Multiple Scattering-Enhanced Fluorescence Within Randomly Oriented Low-Index Polymer Nanofiber Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of PVAc Nanofiber Film
2.2. Microscopy and Optical Measurement
2.3. Preparation for Fluorescence Measurements
2.4. Finite-Difference Time-Domain (FDTD) Simulations
3. Results and Discussion
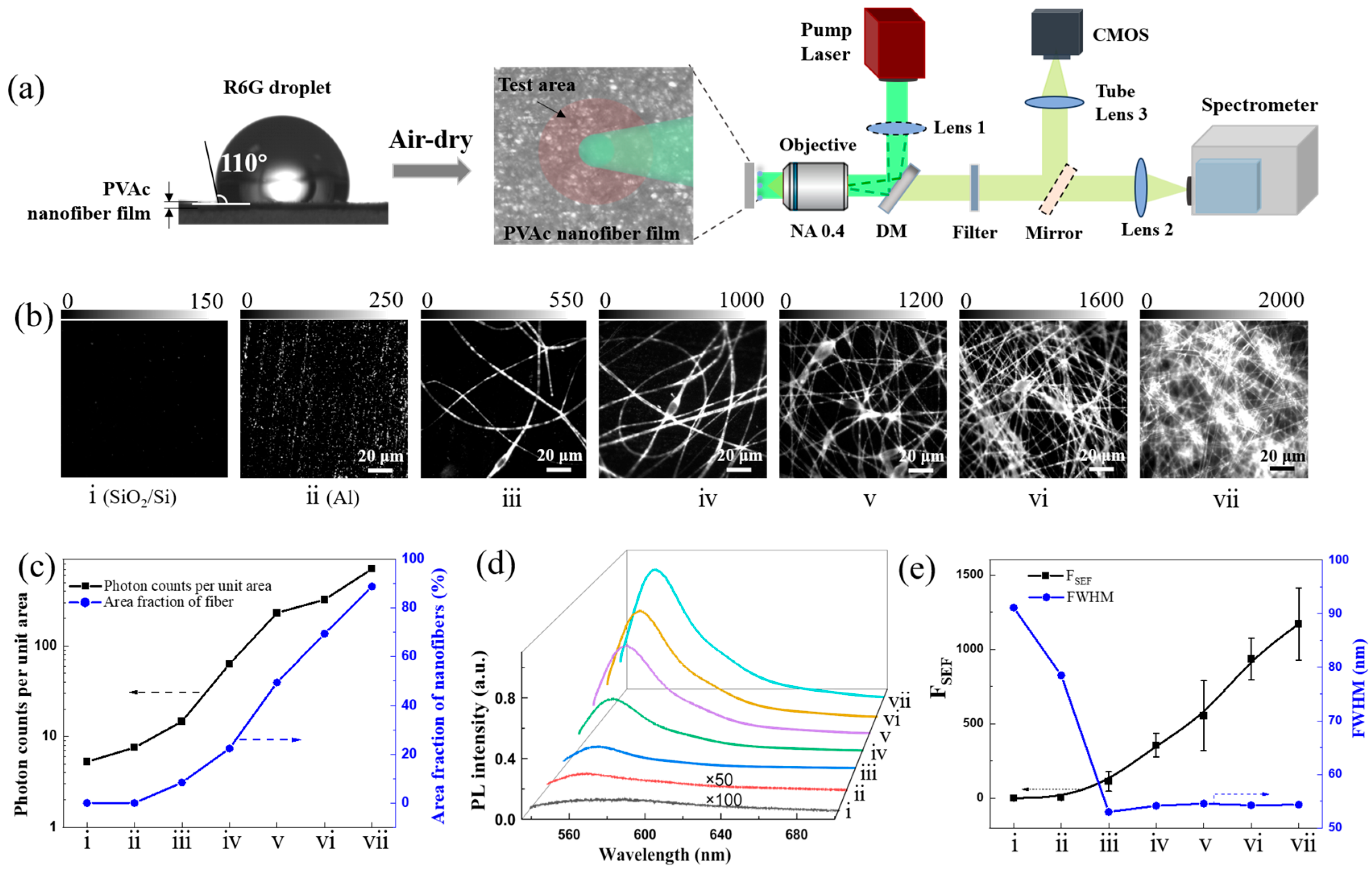
3.1. Multiple Scattering-Enhanced Fluorescence Strategy
3.2. Polymer Nanofiber-Based FSEF
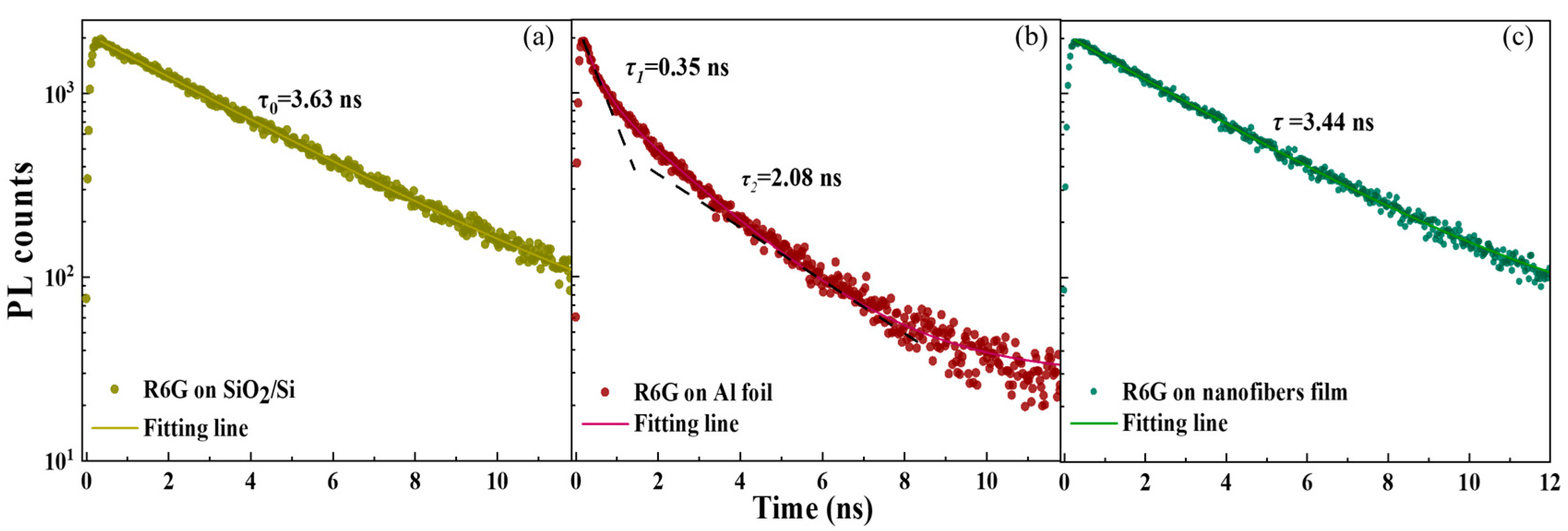
3.3. PL Decay Dynamics and Theoretical FSEF
3.4. Random Lasing and Characteristic of Multiple Scattering
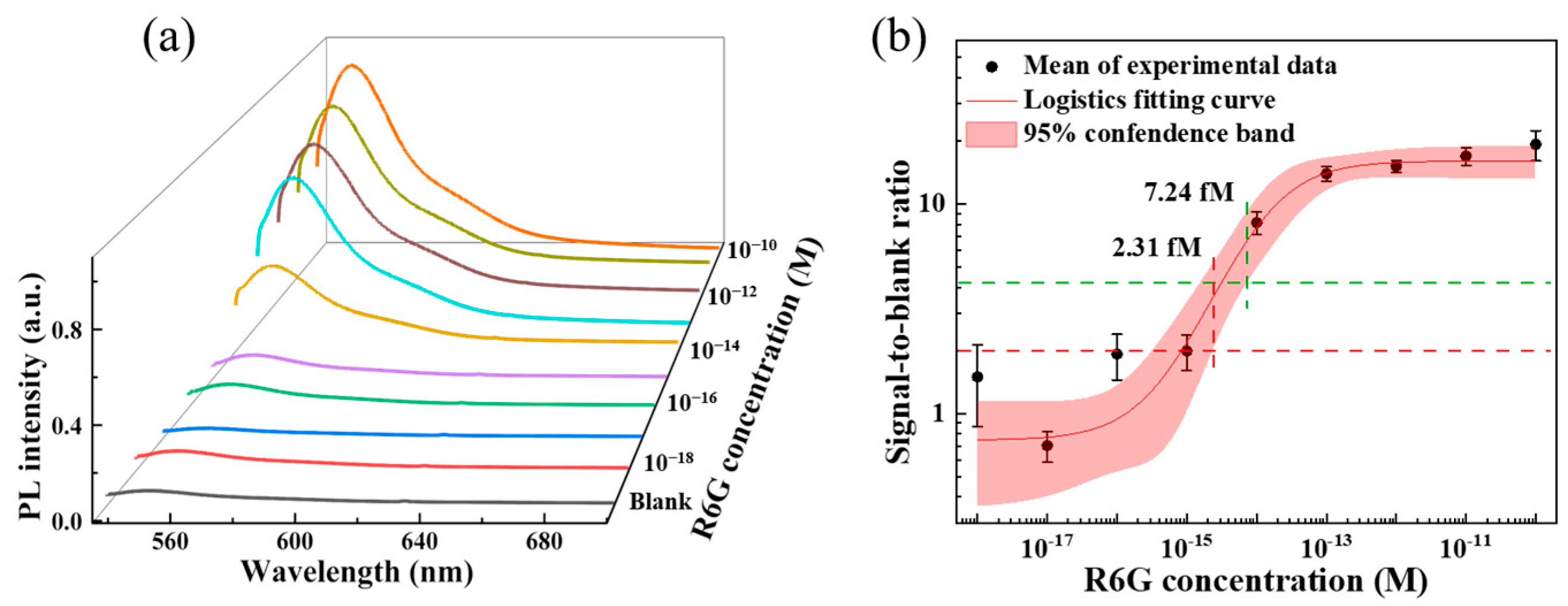
3.5. The Limit of Detection of PVAc Nanofiber Sensor
3.6. Detection of Aptamer-Cy3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Antonio, I.; Fernández, D.; Francisco, J.G.; Luis, M.M. Unrelenting plasmons. Nat. Photonics 2017, 11, 8–10. [Google Scholar]
- Khurgin, J.B. How to deal with the loss in plasmonics and metamaterials. Nat. Nanotechnol. 2015, 10, 2–6. [Google Scholar] [CrossRef]
- Gobin, A.M.; Lee, M.H.; Halas, N.J.; James, W.D.; Drezek, R.A.; West, J.L. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett. 2007, 7, 1929–1934. [Google Scholar] [CrossRef]
- Boyer, D.; Tamarat, P.; Maali, A.; Lounis, B.; Orrit, M. Photothermal imaging of nanometer-sized metal particles among scatterers. Science 2002, 297, 1160–1163. [Google Scholar] [CrossRef] [PubMed]
- Christopher, P.; Xin, H.; Linic, S. Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures. Nat. Chem. 2011, 3, 467–472. [Google Scholar] [CrossRef]
- Johansson, P.; Xu, H.; Kall, M. Surface-enhanced Raman scattering and fluorescence near metal nanoparticles. Phys. Rev. B 2005, 72, 035427. [Google Scholar] [CrossRef]
- Holzmeister, P.; Pibiri, E.; Schmied, J.J.; Sen, T.; Acuna, G.P.; Tinnefeld, P. Quantum yield and excitation rate of single molecules close to metallic nanostructures. Nat. Commun. 2014, 5, 5356. [Google Scholar] [CrossRef]
- Montaño-Priede, J.L.; Zapata-Herrera, M.; Esteban, R.; Zabala, N.; Aizpurua, J. An overview on plasmon-enhanced photoluminescence via metallic nanoantennas. Nanophotonics 2024, 13, 4771–4794. [Google Scholar] [CrossRef]
- Ru, L.; Auguié, B. Enhancement Factors: A central concept during 50 Years of surface-enhanced Raman spectroscopy. ACS Nano 2024, 18, 9773–9783. [Google Scholar]
- Anger, P.; Bharadwaj, P.; Novotny, L. Enhancement and quenching of single-molecule fluorescence. Phys. Rev. Lett. 2006, 96, 113002. [Google Scholar] [CrossRef]
- Staude, I.; Schilling, J. Metamaterial-inspired silicon nanophotonics. Nat. Photonics 2017, 11, 274–284. [Google Scholar] [CrossRef]
- Yang, Y.; Kravchenko, I.I.; Briggs, D.P.; Valentine, J. All-dielectric metasurface analogue of electromagnetically induced transparency. Nat. Commun. 2014, 5, 5753. [Google Scholar] [CrossRef] [PubMed]
- Cambiasso, J.; Grinblat, G.; Li, Y.; Rakovich, A.; Cortes, E.; Maier, S.A. Bridging the gap between dielectric nanophotonics and the visible regime with effectively lossless gallium phosphide antennas. Nano Lett. 2017, 17, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Devilez, A.; Zambrana-Puyalto, X.; Stout, B.; Bonod, N. Mimicking localized surface plasmons with dielectric particles. Phys. Rev. B 2015, 92, 241412. [Google Scholar] [CrossRef]
- Haar, M.A.; Groep, J.; Brenny, B.J.M.; Polman, A. Controlling magnetic and electric dipole modes in hollow silicon nanocylinders. Opt. Express 2016, 24, 2047–2064. [Google Scholar] [CrossRef] [PubMed]
- Evlyukhin, A.B.; Novikov, S.M.; Zywietz, U.; Eriksen, R.L.; Reinhardt, C.; Bozhevolnyi, S.I.; Chichkov, B.N. Demonstration of magnetic dipole resonances of dielectric nanospheres in the vsible region. Nano Lett. 2012, 12, 3749–3755. [Google Scholar] [CrossRef] [PubMed]
- Regmi, R.; Berthelot, J.; Winkler, P.M.; Mivelle, M.; Proust, J.; Bedu, F.; Ozerov, I.; Begou, T.; Lumeau, J.; Rigneault, H.; et al. All-dielectric silicon nanogap antennas to enhance the fluorescence of single molecules. Nano Lett. 2016, 16, 5143–5151. [Google Scholar] [CrossRef]
- Baranov, D.G.; Zuev, D.A.; Lepeshov, S.I.; Kotov, O.V.; Krasnok, A.E.; Evlyukhin, A.B.; Chichkov, B.N. All-dielectric nanophotonics: The quest for better materials and fabrication techniques. Optica 2017, 4, 814–825. [Google Scholar] [CrossRef]
- Caldarola, M.; Albella, P.; Cortés, E.; Rahmani, M.; Roschuk, T.; Grinblat, G.; Oulton, R.F.; Bragas, A.V.; Maier, S.A. Non-plasmonic nanoantennas for surface enhanced spectroscopies with ultra-low heat conversion. Nat. Commun. 2015, 6, 7915. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Wu, Z.; Xia, J.; Wu, J.; Tong, G.; Zhang, H. Large-area all-dielectric metasurface fabricated by an anodized aluminum oxide template. Opt. Express 2021, 29, 10465–10470. [Google Scholar] [CrossRef]
- Guo, L.; Shi, Y.; Liu, X.; Han, Z.; Zhao, Z.; Chen, Y.; Xie, W.; Li, X. Enhanced fluorescence detection of proteins using ZnO nanowires integrated inside microfluidic chips. Biosens. Bioelectron. 2018, 99, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Robbiano, V.; Paternò, G.M.; Carnicella, G.; Debrassi, A.; La Mattina, A.A.; Mariani, S.; Minotto, A.; Egri, G.; Dähne, L.; et al. Nanoscale photoluminescence manipulation in monolithic porous silicon oxide microcavity coated with rhodamine labeled polyelectrolyte via electrostatic nanoassembling. Adv. Opt. Mater. 2021, 9, 2100036. [Google Scholar] [CrossRef]
- Hanatani, K.; Yoshihara, K.; Sakamoto, M.; Saitow, K. Nanogap-rich TiO2 film for 2000-fold field enhancement with high reproducibility. J. Phys. Chem. Lett. 2020, 11, 8799–8809. [Google Scholar] [CrossRef] [PubMed]
- Bhagyaraj, S.; Oluwafemi, O.S.; Krupa, I. Chapter 13–Polymers in optics. In Polymer Science and Innovative Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 423–455. [Google Scholar]
- Mínguez-García, D.; Montava, I.; Bonet-Aracil, M.; Gisbert-Payá, J.; Díaz-García, P. PVA Nanofibers as an Insoluble pH Sensor. Polymers 2023, 15, 4480. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Jiang, H.; Xia, H.; Luo, H.; Xie, K. U-shaped microfiber sensor coated with PVA nanofibers for the simultaneous measurement of humidity and temperature. Sens. Actuators B Chem. 2023, 378, 133203. [Google Scholar] [CrossRef]
- Lv, G.; Huang, D.; Wu, S.; Ren, G.; Yang, W.; Li, T. Random lasing action generation in polymer nanofiber with small diameters. Laser Phys. 2018, 28, 075803. [Google Scholar] [CrossRef]
- Huang, D.; Li, T.; Liu, S.; Yi, T.; Wang, C.; Li, J.; Liu, X.; Xu, M. Random lasing action from electrospun nanofibers doped with laser dyes. Laser Phys. 2017, 27, 035802. [Google Scholar] [CrossRef]
- Matino, F.; Persano, L.; Camposeo, A.; Pisignano, D. Laser systems and networks with organic nanowires and nanofibers. Adv. Opt. Mater. 2019, 7, 1900192. [Google Scholar] [CrossRef]
- Ao, X.; Wang, D.; Odom, T.W. Enhanced fields in mirror-backed low-index dielectric structures. ACS Photonics 2019, 6, 2612–2617. [Google Scholar] [CrossRef]
- Burresi, M.; Cortese, L.; Pattelli, L.; Kolle, M.; Vukusic, P.; Wiersma, D.S.; Steiner, U.; Vignolini, S. Bright-white beetle scales optimise multiple scattering of light. Sci. Rep. 2014, 4, 6075. [Google Scholar]
- Li, W.; Tong, Z.; Xiao, K.; Liu, Z.; Gao, Q.; Sun, J.; Liu, S.; Han, S.; Wang, Z. Single-frame wide-field nanoscopy based on ghost imaging via sparsity constraints. Optica 2019, 6, 1515–1523. [Google Scholar] [CrossRef]
- Smith, D.Y.; Shiles, E.; Inokuti, M. The optical properties of metallic aluminum. Handb. Opt. Constants Solids 1997, 1, 369–406. [Google Scholar]
- Ray, K.; Chowdhury, M.H.; Lakowicz, J.R. Aluminum nanostructured films as substrates for enhanced fluorescence in the ultraviolet-blue spectral region. Anal. Chem. 2007, 79, 6480–6487. [Google Scholar] [CrossRef] [PubMed]
- Fort, E.; Grésillon, S. Surface enhanced fluorescence. J. Phys. D Appl. Phys. 2007, 41, 013001. [Google Scholar] [CrossRef]
- Alhalab, H.; Zaraket, H.; Principe, M. Enhanced photoluminescence with dielectric nanostructures: A review. Results Opt. 2021, 3, 100073. [Google Scholar] [CrossRef]
- Drozdowicz-Tomsia, K.; Xie, F.; Goldys, E.M. Deposition of silver dentritic nanostructures on silicon for enhanced fluorescence. J. Phys. Chem. C 2010, 114, 1562–1569. [Google Scholar] [CrossRef]
- Würth, C.; González, M.G.; Niessner, R.; Panne, U.; Haisch, C.; Genger, U.R. Determination of the absolute fluorescence quantum yield of rhodamine 6G with optical and photoacoustic methods–providing the basis for fluorescence quantum yield standards. Talanta 2012, 90, 30–37. [Google Scholar] [CrossRef]
- Wiersma, D.S.; Albada, M.P.; Tiggelen, B.A.; Lagendijk, A. Experimental evidence for recurrent multiple scattering events of light in disordered media. Phys. Rev. Lett. 1995, 74, 4193–4196. [Google Scholar] [CrossRef]
- Krämmer, S.; Vannahme, C.; Smith, C.L.C.; Grossmann, T.; Jenne, M.; Schierle, S.; Jørgensen, L.; Chronakis, I.S.; Kristensen, I.; Kalt, H. Random-cavity lasing from electrospun polymer fiber networks. Adv. Mater. 2014, 26, 8096–8100. [Google Scholar] [CrossRef]
- Zimnyakov, D.A.; Volchkov, S.S.; Kochkurov, L.A.; Kochubey, V.I.; Melnikov, A.G.; Melnikov, G.V. Speckle patterning of a pumping laser light as a limiting factor for stimulated fluorescence emission in dense random media. Opt. Express 2021, 29, 2309–2331. [Google Scholar] [CrossRef] [PubMed]
- Durian, D.J. Influence of boundary reflection and refraction on diffusive photon transport. Phys. Rev. E 1994, 50, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Gottardo, S.; Sapienza, R.; García, P.; Blanco, A.; Wiersma, D.S.; López, C. Resonance-driven random lasing. Nat. Photonics 2008, 2, 429–432. [Google Scholar] [CrossRef]
- Waszczuk, L.; Ogien, J.; Pain, F.; Dubois, A. Determination of scattering coefficient and scattering anisotropy factor of tissue-mimicking phantoms using line-field confocal optical coherence tomography (LC-OCT). J. Eur. Opt. Soc.-Rapid Publ. 2023, 19, 39. [Google Scholar] [CrossRef]
- Zhang, W.; Cue, A.; Yoo, K.M. Emission linewidth of laser action in random gain media. Opt. Lett. 1995, 20, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, Q.; Miao, B.; Fu, Q.; Zou, G.; Chen, Y.; Luo, Y.; Zhang, D.; Wang, P.; Ming, H.; et al. Coherent random fiber laser based on nanoparticles scattering in the extremely weakly scattering regime. Phys. Rev. Lett. 2012, 109, 253901. [Google Scholar] [CrossRef]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29, S49–S52. [Google Scholar] [PubMed]
- Altug, H.; Oh, S.H.; Maier, S.A.; Homola, J. Advances and applications of nanophotonic Biosensors. Nat. Nanotechnol. 2022, 17, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Kinkhabwala, A.; Yu, Z.; Fan, S.; Avlasevich, Y.; Müllen, K.; Moerner, W.E. Large single-molecule fluorescence enhancements produced by a bowtie nanoantenna. Nat. Photon. 2009, 3, 654–657. [Google Scholar] [CrossRef]
- Lu, X.; Ye, G.; Punj, D.; Chiechi, R.C.; Orrit, M. Quantum yield limits for the detection of single-molecule fluorescence enhancement by a gold nanorod. ACS Photonics 2020, 7, 2498–2505. [Google Scholar] [CrossRef]
- Zhu, Z.; Yuan, P.; Li, S.; Garai, M.; Hong, M.; Xu, Q. Plasmon-enhanced fluorescence in coupled nanostructures and applications in dna detection. ACS Appl. Bio Mater. 2018, 1, 118–124. [Google Scholar] [CrossRef]
- Gartia, M.R.; Eichorst, J.P.; Clegg, R.M.; Liu, G.L. Lifetime imaging of radiative and non-radiative fluorescence decays on nanoplasmonic surface. Appl. Phys. Lett. 2012, 101, 23118–23122. [Google Scholar] [CrossRef]
- Trofymchuk, K.; Kołątaj, K.; Glembockyte, V.; Zhu, F.; Acuna, G.P.; Liedl, T.; Tinnefeld, P. Gold nanorod dna origami antennas for 3 orders of magnitude fluorescence enhancement in NIR. ACS Nano 2023, 17, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, M.; Hironaka, T.; Ikeda, N.; Sugasawa, T.; Takekoshi, K. Metasurface biosensors enabling single-molecule sensing of cell free DNA. Nano Lett. 2023, 23, 5755–5761. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Fujii, M. Colloidal dispersion of subquarter micrometer silicon spheres for low-loss antenna in visible regime. Adv. Opt. Mater. 2017, 5, 1700332. [Google Scholar] [CrossRef]
- Romano, S.; Zito, G.; Manago, S.; Calafiore, G.; Cabrini, E.P.S.; Luca, A.C.D.; Mocella, V. Surface-enhanced raman and fluorescence spectroscopy with an all-dielectric metasurface. J. Phys. Chem. C 2018, 122, 19738–19745. [Google Scholar] [CrossRef]
- Hu, Q.; Iwanaga, M.; Tang, Y. Metasurface platform incorporating aggregation induced emission based biosensor for enhanced human serum albumin detection. Adv. Opt. Mater. 2024, 12, 2400868. [Google Scholar] [CrossRef]
- Iwanaga, M. All-dielectric metasurface fluorescence biosensors for high-sensitivity antibody/antigen detection. ACS Nano 2020, 14, 17458–17467. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Sakamoto, M.; Tamamitsu, H.; Arakawa, M.; Saitow, K. Extraordinary field enhancement of TiO2 porous layer up to 500-fold. Adv. Opt. Mater. 2018, 6, 1800462. [Google Scholar] [CrossRef]
- Sang, C.; Chou, S.; Pan, F.M.; Sheu, J. Fluorescence enhancement and multiple protein detection in ZnO nanostructure microfluidic devices. Biosens. Bioelectron. 2016, 75, 285–292. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Huang, T.; Wang, Z. Multiple Scattering-Enhanced Fluorescence Within Randomly Oriented Low-Index Polymer Nanofiber Sensors. Biosensors 2025, 15, 97. https://doi.org/10.3390/bios15020097
Sun J, Huang T, Wang Z. Multiple Scattering-Enhanced Fluorescence Within Randomly Oriented Low-Index Polymer Nanofiber Sensors. Biosensors. 2025; 15(2):97. https://doi.org/10.3390/bios15020097
Chicago/Turabian StyleSun, Jing, Tao Huang, and Zhongyang Wang. 2025. "Multiple Scattering-Enhanced Fluorescence Within Randomly Oriented Low-Index Polymer Nanofiber Sensors" Biosensors 15, no. 2: 97. https://doi.org/10.3390/bios15020097
APA StyleSun, J., Huang, T., & Wang, Z. (2025). Multiple Scattering-Enhanced Fluorescence Within Randomly Oriented Low-Index Polymer Nanofiber Sensors. Biosensors, 15(2), 97. https://doi.org/10.3390/bios15020097




