholar] [CrossRef] [PubMed]
- Lycke, R.; Kim, R.; Zolotavin, P.; Montes, J.; Sun, Y.; Koszeghy, A.; Altun, E.; Noble, B.; Yin, R.; He, F. Low-threshold, high-resolution, chronically stable intracortical microstimulation by ultraflexible electrodes. Cell Rep. 2023, 42, 112554. [Google Scholar] [CrossRef] [PubMed]
- Rembado, I.; Castagnola, E.; Turella, L.; Ius, T.; Budai, R.; Ansaldo, A.; Angotzi, G.N.; Debertoldi, F.; Ricci, D.; Skrap, M. Independent component decomposition of human somatosensory evoked potentials recorded by micro-electrocorticography. Int. J. Neural Syst. 2017, 27, 1650052. [Google Scholar] [CrossRef]
- Mokienko, O. Brain–computer interfaces with intracortical implants for motor and communication functions compensation: Review of recent developments. Sovremennye Tehnologii v Medicine 2024, 16, 78–89. [Google Scholar] [CrossRef]
- Flesher, S.; Downey, J.; Collinger, J.; Foldes, S.; Weiss, J.; Tyler-Kabara, E.; Bensmaia, S.; Schwartz, A.; Boninger, M.; Gaunt, R. Intracortical microstimulation as a feedback source for brain-computer interface users. In Brain-Computer Interface Research; Springer: Berlin/Heidelberg, Germany, 2017; pp. 43–54. [Google Scholar]
- Meng, S.; Rouabhia, M.; Zhang, Z.; De, D.; De, F.; Laval, U. Electrical stimulation in tissue regeneration. Appl. Biomed. Eng. 2011, 37–62. [Google Scholar]
- Das, K.K.; Basu, B.; Maiti, P.; Dubey, A.K. Interplay of piezoelectricity and electrical stimulation in tissue engineering and regenerative medicine. Appl. Mater. Today 2024, 39, 102332. [Google Scholar] [CrossRef]
- Schröter, M.; Wang, C.; Terrigno, M.; Hornauer, P.; Huang, Z.; Jagasia, R.; Hierlemann, A. Functional imaging of brain organoids using high-density microelectrode arrays. MRS Bull. 2022, 47, 530–544. [Google Scholar] [CrossRef]
- Huang, Q.; Tang, B.; Romero, J.C.; Yang, Y.; Elsayed, S.K.; Pahapale, G.; Lee, T.-J.; Morales Pantoja, I.E.; Han, F.; Berlinicke, C. Shell microelectrode arrays (MEAs) for brain organoids. Sci. Adv. 2022, 8, eabq5031. [Google Scholar] [CrossRef]
- Durens, M.; Nestor, J.; Williams, M.; Herold, K.; Niescier, R.F.; Lunden, J.W.; Phillips, A.W.; Lin, Y.-C.; Dykxhoorn, D.M.; Nestor, M.W. High-throughput screening of human induced pluripotent stem cell-derived brain organoids. J. Neurosci. Methods 2020, 335, 108627. [Google Scholar] [CrossRef] [PubMed]
- Di Lullo, E.; Kriegstein, A.R. The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci. 2017, 18, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Song, H.; Ming, G.-l. Brain organoids: Advances, applications and challenges. Development 2019, 146, dev166074. [Google Scholar] [CrossRef]
- Luo, J.; Li, P. Human pluripotent stem cell-derived brain organoids as in vitro models for studying neural disorders and cancer. Cell Biosci. 2021, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.; Lewis, K.; Saulich, F.; Endres, M.; Boehmerle, W.; Huehnchen, P. Induced pluripotent stem cell-derived brain organoids as potential human model system for chemotherapy induced CNS toxicity. Front. Mol. Biosci. 2022, 9, 1006497. [Google Scholar] [CrossRef]
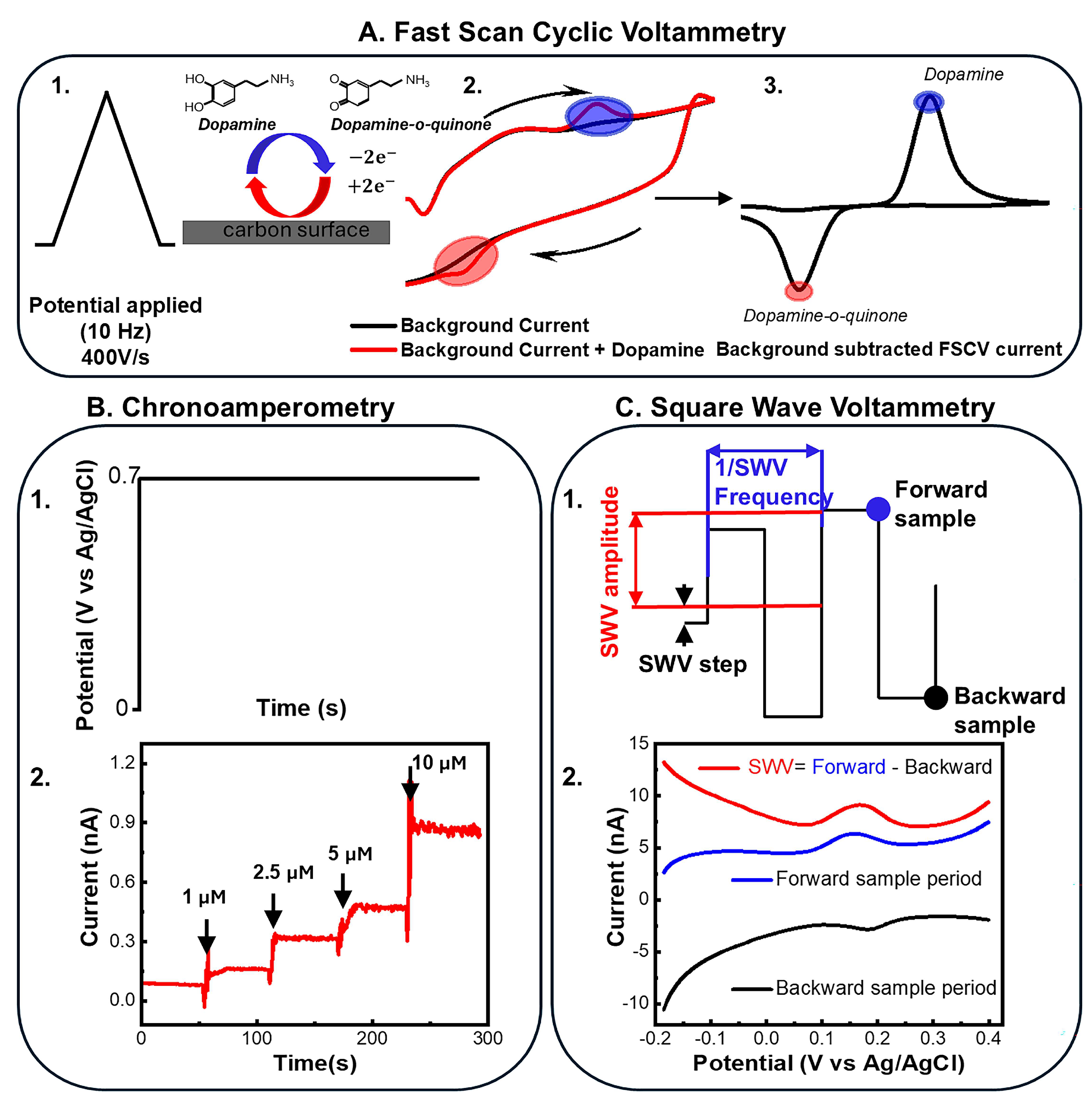
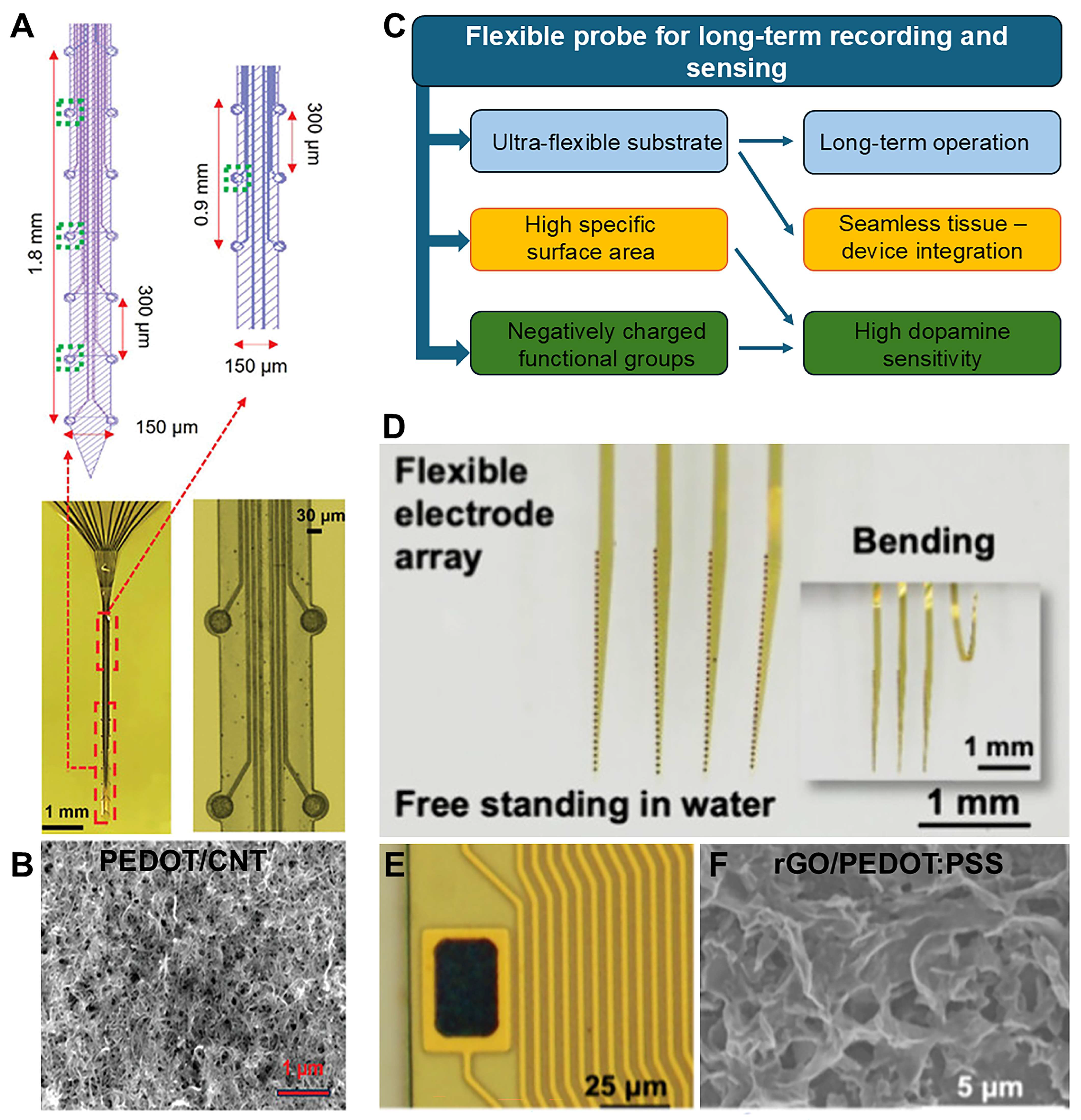
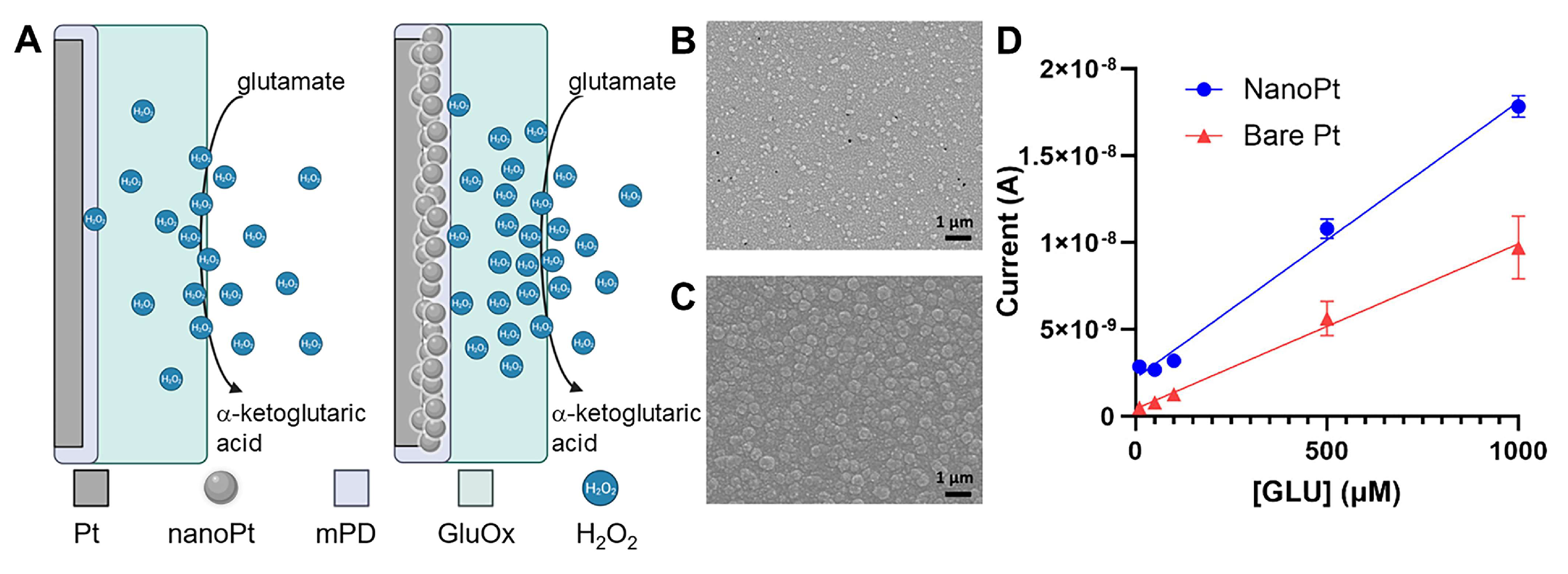
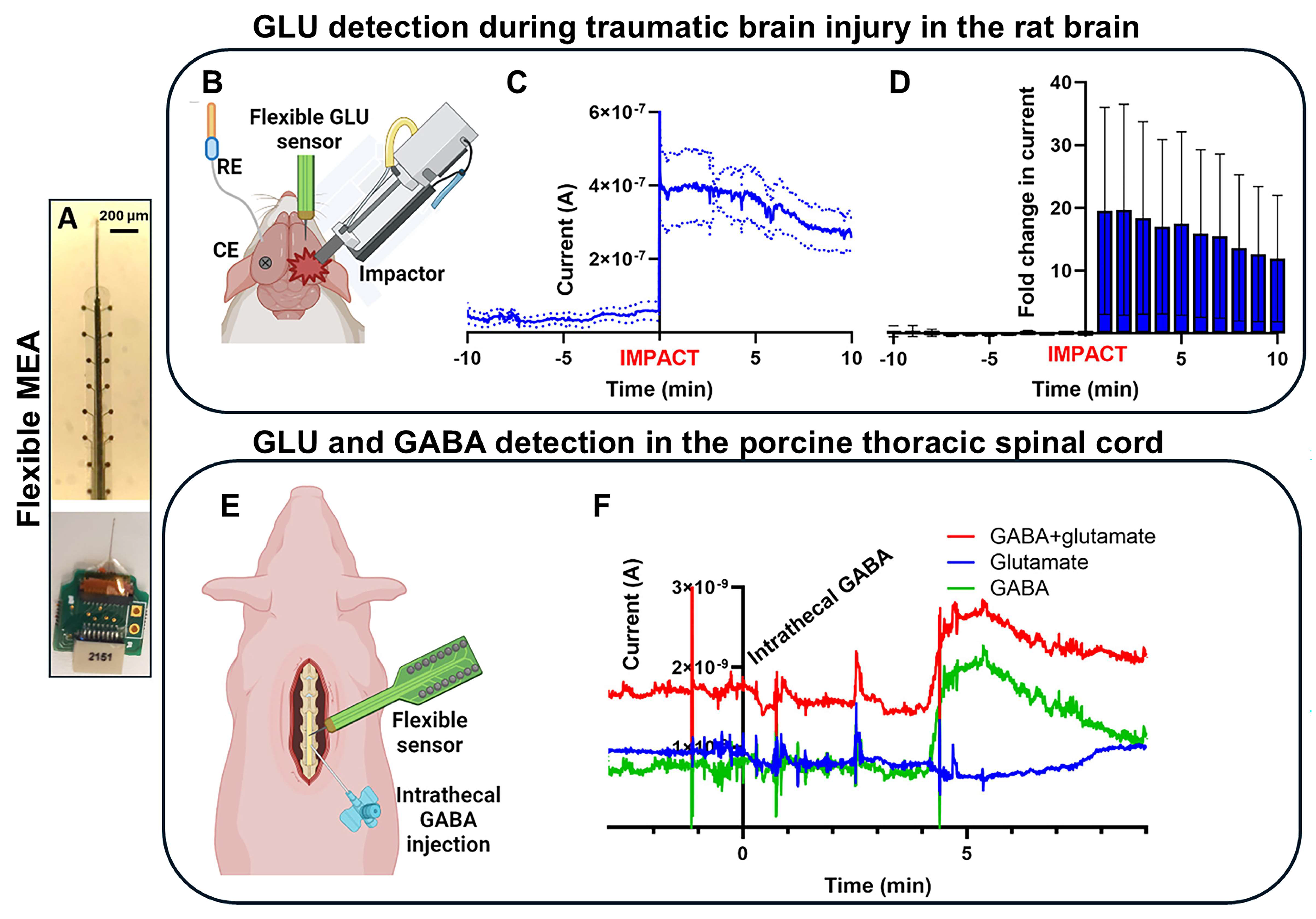
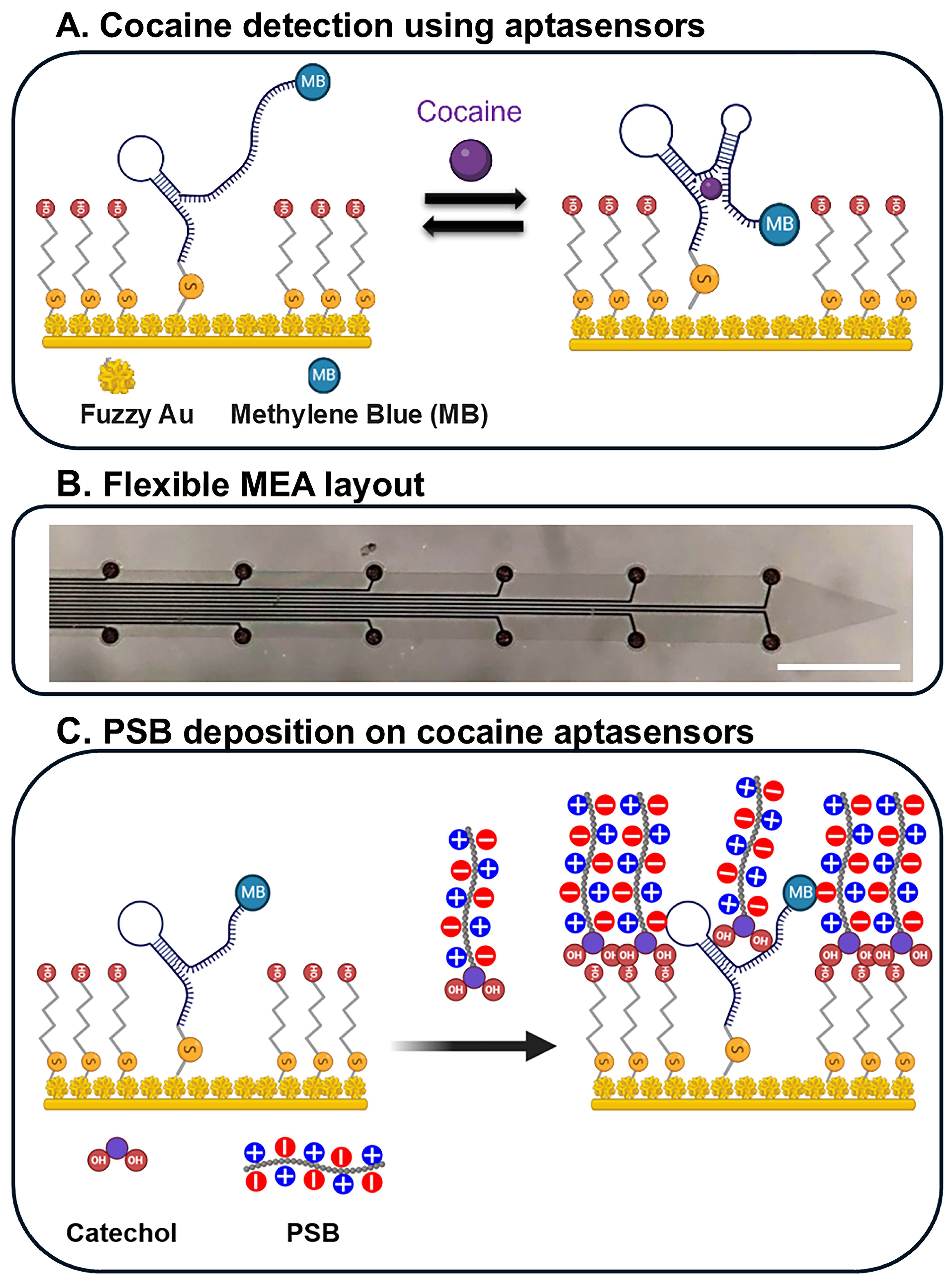
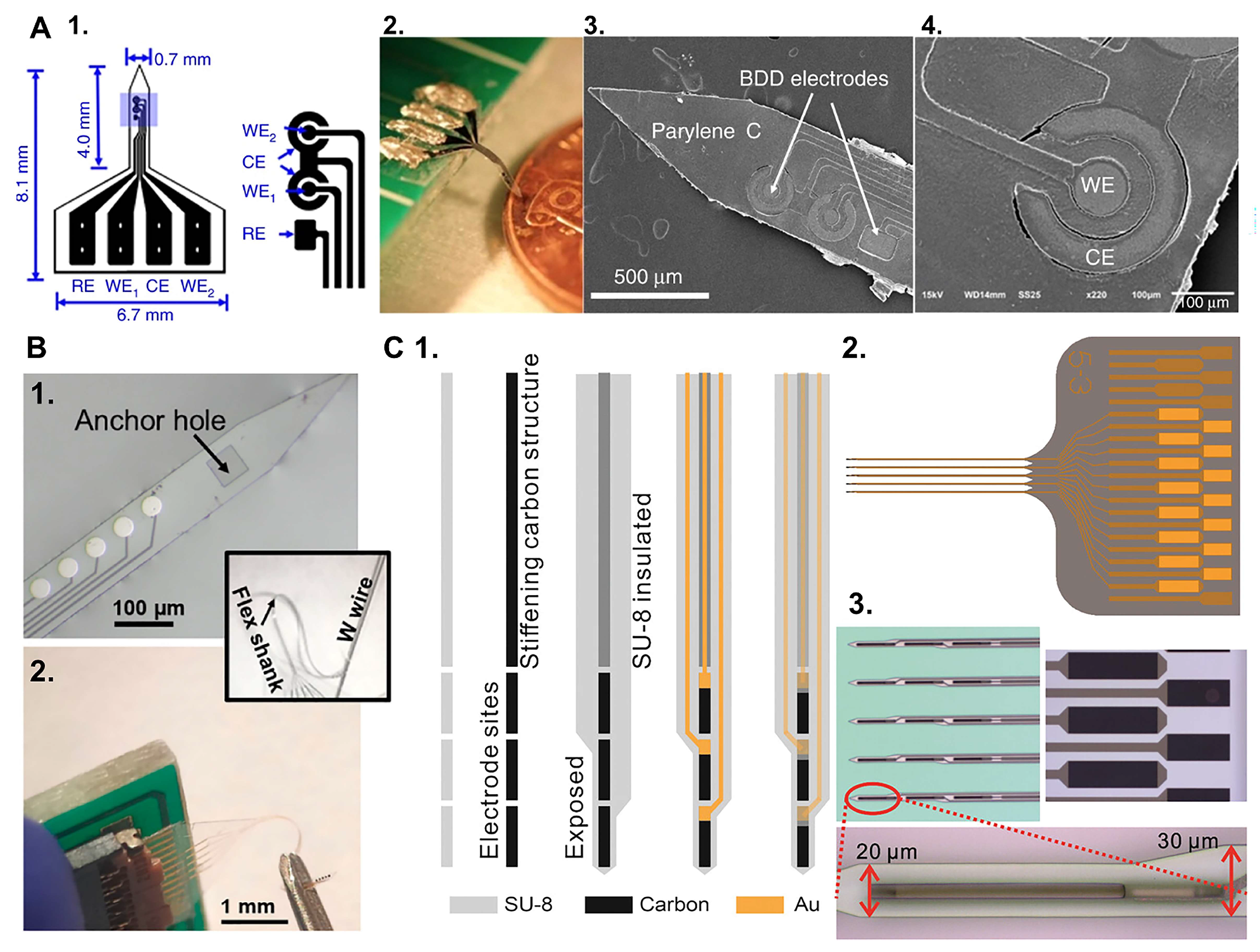
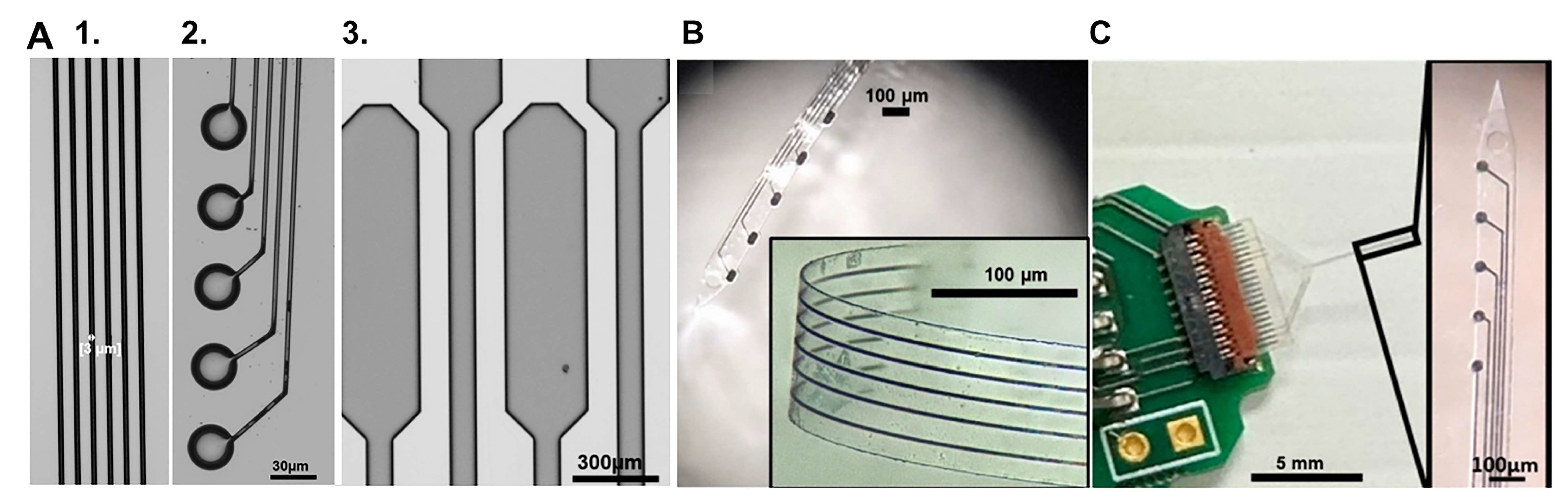
| Device | Coating | Modality | Sensitivity | LOD | Echem Technique | Acute/Chronic | Ref. |
|---|---|---|---|---|---|---|---|
| CFEs and silicon MEAs | PEDOT/CNT | Echem (tonic DA) | 108 ± 9 (CFEs) and 14.7 ± 0.05 nA/μM (MEAs) in a CSF | 2.03 ± 0.09 nM (CFEs) | SWV | Acute (rat brain) | [124] |
| Flexible GC-MEAs | PEDOT/CNT | Echem (tonic and phasic DA) | 55.634 ± 0.001 nA/μM in aCSF | -- | SWV (at PEDOT/CNT) and SWV (at GC) | Chronic SVW (21 days) and acute FSCV (mouse brain) | [43] |
| Flexible GC-MEAs | PEDOT/CNT | Echem (tonic 5-HT) | 17.56 ± 0.01 nA/μM | -- | SWV | Acute and chronic (7 days) (mouse brain) | [107] |
| Silicon MEAs | SWCNTs/PEDOT:PSS | Ephys and Echem (DA) | 217 pA/μM | 10 nM | chronoamperometry | Acute (rat brain) | [130] |
| Flexible MEAs | PtNPs/PEDOT:PSS | Ephys and Echem (DA) | 162.3 pA/μM in PBS | -- | chronoamperometry | Acute (mouse brain) | [132] |
| Flexible MEAs | PEDOT/CNT | Ephys and Echem (tonic DA) | ~150 nA/μM in PBS | ~4.4 nM | SWV | Chronic (28 days) (mouse brain) | [39] |
| Flexible MEAs | rGO/PEDOT:PSS | Ephys and Echem (DA) | 15 pA/μM in PBS with agarose | -- | CPA | Chronic (6 weeks)(mouse brain) | [133] |
| Device | Electrode Material | Modality | Biological Recognition Elements | Sensitivity | Echem Technique | Acute/Chronic | Ref. |
|---|---|---|---|---|---|---|---|
| Silicon MEAs | Pt/rGOs nanoparticles | Ephys and Echem (DA and GLU) | GluOx and mPD on the GLU site | 8.62 ± 1.32 pA/μM for GLU 13.21 ± 2.31 pA/μM for DA (in PBS) | chronoamperometry | Acute (mouse brain) | [131] |
| Ceramic-based MEAs | Pt | Echem (GLU and ACh tonic and phasic) | GluOx and mPD (GLU) ChOx/AChE (ACh) | 4.2 ± 2.0 pA/μM (GLU) 5.8 ± 2.6 pA/μM (ACh) | chronoamperometry | Acute (rat brain) | [89] |
| Silicon MEAs | Pt | Echem (GLU and GABA) | GluOx and mPD GluOx/GABASE and mPD | ~500 nA/μM.cm2 (in PBS) | chronoamperometry | Chronic (11 days) (rat brain) | [52] |
| Flexible MEAs | Echem (GLU and GABA) | GluOx and mPD | -- | chronoamperometry | Acute (pig spinal cord) | [51] | |
| Silicon MEAs | nanoPt | Echem (GLU) | GluOx and mPD | 1.590 ± 0.057 × 10−2 nA/μM (in PBS) | chronoamperometry | Acute and chronic (7 days mouse brain) | [50] |
| Flexible MEAs | nanoPt | Echem (GLU) | GluOx and mPD | 1.590 ± 0.057 × 10−2 nA/μM (in PBS) | chronoamperometry | Acute (TBI rat brain) | [50] |
| Flexible MEAs | nanoPt | Echem (GLU and GABA) | GluOx and mPD GluOx/GABASE and mPD | 1.590 ± 0.057 × 10−2 nA/μM (in PBS) | chronoamperometry | Acute (pig spinal cord) | [50] |
| Bimodal (RTBM) microelectromechanical system (MEMS) neural prob | Pt coated with mPD and OPPy | Ephys and Echem (glucose, lactate, GLU, and choline) | glucose oxidase, LOx, ChOx, GluOx, and mPD | 6.18 ± 0.71 nA mM−1 (glucose) 0.62 ± 0.07 nA mM−1 (lactate) 7.03 ± 1.26 pA μM−1 (GLU) and 19.82 ± 1.09 pA μM−1 (choline) (in aCSF) | chronoamperometry | Acute (mouse brain) | [148] |
| Silicon MEAs | PtNPt | Ephys and Echem (GLU) | GluOx and mPD | 7.807 pA/μM (in PBS) | chronoamperometry | Acute (rat brain) | [149] |
| Silicon MEAs | PtNPt | Ephys and Echem (GLU) | GluOx and mPD | 56 pA µM−1 (in PBS) | chronoamperometry | Acute (rat brain) | [150] |
| Silicon MEAs | dendritic gold | Ephys and Echem (cocaine) | Cocaine-targeting aptamer: 5′-HS-(CH2)6-AGACAAGGAAAATCCTTCAATGAAGTGGGTCG-(CH2)7-MB-3′ and MB | Not linear modified exponential Langmuir model | SWV | Acute (rat brain) | [159] |
| Silicon MEAs | fuzzy gold | Ephys and Echem (cocaine) | Cocaine-targeting aptamer: 5′-HS-(CH2)6-AGACAAGGAAAATCCTTCAATGAAGTGGGTCG-(CH2)7-MB-3′ and MB | Not linear modified exponential Langmuir model | SWV | Acute (rat brain) | [44] |
| Device | Electrode Material | Modality | Sensitivity | LOD | Echem Technique | Acute/Chronic | Ref. |
|---|---|---|---|---|---|---|---|
| FlexibleMEAs | BDD | Ephys and Echem (DA) | 0.9 nA/µM (in PBS) | 830 nM | SWV | Acute ephys in rat | [198] |
| hybrid GC-MEAs | GC | Echem (DA and 5-HT) | 164 nA/µM (DA) and 110 nA/µM (5-HT) using EW −0.4/1 V at 400V/s 354 nA/µM (DA) and 170 nA/µM (5-HT) using EW −0.5/1.3 V at 400 V/s. (in PBS) | 1.11nM (DA) and 1.29 nM (5-HT) using EW −0.4/1 V at 400 V/s. 1.17 nM (DA) and 1.73 nM (5-HT) using EW 0.5/1.3 V at 400 V/s | FSCV | Acute (proof of concept) co-detection of DA and 5-HT in the rat striatum | [200] |
| hybrid GC-MEAs | GC | Echem (DA) | 105.18 ± 6.22 nA/µM (in aCSF) | -- | Multichannel FSCV | Acute mouse DS | [43] |
| hybrid GC-MEAs | GC | Ephys and Echem (DA) | -- | -- | FSCV | Acute, songbird striatum | [204] |
| GCF MEAs | GC | Ephys and Echem (DA and 5-HT) | FSCV: 2.0 ± 0.2 pA µM−1 µm−2 (DA) and 4.0 ± 0.2 pA µM−1 µm−2 (5-HT). SWV: 0.45 µA cm−2 nM−1 (DA) and 1.22 µA cm−2 nM−1 (5-HT) (in PBS) | FSCV: 1.18 (DA) 0.89 nM (5-HT) | FSCV and SWV | Acute, mouse and rat brain | [106] |
| “all” GC-MEAs | GC | Ephys and Echem (DA) | 1.135 nA/nM.cm2 (in PBS) | 10nM | FSCV | Ephys from rats | [136] |
| “all” GC-MEAs | GC | Echem (5-HT) | 122.94 ± 4.36 nA/μM (in PBS) | -- | FSCV | -- | [205] |
| Neuro String | graphene/Fe3O4 nanoparticle network embedded in an elastomer | Echem (5-HT and DA) | -- | -- | FSCV | Chronic detection mouse brain (DA) and acute detection mouse brain (5-HT) and mouse colon and pig gut | [213] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siwakoti, U.; Jones, S.A.; Kumbhare, D.; Cui, X.T.; Castagnola, E. Recent Progress in Flexible Microelectrode Arrays for Combined Electrophysiological and Electrochemical Sensing. Biosensors 2025, 15, 100. https://doi.org/10.3390/bios15020100
Siwakoti U, Jones SA, Kumbhare D, Cui XT, Castagnola E. Recent Progress in Flexible Microelectrode Arrays for Combined Electrophysiological and Electrochemical Sensing. Biosensors. 2025; 15(2):100. https://doi.org/10.3390/bios15020100
Chicago/Turabian StyleSiwakoti, Umisha, Steven A. Jones, Deepak Kumbhare, Xinyan Tracy Cui, and Elisa Castagnola. 2025. "Recent Progress in Flexible Microelectrode Arrays for Combined Electrophysiological and Electrochemical Sensing" Biosensors 15, no. 2: 100. https://doi.org/10.3390/bios15020100
APA StyleSiwakoti, U., Jones, S. A., Kumbhare, D., Cui, X. T., & Castagnola, E. (2025). Recent Progress in Flexible Microelectrode Arrays for Combined Electrophysiological and Electrochemical Sensing. Biosensors, 15(2), 100. https://doi.org/10.3390/bios15020100





