Abstract
This review explores the development, technological foundations, and applications of biosensor technologies across various fields, such as medicine for disease diagnosis and monitoring, and the food industry. However, the primary focus is on their use in detecting contaminants and pathogens, as well as in environmental monitoring for water quality assessment. The review classifies different types of biosensors based on their bioreceptor and transducer, highlighting how they are specifically designed for the detection of emerging contaminants (ECs) and pathogens in water. Key innovations in this technology are critically examined, including advanced techniques such as systematic evolution of ligands by exponential enrichment (SELEX), molecularly imprinted polymers (MIPs), and self-assembled monolayers (SAMs), which enable the fabrication of sensors with improved sensitivity and selectivity. Additionally, the integration of microfluidic systems into biosensors is analyzed, demonstrating significant enhancements in performance and detection speed. Through these advancements, this work emphasizes the fundamental role of biosensors as key tools for safeguarding public health and preserving environmental integrity.
1. Introduction
The global water crisis is critical, as climate change is causing severe meteorological alterations such as prolonged droughts and intense torrential rains. These events lead to a significant reduction in available water resources and a deterioration in their quality. The situation is further exacerbated by the increasing water consumption due to population growth, which has dramatically accelerated water scarcity [1]. Additionally, the limited water available is being contaminated by various chemical compounds, many of which are toxic and harmful to both human health and ecosystems.
Among these contaminants, emerging contaminants (ECs) stand out. ECs refer to synthetic and natural chemical agents, as well as biological agents, whose presence in the environment is not often monitored, but which have the potential to affect ecosystems and public health [2]. These contaminants often lack regulatory standards, and their environmental behavior and toxicological profiles are not fully understood [3]. ECs encompass a wide range of pollutants, including pharmaceuticals, micro- or nanoplastics, engineered nanoparticles, perfluoroalkylated and polyfluoroalkylated substances, pesticides, and industrial chemicals. These substances often resist natural degradation processes and require long-range transport, bioaccumulation, and complex ecosystem interactions, which complicates risk assessment [4].
The importance of studying ECs lies in their intensive use in various fields, such as pharmaceuticals, personal care products, agricultural pesticides, and industrial additives [2,5]. Furthermore, urban and industrial wastewater treatment plants are not efficient enough to eliminate these compounds, resulting in their reintroduction into the environment, where they persist and may accumulate [6]. A study by Fatta-Kassinos et al. [7] highlights that these compounds can persist in the environment and are being reintroduced into the human food chain through drinking water. The release routes of EC to the environment and eventual effects are disclosed in Figure 1. In traditional wastewater treatment plants, pharmaceuticals, disinfectants, and biological contaminants such as antibiotic-resistant bacteria and viruses are not effectively removed. As a result, these pollutants remain untreated, allowing them to enter the environment, where they can accumulate, spread, and pose significant risks to human health and ecosystems [8].
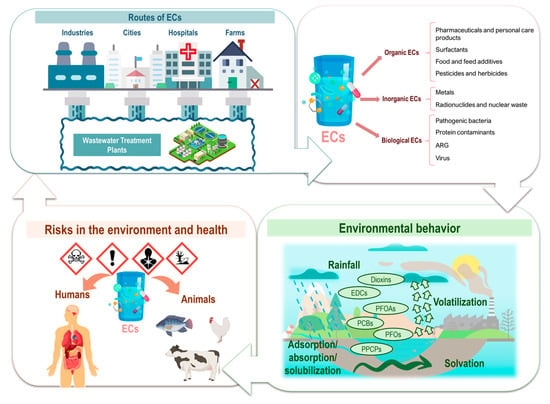
Figure 1.
Release routes of emerging pollutants to the environment and eventual effects. EDCs: endocrine-disrupting chemicals; PFOAs: perfluooctanoic acids; PFOs: perfluorooctane sulfonates; PCBs: polychlorinated biphenyls; PPCPs: pharmaceuticals and personal care products.
The presence of pharmaceutical contaminants in ecosystems not only disrupts aquatic life, but also fosters the development of antibiotic-resistant bacteria (ARB) and antibiotic resistance genes (ARGs) [9,10]. This situation arises because microorganisms exposed to sub-lethal concentrations of antibiotics in the environment can develop resistance mechanisms. Consequently, ARB and ARGs can spread through water systems, potentially reaching human populations and reducing the effectiveness of standard antibiotic treatments. This exacerbates the global health crisis of antimicrobial resistance, leading to higher medical costs, prolonged hospital stays, and increased mortality. The emergence of ARGs and ARBs is recognized as one of the most critical threats to global health. These genetic elements can spread through microbial communities in aquatic systems, which are amplified by anthropogenic activities such as agriculture and aquaculture, where antibiotics are used extensively. This creates reservoirs of resistance that can be reintroduced into human systems through food and water, posing significant public health risks [11,12].
Since the COVID-19 pandemic, interest in monitoring microorganisms and viral RNA in wastewater has increased significantly. This focus, combined with concerns about persistent contaminants such as pharmaceuticals, highlights the importance of monitoring wastewater generated in hospitals. Depending on the region of the world, the volume of wastewater per patient can vary significantly, ranging from approximately 156 L/patient/day in India to 2258 L/patient/day in Germany [13]. This high volume of wastewater generation makes its monitoring essential to understand the associated risks, such as the spread of antibiotic-resistant microorganisms, the presence of viruses and pathogenic bacteria, and the environmental and health impacts of these contaminants. In addition, pathogens such as cyanobacteria, which produce harmful cyanotoxins, are becoming increasingly relevant. Eutrophication and climate change have intensified harmful algal blooms, resulting in toxins that bioaccumulate in food webs and pose serious risks to aquatic organisms, humans, and animals [14,15].
In this context, the development of new analytical techniques that can quickly and effectively quantify these contaminants in various water resources is essential. While numerous analytical techniques exist, biosensors have seen a significant surge in development in recent years, emerging as a promising alternatives for contamination and pathogen monitoring. Traditionally recognized for their applications in the biomedical field, biosensors are now being increasingly adapted and optimized to detect pollutants in air, soil, and water [16]. They offer the potential for sensitive, rapid, and cost-effective detection of ECs and pathogens in wastewater and natural water bodies. Emerging techniques such as high-resolution mass spectrometry and advanced biosensors have greatly improved contaminant identification. In addition, bioanalytical tools such as aptamer-based biosensors and molecular diagnostics offer increased sensitivity for the real-time monitoring of chemical and biological contaminants. These advances are crucial to addressing the persistent and evolving challenges posed by emerging contaminants [17,18,19].
For this reason, this review aims to provide a comprehensive and accessible overview of biosensors, making their basic principles, significance, and applications understandable to a wide audience. It highlights their growing role in several aspects including contaminants quantification, as ECs and pathogens in water, while also showcasing recent technological advancements and how they have improved existing detection methods. Additionally, the review explores emerging trends, current challenges, and the future perspectives of biosensors, addressing key innovations and potential solutions that will shape their development in the coming years.
1.1. The Role of Biosensors
Traditional methods for detecting ECs and pathogens in wastewater, such as chromatography and culture-based techniques, are often time-consuming, labor-intensive, and require sophisticated laboratory equipment. For this reason, in recent years, there has been a growing interest in the application of biosensors for the quantification of these environmental contaminants [20]. Unlike the traditional analytical methods mentioned above, biosensors offer several advantages, including the following:
- Rapid detection: Biosensors deliver real-time or near-instant results, facilitating faster decision making. They serve as a cost-effective and efficient alternatives to traditionally slow and labor-intensive methods for food and water pathogen detection, making them ideal for large-scale monitoring applications [21].
- High sensitivity and specificity: The incorporation of specific biorecognition elements to biosensors ensure high precision and enhanced sensitivity in the detection of target analytes. For instance, aptamer-based biosensors have proved remarkably efficiency in the identification of pharmaceuticals, heavy metals, endocrine-disrupting chemicals, and agricultural contaminants in environmental samples [22].
- On-site monitoring: The portability of biosensors allows on-site monitoring, which reduces the need to transport samples and allows immediate analysis. For example, enzyme-based electrochemical biosensors have been developed for microfluidic applications, facilitating the in situ detection of contaminants [21].
Based on these benefits, biosensors have become powerful tools for monitoring the presence in wastewater of contaminants and pathogens, contributing substantially to safeguard human health and the environment [23]. Therefore, understanding their nature, structure and function is essential to appreciating their impact. Essentially, they are devices that integrate a biological recognition element with a transducer that converts the resulting physicochemical reaction into a measurable signal [24,25]. These devices usually comprise a bioreceptor, a transducer, and a signal processor as main components (Figure 2) [26].
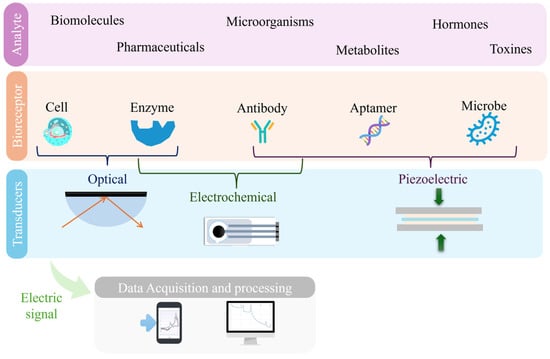
Figure 2.
Simplified schematic illustrating the key components of a biosensor.
The bioreceptor, a crucial component, is typically composed of a layer of macromolecules as antibodies, nucleic acid probes, or viral proteins, specifically designed to bind to the target analyte [27]. This interaction generates a signal, which is then captured by the transducer and converted into an electrical response [20,24,25]. The processed signal is subsequently analyzed and displayed as a quantifiable digital output, allowing for the accurate and real-time detection of contaminants.
To enhance the interaction between the analyte and the bioreceptor, additional modifications are often required. One notable example is the integration of nanomaterials, which significantly improves biosensor performance by increasing sensitivity [28,29]. Nanomaterials, due to their high surface area and unique physicochemical properties, enhance the interaction between biosensors and target pathogens, leading to greater sensitivity and selectivity in detection [30]. The incorporation of nanomaterials also enables biosensors to achieve faster response times, which is critical for the timely detection of pathogens in water samples [31].
The necessity of rapid contaminant detection is evidenced in cases where timely identification has enabled effective interventions. Wu et al. developed a whole-cell biosensor for detecting Pseudomonas aeruginosa and Burkholderia pseudomallei, two key waterborne pathogens [32]. Using a QscR quorum sensing system in an E. coli host, this biosensor expresses an enhanced green fluorescent protein upon detecting quorum sensing molecules, facilitating cost-effective and rapid detection. Unlike traditional methods, which require hours or days, this biosensor provides results within minutes, allowing for swift responses to contamination events [33].
Similarly, the COVID-19 pandemic highlighted the urgent need for rapid and accessible diagnostic tools. While reverse transcription polymerase chain reaction (RT-PCR) remains the gold standard, it requires specialized equipment, trained personnel, and long processing times, limiting its practicality for large-scale screening. In contrast, biosensors have emerged as fast, sensitive, and on-site alternatives [34]. Technologies such as aptamer-based biosensors, which utilize single-stranded DNA or RNA molecules to selectively bind to SARS-CoV-2 proteins, have demonstrated high specificity and efficiency, offering a reliable mechanism for real-time virus detection [4,35].
For example, electrochemical biosensors have been developed to detect SARS-CoV-2, the virus responsible for COVID-19. These biosensors provide benefits such as speed, accuracy, portability, and real-time results. They can detect viral components, such as proteins or nucleic acids, directly from patient samples, significantly reducing the time required for diagnosis [36]. A notable innovation is the use of a DNA biosensor leveraging Au@Pt/Au core@shell nanoparticles, as described by Martínez-Periñán et al. [37]. These nanoparticles serve as dual-function components, combining excellent electrocatalytic activity for the oxygen reduction reaction with their ability to bioconjugate with thiolated DNA probes. The resulting biosensor demonstrated a low detection limit of 32 pM for SARS-CoV-2 RNA and facilitated the direct analysis of patient samples without requiring amplification processes. This approach provided a robust and highly sensitive platform for detecting viral RNA, yielding results comparable to RT-qPCR but with a significantly reduced processing time. Such advancements exemplify the growing potential of biosensors to deliver rapid, reliable, and scalable diagnostic solutions in public health crises.
Nanobiosensors incorporating nanomaterials have significantly enhanced the sensitivity and specificity of COVID-19 detection, enabling early diagnosis and timely intervention. The integration of nanotechnology has led to the development of fast, accurate, and cost-effective point-of-care devices, particularly beneficial in resource-limited settings [38]. For instance, carbon-based nanomaterials like graphene improve electrical conductivity and signal amplification, achieving unprecedented sensitivity in detecting RNA or viral antigens [18,39].
The application of biosensors in the diagnosis of COVID-19 exemplifies their potential to revolutionize pathogen detection, providing a means for rapid and reliable diagnosis that is crucial for controlling infectious disease outbreaks and safeguarding public health [40]. These advances not only highlight the versatility of biosensors, but also underscore their role in real-time epidemiological surveillance, enabling proactive measures to be taken during pandemics and reducing the burden on healthcare systems [41,42]
1.2. Bioreceptor Immobilization: The Critical Step in Biosensor Development
The immobilization of the bioreceptor onto the transducer is one of the most critical steps in biosensor development, as it directly influences the sensor’s sensitivity, specificity, and overall performance. Effective immobilization is essential to ensure that the bioreceptor retains its biological activity while achieving a stable and robust attachment to the transducer surface. The main challenge lies in maintaining a delicate balance between achieving strong immobilization and preserving the native functionality of the bioreceptor, such as enzyme activity, antigen–antibody recognition, and nucleic acid hybridization. Some of the most commonly employed immobilization techniques include adsorption, covalent bonding, cross-linking, and entrapment [43,44,45,46].
The first technique, adsorption, relies on the physical adsorption of the bioreceptor onto the surface of the transducer via van der Waals forces, hydrogen bonding, or electrostatic interactions. This method is simple and cost-effective; however, it may lead to disruption or the loss of activity under changing environmental conditions. Another widely used technique involves covalent bonding and its related method, cross-linking. Both are based on covalent interactions, but the key difference lies in how the interaction occurs. Covalent bonding directly links the bioreceptor to the transducer through functional groups such as amines, carboxyls, or thiols, while cross-linking uses bifunctional reagents to form a stable network between the bioreceptor and the transducer.
Another important immobilization technique is entrapment and microencapsulation, where the bioreceptor is physically enclosed within a polymeric matrix or membrane. This method ensures that the bioreceptor remains near the transducer, providing a protective environment. However, it may limit substrate accessibility and diffusion, potentially reducing the overall efficiency of the biosensor. In the case of covalent bonding, the bioreceptor is directly attached to the transducer through specific functional groups, such as amines, carboxyls, and thiols. This method ensures strong and stable immobilization, minimizing the risk of bioreceptor leakage. However, precise control is required during the process to prevent potential damage that could compromise the biological activity of the bioreceptor.
On the other hand, cross-linking employs bifunctional reagents that act as intermediaries to facilitate the formation of covalent bonds between the bioreceptor and the transducer. These agents are essential because without them, the bond between these two elements would not occur. While this technique provides excellent stability, improper cross-linking can reduce the biological activity of the bioreceptor, thereby affecting the overall performance of the biosensor.
The selection of an immobilization method relies on the bioreceptor type, transducer material, and biosensor application. Each one presents advantages and drawbacks, requiring optimization to ensure maximum performance and reliability. The enhancement of the immobilization strategies improves the biosensor analytical capabilities for their use in environmental monitoring, medical diagnostics, and industrial applications.
1.3. Types of Biosensors
As discussed in Section 1.1, biosensors are sophisticated analytical devices that integrate a biological recognition element with a physicochemical transducer to detect specific analytes (Figure 2).
The biological recognition component, often referred to as a bioreceptor, may include entities such as enzymes, antibodies, aptamers, or even tissues and whole-cells, each selected for its specific interaction with the target analyte. In some cases, bioreceptors can be derived from microbes, which encompass bacteria, viruses, protozoa, fungi, and archaea, due to their ability to bind selectively to specific molecules or structures. After interacting with the analyte, the bioreceptor undergoes a biochemical change, which the transducer converts into a measurable signal, either electrical, optical, or mechanical [47,48]. This conversion facilitates the quantification or qualitative evaluation of the presence of the analyte [49]. The design and performance of biosensors are heavily determined by the characteristics of the bioreceptor and the transduction and immobilization techniques [27]. Consequently, biosensors are commonly classified based on two fundamental components: the type of biorecognition element and the detection technique employed. The detection techniques, which include electrical/electrochemical, optical, and piezoelectric methods, are implemented through various transducers such as electrodes, optical fibers, and oscillators. This classification framework not only aids in understanding the operating principles of different biosensors but also guides their application in diverse fields, including medical diagnostics, environmental monitoring, and food safety [50].
2. Biorecognition Elements
The biorecognition element is a fundamental component that directly influences the specificity, sensitivity, and functionality of the biosensor [51]. Selecting the right biorecognition element is crucial to ensure optimal performance in various applications, including healthcare, environmental monitoring, and food safety. The main types of biosensors categorized by their biorecognition elements include enzymes, antibodies, DNA, whole cells, aptamers, antimicrobial peptides, and artificial binding proteins [51,52].
2.1. Enzyme-Based Biosensors
They use enzymes to catalyze specific biochemical reactions with the target analyte, generating a measurable signal [53]. Many enzymes can interact with a wide range of substrates. The selectivity of enzyme-based biosensors varies and is influenced by factors such as the sensor design, the detection technique, and key elements such as the immobilization matrix, the operating conditions, and the specific enzyme used [54].
These biosensors are characterized by several properties such as (i) high specificity for its substrates, which allows the accurate detection of analytes such as glucose and urea; (ii) wide applicability in clinical diagnosis; and (iii) extended utility in environmental monitoring, detecting toxins such as organophosphates and heavy metals [55]. Thus, enzymes such as laccase and tyrosinase are used to detect phenolic compounds in wastewater [56,57] or radical species such as superoxide, as illustrated in Figure 3 below.
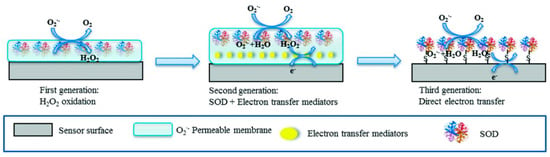
Figure 3.
Principle of detection and the generational evolution of superoxide biosensors utilizing the superoxide dismutase enzyme. The figure illustrates the progression from first-generation biosensors, relying on direct enzyme–substrate interactions, to second- and third-generation biosensors, which incorporate advanced materials and electron transfer strategies to enhance sensitivity, specificity, and stability. Reprinted with permission from Liu et al. [42]. Copyright 2015 American Chemical Society.
Enzyme-based biosensors have always represented a promising technology for environmental monitoring, particularly in the assessment of water quality and the detection of pollutants [58]. The functionality of these biosensors is based on the principle of enzymatic inhibition, which involves enzymes such as urease, horseradish peroxidase, tyrosinase, and cholinesterase [59]. Certain pesticides, such as organophosphates and carbamates, inhibit enzymes like acetylcholine esterase by binding to their active sites, thereby preventing the hydrolysis of acetylcholine into acetate and choline [60]. This enzymatic inhibition has been widely utilized as a reliable mechanism for detecting these toxic substances in environmental samples. Early works in this field include the study by La Rosa et al. [61], who demonstrated an acetylcholinesterase-based amperometric biosensor using 4-aminophenyl acetate as a substrate. This approach achieved the highly sensitive detection of organophosphorus and carbamate pesticides by monitoring the inhibition of enzymatic activity. Similarly, Abad et al. [62] developed a piezoelectric biosensor for pesticide detection, which relied on frequency changes induced by enzymatic reactions on immobilized acetylcholine esterase. The system achieved remarkable detection limits, as low as 5.0 × 10⁻2 µM for paroxon.
The integration of these enzyme systems with electrochemical and piezoelectric transducers offers several advantages, including rapid, in situ analysis with minimal sample preparation, representing a significant improvement over traditional laboratory-based methods [63]. By combining the specificity of enzyme inhibition with the sensitivity of modern transducers, these biosensors provide a promising tool for environmental monitoring of pesticide residues.
Recent advancements in biosensor technology have led to the development of more innovative and practical solutions for organophosphate detection. For instance, Mool-am-kha et al. [64] introduced a fluorescence-based biosensor to detect organophosphates, such as malathion, by leveraging the inhibition of alkaline phosphatase. The biosensor converts fluorescence signals into RGB values using a portable device connected to a smartphone, achieving a detection range of 0.30–3.03 µM with a limit of detection as low as 0.15 µM. This system demonstrated high precision in vegetable sample analysis, offering a cost-effective and portable solution for the real-time monitoring of pesticide residues. Similarly, Miglione et al. [65] developed a portable electrochemical biosensor integrated into gloves for the detection of organophosphates, such as dichlorvos, directly on fruit peels [58]. This innovative design employs butyrylcholinesterase as the bioreceptor, whose activity is inhibited by organophosphates, achieving a detection limit in the nanomolar range with excellent repeatability. The glove-integrated sensor offers a practical and efficient tool for on-site monitoring, particularly in precision agriculture.
For the detection of herbicides such as triazines and phenylureas, which disrupt the photosynthetic process, biosensors have been developed that incorporate chloroplast or thylakoid membrane receptors, as well as unicellular algae [66,67]. These biological components enable direct interaction with herbicides, facilitating sensitive detection. Additionally, enzymes such as urease, organophosphorus hydrolase, and cholinesterase continue to play a critical role in the design of electrochemical biosensors for pesticide detection [68].
To detect heavy metals, enzyme-based biosensors have proven effective using the inhibitory effects of metals, such as copper, lead, and cadmium, on enzymatic activity to form the basis for their detection [69]. Additionally, enzymes such as laccase and tyrosinase facilitate the oxidation and detection of phenolic compounds [69]. This approach not only demonstrates the versatility of enzyme-based biosensors for detecting specific analytes but also underscores their usefulness for monitoring toxic substances such as mercury in environmental samples [70]. Yang et al. [71] developed a potentiometric biosensor for mercury detection, utilizing the inhibition of the urease enzyme as a detection mechanism. The biosensor demonstrated a linear detection range of 0.09–1.99 µM and achieved an impressive detection limit of 0.05 µM. This biosensor works based on the enzymatic hydrolysis of urea, which produces ammonia and carbon dioxide, causing an increase in the pH of the solution. In this case, it is necessary to use a pH-sensitive polyvinyl chloride (PVC) membrane electrode to monitor changes in the concentration of hydrogen ions. As the urea concentration increases, the potential response of the biosensor changes, accordingly, allowing the quantification of urea levels [72].
Advances in nanotechnology have further enhanced the capabilities of enzyme-based biosensors. The incorporation of nanoparticles with enzymes not only significantly improves their stability but also increases the active electrode surface area, thereby enhancing their sensitivity [73]. Nanostructured materials, such as metal nanoparticles and metal oxides, have been effectively combined with immobilized enzymes to detect contaminants such as nitrates and pesticides in water samples. These hybrid systems achieve high accuracy and low detection limits, underscoring their potential for fast and reliable environmental monitoring [59].
Despite these advances, significant challenges remain. Enzyme stability in complex matrices, such as those with varying pH or ionic strength, can compromise sensor performance. In addition, the interference of non-target compounds in environmental samples can reduce the detection accuracy [74]. Addressing these limitations requires ongoing research in enzyme immobilization techniques (Section 1.2), the use of extremozymes to improve durability, and the integration of biosensors with advanced technologies.
Extremozymes, derived from thermophilic organisms, offer unique advantages to overcome these challenges due to their exceptional stability under adverse conditions. A series of specific structural characteristics of thermostable enzymes prevents denaturation at high temperatures and maintains enzymatic activity [75]. These adaptations include an increased number of salt bridges and hydrogen bonds between side chains, improved hydrophobic interactions, and a more rigid overall structure. In addition, thermophilic proteins typically exhibit a higher content of Arg and Tyr residues and fewer thermolabile residues, such as Asn and Gln, compared to their mesophilic counterparts.
Beyond thermal stability, extremozymes resist proteolysis and demonstrate high chemical and pH stability, which makes them ideal candidates for applications in biosensors designed for environmental monitoring. Recent developments have shown that thermophilic microorganisms, such as bacteria and fungi, are a significant source of novel catalysts of industrial interest. These extremozymes can replace mesophilic enzymes in applications that require high-temperature processes, reducing the risks of microbial contamination, improving the solubility of the substrate and improving reaction rates. In addition, the heterologous overexpression of extremozymes in Escherichia coli (E. coli) facilitates the production of large amounts of enzymes, which are easily purified by heat treatment.
By integrating extremozymes into biosensor designs, researchers can achieve robust performance and reliability, even under challenging environmental conditions. This integration represents a critical step forward in the development of biosensors capable of accurately detecting long-term contaminants in complex environmental matrices.
By leveraging enzyme inhibition principles, advances in nanotechnology and innovative sensor designs, enzyme-based biosensors provide a powerful and adaptable solution for long-term environmental monitoring. Its development continues to bridge the gap between laboratory-based methods and field-ready technologies, ensuring cost-effective and reliable contaminant detection.
2.2. Antibody-Based Biosensors
Also known as immunosensors, antibody-based biosensors take advantage of the high specificity of antigen–antibody interactions to detect pathogens, toxins, and biomarkers. These biosensors are essential tools in medical diagnosis and food safety. Recent advances, such as the development of monoclonal and recombinant antibodies, have significantly improved the reproducibility and specificity of immunosensory assays. In addition, its multiplexing capabilities allow the simultaneous detection of multiple antigens, which is particularly valuable in complex diagnostic scenarios, including the analysis of cancer biomarkers and the monitoring of multiple pathogens. These characteristics underline the fundamental role of immunosensors in the advancement of precision medicine and the guarantee of public health safety [76].
Most biosensors of this type commonly use self-assembled monolayers (SAMs) to enhance the immobilization of antibodies. Essentially, a SAM is an organized molecular film that spontaneously forms on the surface of a solid material, typically a metal such as gold but can be other material as fluorine-doped tin oxide thin film [77]. Its main advantage lies in providing controlled functional surfaces, which improve the immobilization of biomolecules (such as antibodies or enzymes) and facilitate biological interactions [78,79].
As schematically shown in Figure 4a, SAMs typically incorporate chemical compounds with an affinity for the material’s surface, forming covalent or semi-coordinated bonds. These compounds contain functional groups such as thiols, amines, acids, disulfides, and silanes [80,81]. For example, in gold electrodes used as working electrodes, thiol-based chemistry is commonly employed. To facilitate the immobilization process, linking molecules such as glutaraldehyde can be used as auxiliaries in this step. Another strategy involves using polymeric matrices with reactive functional groups (amino and hydroxyl), which are suitable for immobilizing biomolecules [82]. As the final step in the synthesis, bovine serum albumin (BSA) is frequently used to block the electrode surface, preventing non-specific interactions [83,84]. This step contributes to improving the selectivity and accuracy of the system.
As illustrated in Figure 4b, antibody-based biosensors (also known as immunosensors) can incorporate nanomaterials to enhance recognition and amplify the obtained signal, as demonstrated by the work of Chang et al. [85]. In that study, the authors developed an ultrasensitive biosensor for the detection of clinical biomarkers, using prostate-specific antigen (PSA) as an example—a biomarker associated with prostate cancer. The immunosensor employs a sandwich-type reaction based on biotinylated antibodies. A primary antibody is immobilized on a gold electrode modified with SAM, while a secondary antibody functionalized with a metal–organic framework (MOF) loaded with pyrroloquinoline quinone (PQQ) enables signal amplification. PSA detection is achieved through a redox cycling system that uses PQQ as a catalyst to accelerate the oxidation of tris(2-carboxyethyl)phosphine, a chemical reducing agent. This design facilitates the detection of PSA with high sensitivity (LOD: 2.94 × 10−11 µM) and specificity, thanks to the biotin-streptavidin interaction and the use of MOFs, which minimize interference and generate a precise electrochemical signal.
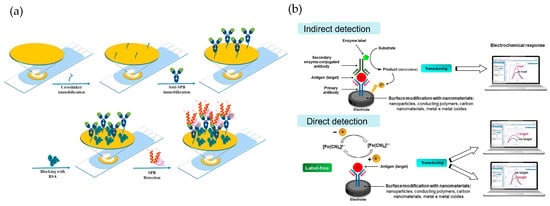
Figure 4.
(a) Schematic representation of the SAM formation process in antibody-based biosensors. This diagram is adapted from the work of Ben Messaoud et al. [86]. Copyright 2023, Elsevier. (b) overview of electrochemical biosensor detection strategies, featuring indirect detection (top) with enzyme-labeled antibodies and direct detection (bottom) via label-free antigen recognition, both enhanced by nanomaterial surface modifications. This diagram is adapted from the work of Ferreira et al. [87], copyright 2023 MDPI (Basel, Switzerland).
On the other hand, a remarkable example of the use of biosensors in environmental applications, specifically for seawater analysis, is the one developed by Ben Messaoud et al. [88]. Their device employs monoclonal antibodies specific to Aeromonas salmonicida, immobilized on a gold electrode through covalent bonding. The design incorporates an integrated electrochemical chip that, using the technique of electrochemical impedance spectroscopy (EIS), enables the ultrasensitive detection of this bacterium. The biosensor can detect the presence of Aeromonas salmonicida at concentrations as low as one colony-forming unit per milliliter (1 CFU/mL) directly in seawater, demonstrating exceptional sensitivity and specificity for applications in aquaculture and environmental monitoring.
2.3. DNA-Based Biosensors
Highly versatile analytical devices that use nucleic acid sequences to detect complementary strands of DNA or RNA, making them indispensable in various genetic and diagnostic applications. Its importance lies in its ability to facilitate mutation detection, pathogen identification, and forensic analysis, addressing critical health and safety needs. Recent advances, such as the integration of CRISPR-Cas systems, have revolutionized these biosensors by allowing the detection of highly precise and specific nucleic acids, even at low concentrations [89]. In addition, DNA biosensors have been proven to be fundamental in decentralized test scenarios, particularly for epidemic monitoring and zoonotic disease detection. Its fast and accurate results significantly reduce response times during outbreaks, offering invaluable support for public health interventions.
However, in the field of water quality monitoring, these biosensors are increasingly being used for the quantification of heavy metals. This is primarily due to their operating principle, which is based on the affinity of certain metal ions for forming stable structures with specific DNA bases [90,91]. For instance, mercury ions (Hg2⁺) can form specific bonds with thymine base pairs, while silver ions (Ag⁺) preferentially interact with cytosine, generating highly stable metal-specific structures under aqueous conditions [92,93]. This behavior enables the development of DNA-based biosensors that leverage this affinity to detect and quantify trace amounts of metals in water with high selectivity and sensitivity. Moreover, the use of DNA as a sensing element not only provides chemical specificity but also offers thermal stability and ease of modification to optimize the biosensor’s performance across different environmental matrices [92].
2.4. Whole-Cell Biosensors
Unique analytical tools that use living cells to detect and quantify analytes, offering remarkable versatility in environmental monitoring, healthcare, and industrial applications. These biosensors take advantage of the natural biochemical and physiological responses of cells, which allows the real-time detection of pollutants and their bioavailability. For example, designed bacterial biosensors that emit luminescence in the presence of heavy metals such as mercury and arsenic provide critical information about water quality and ecosystem health [94]. Advances in synthetic biology have significantly expanded its functionality, allowing the design of cells with genetic circuits that respond to specific emerging pollutants, such as endocrine disruptors, through fluorescent or colorimetric signals [95]. These biosensors also play a vital role in assessing the cumulative effects of pollutant mixtures, offering information on their synergistic impacts on cellular metabolism and DNA integrity. Although challenges such as cell viability and reproducibility persist, continuous innovations in encapsulation, microfluidic, and machine learning techniques promise to improve their stability and analytical accuracy. As tools for comprehensive pollution monitoring and impact assessment, whole-cell biosensors are invaluable for addressing modern environmental challenges and promoting sustainable solutions [96].
Building on this versatility, novel applications of whole-cell biosensors for detecting specific environmental contaminants can be explored. For instance, a transcription factor-based biosensor has been developed by Dey et al. [97] for the detection of Sodium Dodecyl Sulfate (SDS), a widely used anionic detergent known for its harmful effects on aquatic ecosystems. This biosensor employs Pseudomonas aeruginosa PAO1 as the chassis organism, utilizing the sdsB1 activator protein and the SDS-responsive sdsA1 promoter to regulate the expression of green fluorescent protein as a transducer. The system demonstrates a linear response in the range of 1.39 to 216.7 µM SDS, with a detection limit of 0.35 µM, showcasing high specificity and minimal interference from other surfactants or metal ions.
2.5. Aptamer-Based Biosensors
Aptamers are short single-stranded nucleic acid sequences (DNA or RNA) that adopt unique three-dimensional structures, enabling them to selectively bind to a wide variety of targets, such as proteins, small molecules, and even entire cells [98]. Compared to traditional antibody-based biosensors, aptamer-based systems offer significant advantages, including high affinity and specificity, enhanced chemical and thermal stability, and lower production costs. Aptamers can also be chemically modified to improve their resistance to enzymatic degradation, enhancing their performance in complex environmental matrices [99,100]. A clear example of the synthesis methodology for an aptamer-based biosensor is shown in Figure 5a, where a biosensor construction process for Legionella pneumophila detection, using thiolated aptamers immobilized on a gold electrode is represented [101]. It consisted of the immobilization of a thiolated aptamer on a gold screen-printed electrode by self-assembly, followed by blocking the gold-free sites with mercaptohexanol. Detection was based on the specific binding of L. pneumophila to the immobilized aptamer, which generated an impedance in the charge transfer of the redox probe, monitored by square wave voltammetry.
Recent advancements in systematic evolution of ligands by exponential enrichment (SELEX, Figure 5b) technology have further refined aptamer selection processes [99,102]. For example, Zhang et al. [102] introduced a microfluidic-based SELEX system, which enables faster and more efficient aptamer screening for small molecules, such as advanced glycation end products, significantly reducing the time and cost associated with traditional SELEX methods [92]. These platforms streamline the SELEX process while allowing the real-time monitoring of enrichment rates, ensuring the development of high-quality aptamer candidates for biosensor applications.
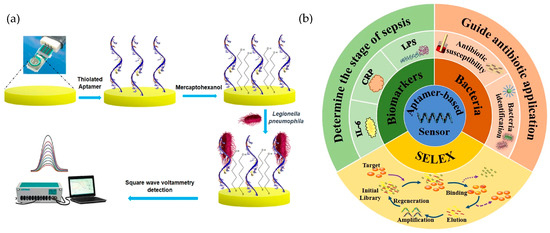
Figure 5.
(a) Illustration of a biosensor construction process for Legionella pneumophila detection, using thiolated aptamers immobilized on a gold electrode. Adapted with permission from Shaukat et al. [101], copyright 2024; Springer Nature. (b) Schematic representation of the SELEX process and its applications in aptamer-based biosensors for sepsis detection, targeting biomarkers (CRP, IL-6, LPS) and bacteria to guide antibiotic use. Reproduced with permission from Lui et al. [103], copyright 2021; Springer Nature.
A study by Zandieh et al. [104] highlights the selection of specific DNA aptamers for detecting microplastics, particularly PVC and polystyrene (PS). Using the SELEX method, the authors isolated DNA sequences enriched in pyrimidines, predominantly cytosine (50%) and thymine (37%), with minimal purine content. The most effective sequence, named PVC1, exhibited an exceptional binding affinity to PVC, demonstrating a six-times-higher efficiency compared to a random DNA sequence of the same length. The recognition mechanism relies primarily on van der Waals forces, enhanced by the aptamer’s flexible structure, which optimizes surface contact with the plastic. The aptamer PVC1 achieved selective detection of PVC and PS with detection limits of 8.0 µmol and 5.76 µmol, respectively. Using a fluorescence-based assay, the researchers successfully distinguished microplastics even in complex samples, such as wastewater, without interference from other materials like silica. This work offers a sensitive and robust method for environmental monitoring, providing a reliable solution for detecting and quantifying microplastics in real-world scenarios.
In the field of pesticide detection, Feng et al. [105] developed a fluorescence-based aptasensor for detecting acetamiprid, a neonicotinoid pesticide. The biosensor uses oxidized carbon nanohorns as the signal amplification platform and an aptamer as the biorecognition element. The system achieved an impressive detection limit as low as 2.84 × 10−8 µM and demonstrated high selectivity, with no significant interference from other pesticides. The signal amplification was enhanced with cryonase enzymes, further improving the sensitivity of the detection system. Similarly, Thai Duong et al. [106] introduced a label-free biosensor based on liquid crystals for detecting acetamiprid. In their system, the binding of the pesticide to its specific aptamer induces changes in the orientation of the liquid crystals, producing measurable optical signals. The sensor achieved an exceptional detection range of 5 × 10−6 µM to 0.070 µM, with a detection limit as low as 0.69 × 10−6 µM.
2.6. Antimicrobial Peptide (AMP)-Based Biosensors
These sensors utilize natural immune defense molecules to detect bacteria and other pathogens with remarkable efficiency. Their fast-binding kinetics and broad specificity make them ideal for applications that require fast and accurate microbial detection. AMP are commonly integrated into impedimetric biosensors, enabling sensitive, real-time bacterial detection for clinical diagnosis and food safety monitoring. In addition, its relatively simple structure allows for extensive customization, facilitating the design of biosensors adapted to specific applications and target analytes. These attributes position AMP-based biosensors as a valuable tool to address critical challenges in healthcare, food safety, and environmental monitoring [106].
2.7. Biosensors Based on Artificial Binding Proteins
Biosensors utilizing artificial bindings proteins, such as domain antibody-related proteins and non-immunoglobulin scaffold proteins, overcome several limitations of traditional antibodies, offering greater stability and scalability for large-scale applications [107]. These modified proteins are designed for the rapid and precise detection of a wide range of analytes, from small molecules to complex proteins, ensuring high specificity and sensitivity. Their robustness and adaptability have allowed their integration into portable devices, which makes them very suitable for point-of-care diagnostics and field applications. These features underscore the transformative potential of biosensors based on artificial binding proteins to advance healthcare accessibility, environmental monitoring, and real-time analytical solutions [108].
These diverse biorecognition elements highlight the adaptability and accuracy of biosensors in a variety of applications. Leveraging advances in biotechnology and synthetic biology, biosensors are becoming indispensable tools to address critical challenges in the fields of healthcare, environmental monitoring, and food safety. Continued research and innovation promise to expand their capabilities and strengthen their role in solving modern analytical challenges.
3. Transduction Mechanism
Both the bioreceptor and the transducer employed are fundamental components for determining the sensitivity, specificity, and suitability of the biosensor for various applications. The transducer, in particular, is responsible for converting the interaction into a measurable signal. The primary transduction mechanisms include electrochemical, optical, and piezoelectric methods.
Regarding the first type of transducer, it is based on biochemical interactions being transformed into measurable electrical signals. This process relies on detecting changes in electrical properties, such as current, potential, and conductance, resulting from specific chemical reactions between the analyte and the bioreceptor. For this reason, these transducers are typically classified based on the type of electrical signal they measure, and the technique used for detecting biochemical interactions, with the most used types being amperometric, potentiometric, conductometric, and impedimetric transducers.
For optical transducers, this interaction is converted into measurable optical signals based on changes in the properties of light, such as intensity, phase, polarization, and wavelength. Therefore, the most common classification of this type of transducer is based on the optical principle they employ. The most widely used optical transducers in biosensors include those based on surface plasmon resonance (SPR), fluorescence, and absorbance as measurement techniques.
As for the piezoelectric transducer, it is primarily based on the mass change occurring on the surface of the piezoelectric crystal, which oscillates at a specific frequency depending on this mass. Thus, the frequency change is proportional to the amount of analyte present. Among these techniques, the most well-known and widely used transducer is the Quartz Crystal Microbalance (QCM), but surface acoustic wave and bulk acoustic wave devices can also be employed in biosensors.
3.1. Electrochemical Biosensors
Electrochemical biosensors, known for their ability to detect biochemical reactions produced by one or more analytes through discrete or continuous signals, are highly sensitive and versatile, making them ideal for numerous applications (Figure 6a). These devices operate based on different electrochemical principles, such as amperometry, potentiometry, conductometry, and impedance spectroscopy (Figure 6b) [53].
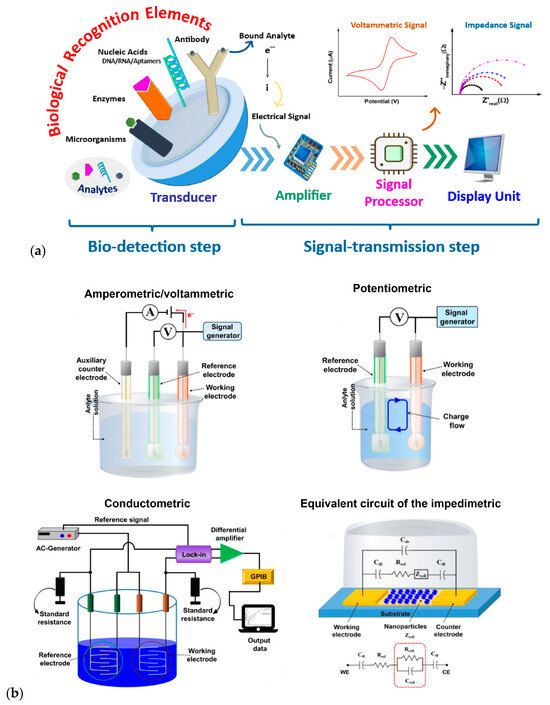
Figure 6.
(a) Stages in the functional mechanism of an electrochemical biosensor. This diagram is reprinted from [53], copyright 2023, with permission from Elsevier. (b) Schematic diagrams of various electrochemical transducers. This diagram was adapted from Naresh and Lee [47]. Copyright 2021 MDPI (Basel, Switzerland).
As early as 1956, Professor Leland C. Clark, Jr., known as the biosensor concept creator, introduced the use of electrochemical biosensors with the publication of his article on oxygen electrodes for the continuous measurement of arterial oxygen tension [109]. This discovery laid the groundwork for the proposal that Clark and Lyons published in 1962, attempting to expand the range of analytes that could be measured through so-called “enzyme electrodes” [110,111]. In this study, glucose oxidase was immobilized on an oxygen electrode using a dialysis membrane, with a proportional relation between the decrease in oxygen and the glucose concentration. The significance of Clark’s design was so notable that many studies continue to use the oxygen measurement concept [112].
Today, advances in micro- and nanoscale manufacturing technologies have facilitated the development of electrochemical biosensors designed for point-of-care use [113]. This progress has allowed the miniaturization and integration of complex systems into portable and easy-to-use tools [114]. For example, conventional potentiostats, traditionally large and limited to the laboratory, have been transformed into compact devices the size of a cell phone. Despite their small size, these miniaturized potentiostats retain all their functionality as electroanalytical instruments, allowing their deployment in the field with minimal training [115].
3.1.1. Amperometric Sensors
These sensors measure the current resulting from the oxidation or reduction of electroactive species. The produced current is directly proportional to the concentration of the target analyte. Amperometric biosensors are widely used in clinical diagnostics, such as glucose monitoring in diabetes management [116]. Beyond their clinical applications, these biosensors have been explored for the detection of emerging contaminants of diverse nature (Table 1).
A remarkable case is the detection of chlorpyrifos, an organophosphate pesticide widely used in agriculture whose accumulation can cause serious risks to human health and environment [117]. In this framework, amperometric enzyme biosensors are presented as a potential tool for chlorpyrifos detection due to their rapidity, in situ analysis and high sensitivity. These devices detect the current generated by the oxidation of thiocholine at the working electrode, which occurs through the hydrolysis of acetylcholine chloride catalyzed by the enzyme acetylcholinesterase (AChE), immobilized on the electrode. When chlorpyrifos is present, it inhibits the activity of the enzyme, reducing the production of thiocholine and, thus, the current generated, which allows the pesticide to be detected [118].

Table 1.
Classification of electrochemical biosensors for pathogens and emerging pollutants in terms of target, electrochemical method, working electrode, biorecognition element, and limit of detection (LOD).
Table 1.
Classification of electrochemical biosensors for pathogens and emerging pollutants in terms of target, electrochemical method, working electrode, biorecognition element, and limit of detection (LOD).
| Target | Working Electrode | Measurement Technique | Biorecognition Element | LOD | Ref. |
|---|---|---|---|---|---|
| Pathogens | |||||
| Salmonella | CoFe2SO4/SWCNT modified with TEOS/APTES | CV/EIS | Functionalized DNA probe | 2.34 × 10−10 µM | [119] |
| E. coli | Ag-Carbon | CV | E. coli-aptamer | 34 CFU/mL | [120] |
| SARS-CoV-2 | Modified PCB | CV/DPV | DNA-aptamer | 1.7 × 10−9 µM | [121] |
| Pharmaceuticals | |||||
| Sulfamethoxazole | Au/SPE | DPV | Tyrosinase enzyme | 22.6 µM | [122] |
| Bevacizumab | OG | DPV | Anti-bevacizumab | 0.02 µg/mL | [123] |
| Lyncomycin Neomycin | Au/CNF/SPE | SWV | DNA-aptamer | 4.92 × 10−8 µM 5.55 × 10−8 µM | [124] |
| Pesticides and agrochemicals | |||||
| Atrazine | Au 5 Ir@RFBP-GQD | CV/DPV | Hairpin DNA | 3.4 × 10−13 µM | [125] |
| Chlorpyrifos | SPCE modified with AChE/CS-GO/GO/CNF | SWV | AChE enzyme | 2.2 × 10−3 µM | [126] |
| Chlorpyrifos | SPCE modified with CuNWs/rGO | CV | AChE enzyme | 8.84 × 10−3 µM | [127] |
| Methyl parathion Chlornitrofen | GC modified with CRL@MAC-ZIF-8/CS | CV/EIS | CRL enzyme | 0.06 µM 0.03 µM | [128] |
| Endocrine disruptors | |||||
| 4 n-nonylphenol 4-t-octylphenol | SPE modified with [BMIM][PF6] | CV/EIS | Horseradish peroxidase enzyme | 1.1 µM 0.4 µM | [129] |
| 17β-estradiol | Au/NiHCF NPs | DPV | Aptamer | 0.8 × 10−6 µM | [130] |
| Persistent organic compounds | |||||
| BDE-100 PBB-1 PCB-1 PCB-28 PCB-101 | Pt/PANI | Amperometry | Horseradish peroxidase enzyme | 2.48 × 10−5 µM 7.72 × 10−5 µM 1.17 × 10−4 µM 6.21 × 10−5 µM 5.82 × 10−5 µM | [131] |
The following acronyms are used in Table 1: SWCNTs: single-walled carbon nanotubes; TEOS/APTES: tetraethyl orthosilicate/(3-amonopropyl)triethoxysilane; PCB: printed circuit board; SPE: screen-printed electrodes; GO: graphene oxide; CNF: carbon nanofiber; GQD: graphene quantum dot; AChE: acetylcholinesterase; CS: chitosan; NWs: nanowires; rGO: reduced graphene oxide; GC: glassy carbon; CRL: Candida rugosa lipase; MAC-ZIF-8: macro-microporous ZIF-8 nanofibers; [BMIM][PF6]: 1-butyl-3metthylimidazolium hexafluorophosphate; PANI: polyaniline; NiHCF NPs: nickel hexacyanoferrate nanoparticles; PBB-1: 2-Bromobiphenyl; PCB-1: 2-Chlorobiphenyl; PCB-28: 2,4,4′-Trichlorobiphenyl; PCB-101: 2,2′,4,5,5′-Pentachlorobiphenyl; BDE-100: 2,2′,4,4′,6-Pentabromodiphenyl ether; CV: cyclic voltammetry; DPV: differential pulse voltammetry; EIS: electrochemical impedance spectroscopy; SWV: square wave voltammetry.
However, the low conductivity of AChE needs to be compensated for by obtaining a functional biosensor, so the use of conductive nanomaterials has become increasingly popular. Thus, the incorporation of nanomaterials in electrochemical biosensors has opened a plethora of possibilities to improve their performance. These nanoscale structures possess unique properties, such as a high surface-to-volume ratio, which provides a larger active area for biomolecular interactions. In addition, nanomaterials can be functionalized with various molecules, which allows the creation of highly selective and sensitive biosensors [132].
For example, Suwannachat et al. [127] performed a simple modification on screen-printed carbon electrodes (SPCEs) with copper nanowires and reduced graphene oxide, improving the electronic properties of an SPCE and facilitating the immobilization of AChE. Similarly, Tun et al. [126] also modified an SPCE with graphene oxide, a graphene oxide–chitosan composite, AChE, and cellulose nanofibers. In both cases, the pesticide detection limit remained in the order of 10−3 µM, but Zhimin et al. [118] were able to achieve an LOD in the order of 10−7 µM using a biosensor based on the concept of nanoarchitectonics, which allowed them to eliminate the structural and chemical limitations of nanomaterials. Thus, a glassy carbon electrode was modified with nitrogen-doped nanoporous carbon nanocomposites ZnO@CoO hollow core–shell, achieving high values of surface area, graphitization level, and conductivity.
Other examples are the use of enzymes such as laccase and tyrosinase, which are used to detect phenolic compounds in wastewater through amperometric biosensors [131]. These biosensors measure the current changes associated with oxidation or reduction reactions, offering a sensitive method for the detection of contaminants [56].
Advances in electrochemical biosensors have leveraged innovative nanomaterials to improve the detection not only of emerging pollutants, but also pathogens as E. coli O157:H7. For example, sandwich-type electrochemical immunosensors incorporating nanocomposites into modified electrodes have shown promising results. In addition, magnetic and bimetallic nanoparticles have been used in point-of-care testing to achieve sensitive and rapid detection [133].
3.1.2. Potentiometric Sensors
Potentiometric sensors measure the potential difference between a working electrode and a reference electrode, with no significant current flow. The measured potential correlates with the logarithm of the analyte concentration, following the Nernst equation. Potentiometric biosensors are commonly used for detecting ions and small molecules [134] and are recognized for their affordability, compactness, and high sensitivity and selectivity. These sensors take advantage of ion-selective electrodes (ISEs) and ion-sensitive field-effect transistors (ISFETs) to effectively collect analytical data [135]. Recent advances have expanded their usefulness in various configurations, including light-addressable potentiometric systems that provide improved spatial and temporal control [136]. For example, a recent innovation presented by Shaibani et al. [137] demonstrated the use of a portable nanofiber light-addressable potentiometric sensor (NF-LAPS) to detect E. coli in orange juice. This sensor employed electrospun pH-sensitive polyvinyl alcohol/polyacrylic acid (PVA/PAA) hydrogel nanofibers as the detection layer. Surprisingly, they achieved a detection limit of 100 CFU/mL in one hour, demonstrating a fast and selective performance level. This highlights the potential of light-assisted potentiometric biosensors to meet real-world analytical challenges.
In addition, advances in potentiometric biosensors continue to diversify their applications. Beyond ion detection, they have been integrated with photoelectrochemical systems to improve signal transduction, and molecularly printed polymers to improve analyte specificity and portable configurations for continuous monitoring [136]. Recent advances have explored the use of potentiometric aptasensors integrated with electrogenerated chemiluminescence systems for the detection of E. coli. By using SWCNTs with aptamer molecules, these sensors achieve the highly selective detection of bacteria with exceptional sensitivity. The incorporation of Ru(bpy)32⁺ modified electrodes enhance signal amplification, enabling the detection of E. coli O157:H7 at concentrations as low as 2 CFU/mL [138]. These systems also use innovative fluid flow designs to improve mass transport, facilitating more efficient detection processes even in complex matrices such as seawater [125]. Novel configurations have been developed, such as the incorporation of Chlamydomonas reinhardtii algae cells immobilized on carbon black nanomodified SPCE into an electrochemical transduction system [139]. This configuration records the updrafts under light illumination and the applied potential, correlating with the production of oxygen derived from algae in the presence of bacteria E. coli [126].
3.1.3. Impedimetric Sensors
Impedimetric sensors are among the first approaches developed for the rapid detection of pathogens. Unlike other electrochemical techniques, they focus on measuring impedance, which reflects changes in the conductivity of the solution or the transfer of electrons on the electrode surface [140]. The most widely used technique for these biosensors is electrochemical impedance spectroscopy (EIS), which applies to analyzing the properties of the system [141]. A notable example is the development of an impedimetric biosensor based on indium tin oxide (ITO) electrodes for the detection of bacteria, including E. coli. The immobilization of anti-E. coli antibodies on electrodes exhibited high specificity, as evidenced by their ability to distinguish E. coli from Salmonella typhimurium and other non-specific bacteria [142]. Another breakthrough is the impedance biosensor that incorporates immunomagnetic nanoparticles (MNPs) and urease for the amplification of biological signals [143]. The system combines MNPs with biotinylated antibodies and urease-modified gold nanoparticles, creating a bacteria-specific complex. The impedance decreases as the urease catalyzes the hydrolysis of urea, improving the detection signal.
Recent developments, reported by Cimafonte et al. [144], include an innovative impedimetric biosensor using a simple and cost-effective approach with screen-printed gold electrodes for the rapid detection of E. coli in water. The sensor employs a photochemical immobilization technique to covalently attach anti-E. coli antibodies to the gold surface, ensuring the vertical orientation of the antibodies for effective interaction. Impedance measurements were performed in 0.01 M phosphate-buffered solution containing Fe(CN)63−/Fe(CN)64− 10 mM as a redox probe. Nyquist plots were analyzed using a modified Randles circuit, identifying resistance to charge transfer as a critical parameter influenced by antibody immobilization, bovine serum albumin blockade and E. coli binding [144].
In addition to pathogen detection, impedimetric biosensors have been adapted to detect chemical contaminants such as diazinon, an organophosphate pesticide known for its toxicity and persistence in the environment. A novel impedimetric biosensor has been developed using multi-walled carbon nanotubes (MWCNTs) functionalized with poly-L-lysine to immobilize double-stranded DNA (ds-DNA) on the surface of a glassy carbon electrode. This configuration takes advantage of the interaction between diazinon and ds-DNA, resulting in measurable changes in interfacial resistance to charge transfer (Rct). When diazinon intercalates with the DNA helix, it reduces the Rct, creating a reliable signal for quantification. The biosensor demonstrated exceptional sensitivity, reaching a detection limit as low as 3 × 10−4 µM and a wide linear dynamic range (0.001–100 μM). Its performance was validated on real samples, including river water and agricultural wastewater, showing high accuracy and recovery rates, underlining its practicality for environmental monitoring [145].
3.1.4. Conductometric Sensors
These sensors assess changes in the electrical conductivity of a solution due to biochemical reactions. The interaction between the biorecognition element and the analyte alters the ionic composition, leading to measurable conductivity changes. Conductometric biosensors are utilized in environmental monitoring and industrial process control [146]. Enzymatic processes are particularly suitable for conductometric biosensors, as they can significantly alter the conductivity and ionic strength of a solution. By incorporating zeolite imidazolate frameworks as an electrolytic material, conductometric devices can further improve their sensitivity and selectivity [147,148]. This approach facilitates the study of kinetics and enzymatic processes, which makes them valuable tools in both research and industrial settings. Conductometric biosensors have found widespread applications in environmental monitoring and industrial process control, where they can be used to detect contaminants, monitor fermentation processes, and evaluate the quality of various products [147].
A notable advance is the use of conductometric immunosensors for the detection of E. coli O157:H7 in water. These sensors employ chemically functionalized interdigitated electrodes (IDEs) to improve the binding specificity toward E. coli O157:H7. By modifying the electrode surface with 3-aminopropyl triethoxysilane and binding a carboxylated DNA probe specific to the target bacterium, these sensors achieve high sensitivity and selectivity. The interaction between bacterial DNA and the immobilized probe causes detectable changes in electrical conductivity. This method demonstrated an impressive detection range of 1 × 10−9 µM to 1 × 10−3 µM and a minimum detection limit of only 1 × 10−9 µM, making it suitable for early-stage contamination monitoring in water systems [149].
In addition, the integration of advanced microfabrication techniques into IDE biosensors has improved their performance. For example, aluminum-based IDE designs offer advantages such as low cost, high conductivity, and biocompatibility. These features, combined with an accurate photolithographic pattern, allow sensor designs to be optimized to minimize interference and maximize detection capability. These innovations have proven effective in differentiating complementary, non-complementary, and mismatched DNA sequences, ensuring reliable detection even in complex water simples [150,151].
3.2. Optical Biosensors
Optical biosensors detect changes in light properties resulting from the interaction between the analyte and the biorecognition element. They offer high specificity and are widely used in various fields. Key techniques include surface plasmon resonance (SPR), ratiometric fluorescence, and Raman spectroscopy.
Furthermore, Surface-Enhanced Raman Scattering (SERS), an enhancement over the Raman technique, is emerging as a potential tool for detecting contaminants and infectious agents in wastewater, as it facilitates the identification of unique vibration patterns associated with biomarkers and chemicals, even simultaneously. This technique is characterized by rapid result acquisition, ease of transport, high sensitivity, and good capability for analyzing complex matrices [152]. However, despite its high sensitivity, this technique has encountered its main limitation in sample preparation and the reproducibility of the generated signal. As a result, numerous strategies have been developed to overcome these challenges, with methodologies based on the creation of well-ordered periodic arrays of metallic nanoparticles standing out [153]. These nanoparticles, especially those made of Ag and Au, significantly amplify the Raman cross-section of molecules close to them due to the strong electromagnetic field induced by localized SPR [154]. This leads to a significant improvement in signal reproducibility and intensity, enabling the detection of individual molecules and trace-level analytes. This was demonstrated in the study developed by Zhao et al. [154], where a core–satellite protein nanostructure was created by assembling BSA onto the core surface of Ag nanoparticle. This arrangement allowed BSA to anchor many triclosan molecules near the surface of the Ag nanoparticles, resulting in amplified SERS signals. The method enabled the detection of triclosan in pond water within 30 min, with an LOD of 0.05 µM.
Table 2 lists some significant examples of detection of pathogens and emerging contaminants in aqueous environments by optical biosensors according to the type of signal used.

Table 2.
Classification of optic biosensors for pathogens and emerging pollutants in terms of target, optical signal, biorecognition element, and LOD.
SPR is a highly versatile optical technique that measures the changes in refractive index near a metal surface, typically gold or silver, upon binding of an analyte. This tagless method allows the real-time monitoring of biomolecular interactions, providing detailed information on binding kinetics, affinity, and concentration. SPR has become an indispensable tool in drug discovery and molecular biology research due to its ability to provide quantitative and qualitative data without the need for additional labeling [165]. In addition, it is widely used in the study of protein–protein interactions, particularly to determine dissociation constants at antigen–antibody binding. This is crucial to characterize the affinity and specificity during the development of monoclonal antibodies [166]. Advances in SPR technology have expanded its applications to the detection of multiple analytes, significantly increasing the efficiency in biomolecular studies. This expansion has also been applied to the environmental field, enabling the detection of pollutants of different nature, including nanoplastics, which represent one of the most dangerous sources of pollution in water. A recent study of Seggio et al. [164] developed an SPR-based biosensor functionalized with an estrogen receptor as the biorecognition element. To enhance its detection capability, the receptor was coupled to a polymer-based gold nanograting plasmonic platform, achieving a limit of detection LOD of 1.02 × 10−3 µg/mL for polymethyl methacrylate, a model nanoplastic. The study utilized simulated seawater as the medium to mimic environmental conditions, reflecting the growing concern over nanoplastic pollution, especially due to the increased production of plastics during the COVID-19 pandemic.
Among the most widely used transducers in biosensors are those based on fluorescence phenomena, which can detect a wide range of analytes through changes in fluorescence intensity. This occurs due to physicochemical changes on the biosensor surface, affecting the light properties that interact with it, when the analyte binds to the biorecognition element. Some examples include variations in the signal produced by the chemical interaction between an antigen and an antibody, and changes in the signal generated by a fluorophore when an interaction occurs between a chain of genetic material (DNA or RNA) and the analyte of interest [167]. However, the main limitation of fluorescence-based platforms lies in the evaluation of a single emission wavelength, which can lead to autofluorescence interferences that compromise the accuracy and reliability of measurements, especially in complex matrices such as food or biological samples. The ratiometric fluorescence method has emerged as an improved solution to address this problem. This technique allows the measurement of variations in emission intensity at two or more different wavelengths, leading to a self-calibration process that effectively reduces interference and improves both the accuracy and biosensor dynamic range [168].
A notable example of this improvement is the competitive ratiometric fluorescence biosensor developed by Huang et al. [158] for the rapid detection of azithromycin. In this setup, thioflavin T (ThT) is used as fluorophore, GO is employed, and an affinity aptamer, selected through magnetic bead-SELEX, serves as the biorecognition element. Briefly, when GO binds to the affinity aptamer, the fluorescence of ThT decreases, while in the aptamer binds to ThT, the fluorescence signal increases. Therefore, if azithromycin is present in the medium, it will bind to the aptamer, preventing its binding to the GO and causing a variation in the output signal, which will be stronger, and achieve an LOD of 9.78 × 10−3 µM.
3.3. Piezoelectric Biosensors
Piezoelectric biosensors take advantage of the piezoelectric effect, in which certain materials generate an electrical signal in response to mechanical stress, to detect mass changes on the surface of a sensor due to analyte binding. A prominent example is the QCM, which measures frequency changes corresponding to mass variations. The QCM works by oscillating a quartz crystal at a specific frequency; when an analyte binds to the sensor surface, the added mass causes a measurable decrease in the oscillation frequency, which correlates directly with the amount of bound material.
These biosensors are particularly effective for detecting biomolecular interactions, offering real-time and label-free analysis. This capability is invaluable in healthcare applications, including pathogen detection and biomarker analysis [169,170]. For example, piezoelectric immunosensors have been developed for the detection of various pathogens by immobilizing specific antibodies on the sensor surface. After the binding of the target pathogen, the resulting mass change is detected by the piezoelectric sensor, which facilitates rapid and sensitive identification. In addition, piezoelectric biosensors have been used to monitor biomolecular interactions, such as protein–protein and protein–DNA interactions, which are crucial for understanding the mechanisms of the disease and developing therapeutic strategies [171].
The advantages of piezoelectric biosensors include their high sensitivity, fast response times, and the ability to perform real-time and label-free detection, making them suitable for a wide range of applications. However, challenges such as the need for the precise control of sensor surface properties and possible interference from non-specific junctions must be addressed to ensure accurate and reliable measures.
Although no widespread application of piezoelectric biosensors for pollutants detection in water is known, a meaningful example is the regenerative biosensor proposed by Mazouzi et al. [157] for the detection of DFC in river water. Their biosensor used a technique that combined QCM-D and LSPR. In the study, the surface of the quartz crystal was modified by an optimized activation process to allow the efficient binding of DCF, and an anti-DCF monoclonal antibody was immobilized on it. The binding of DCF to the immobilized antibody causes a shift in the standard resonance frequency associated with QCM-D, which allowed the detection of the contaminant with an LOD of 9.49 × 10−3 µM. Furthermore, the LSPR technique provides additional information on the coverage and hydration level of the antibody monolayer, which serves as an efficiency indicator and biosensor stability.
4. Environmental Real Water Analysis with Biosensors
Environmental sample analyses beyond simple proof-of-principle in ideal conditions have also been performed using biosensors. Numerous publications have focused on the detection of compounds belonging to pharmaceuticals of pesticides in wastewater, river water, and tap water.
Among the most common pharmaceutical groups present in wastewater, and therefore the ones which aroused the greatest detection interest, are the antibiotics employed in treating bacterial diseases [172] such as tetracycline derivatives [173], ampicillin [174,175], kanamycin [176], quinolones [177], and ciprofloxacin [178]. For this purpose, aptamers have been highlighted as the main biorecognition element due to numerous advantages for these types of molecules, such as high specificity, easy synthesis, low cost, and good stability, in contrast with other bioreceptors, such as antibodies and enzymes, which offer high specificity but with significant limitations in terms of stability and reusability [179]. A similar trend is also observed in pesticide detection with aptamer application, especially in the case of organophosphorus compounds, which constitute the largest group of insecticides employed. Thus, aptamers have been applied as a biorecognition element in the case of atrazine [180], imidacloprid [181], acetamiprid and paraquat [182]. However, the main limitation associated with these bioreceptors is the limited interaction between the functional groups of organophosphorus compounds and the aptamer sequences, due to the small molecular structure of the target compounds [183].
All the above-mentioned transduction technologies have found their application in the detection of organic pollutants in water. Optical biosensors show high selectivity, simplicity in operation conditions, stability, and fast response in measurement. However, the detections limits of this technique are conditioned by external factors such as pressure, temperature, and chemical composition of the sample [184]. Similar limitations are found in the case of piezoelectric biosensors, which despite having a high response speed and selectivity, are vulnerable in wet environments or at high temperatures [185], making in situ measurements harder to perform. Regarding electrochemical biosensors, their exceptional sensitivity and high selectivity, as well as their fast response time, are complemented by easy preparation and good portability [186], which has made them one of the most studied alternatives and has attracted the greatest interest in detecting pollutants in real environments, although interference with organic matter and other ions still presents challenges for their application.
Nevertheless, the inclusion of nanomaterials in the design of biosensors has led to a collective and significant improvement in pollutant detection for real water matrices, regardless of their nature. In this way, Talari et al. [187] achieved an LOD lower than 0.01 µM in tap, river, and runoff waters for the detection of diazinon pesticide thanks to an optical apta-nanobiosensor based on reduced graphene quantum dot fluorescence emission, prepared in two steps and with MWCNTs added to improve the detection accuracy. In a similar approach, Hatamluyi et al. [188] succeeded in further increasing the diazinon LOD reaching a limit of 1.02 × 10−8 µM in tap and river water by another amperometric apta-nanobiosensor. In this case, it was developed from a zeolitic imidazolate framework-derived nanoporous carbon platform functionalized with aptamers and poured on an SPCE decorated with hierarchical flower-like gold nanostructures. In the field of pharmaceutical detection, a particularly noteworthy case, performed through an electrochemical transduction mechanism, was reported by Roushani et al. [189] in the detection of ibuprofen, reaching an LOD of 9.80 × 10−8 µM in wastewater. This ultrasensitive apta-nanobiosensor was designed from nitrogen-doped GQD (N-GQD) and gold nanoparticles (AuNPs) retained over GCE, forming an AuNPs@N-GQD/GCE electrode. Subsequently, the amino capture probe (NH2-ssDNA1) was added forming a covalent bond with the AuNPs and the specific ibuprofen aptamer was included. The results show the effective use of cheap and simple apta-nanobiosensors with enough selectivity and sensitivity for the detection of contaminants in real samples.
5. Integration with Microfluidic Systems
In recent years, the development of microfluidic devices has revolutionized analytical chemistry, enabling high-resolution separations, the synthesis of monodisperse colloidal systems, and the rapid, cost-effective detection of multiple analytes [190,191]. Using this technology, small volumes of fluids can be controlled and analyzed at the scale of micrometers. Their high surface-to-volume ratio plays a critical role in enhancing mass transfer and reaction kinetics, and optimizing chemical and biological processes for sensitive and efficient detection [192]. This characteristic is particularly relevant in water quality monitoring, where microfluidic platforms facilitate the real-time detection of contaminants with minimal sample volumes, increased precision, and automated data acquisition [193].
The foundation for these advancements can be traced back to pioneering works in microfluidics, such as the study by Wang et al. [194]. Their work demonstrated the feasibility of fully disposable microfluidic platforms with high analytical performance, sensitivity, and reproducibility, paving the way for the practical implementation of “lab-on-a-chip” technologies in clinical and environmental analysis. Along with other works from this era, it laid the groundwork for the development of this transformative technology.
Microfluidic systems significantly improve biosensor performance by addressing key challenges in water analysis, including low contaminant concentrations, complex sample matrices, and the need for rapid on-site measurements. The integration of miniaturized microchannels optimizes fluid dynamics, ensuring enhanced mixing, controlled reaction times, and efficient analyte transport to detection zones [195]. For this reason, microfluidic devices consist of several interconnected sections (Figure 7a). The process begins at the inlets, where fluids, such as reagents or biological samples, are introduced. These fluids then move through a mixing channel designed to combine liquids homogeneously [196]. Next, they pass through the incubation channel, which extends the residence time to complete chemical or biological reactions [197]. Finally, in the detection chamber, integrated sensors analyze specific parameters, such as molecular concentrations, before the processed fluid exits via the outlet [198]. These configurations can vary depending on the design, with straight, Y-shaped, or T-shaped flow geometries [196,197,198,199].
Figure 7.
(a) General representation of a microfluidic device, illustrating key components for fluid mixing, incubation, reaction, and sensing, which are essential for analytical applications. Reprinted from Weng et al. [200], copyright 2015, Springer Nature. (b–d) Different configurations of microfluidic devices integrated with biosensors, depending on the detection method used: (b) illustration of SAW-based detection, reproduced from Tsougeni et al. [201], copyright 2020, Elsevier; (c) representation of a biosensor utilizing electrochemical measurements, adapted from Yao et al. [202], copyright 2015, Springer Nature; (d) biosensor based on SERS spectroscopy, adapted from Xiao et al. [203], copyright 2020, Elsevier.
By leveraging precisely controlled fluid dynamics, microfluidic biosensors for water quality monitoring achieve enhanced detection sensitivity, rapid response times, and cost-effective operation. The ability to integrate multi-analyte detection on a single chip, coupled with automated sample preparation and analysis, further demonstrates the transformative potential of microfluidics in environmental monitoring.
5.1. Materials and Fabrication Techniques
One of the most critical aspects in the development of microfluidic devices is material selection, as it directly influences biosensor biocompatibility, performance, reproducibility, and durability. Commonly used materials include rigid polymers, polydimethylsiloxane (PDMS), glass, silicon, paper, and 3D-printed composites [199]. PDMS is highly valued for its flexibility, optical transparency, and compatibility with micromolding techniques, facilitating the creation of high-resolution microchannels. However, its tendency to absorb hydrophobic compounds and high gas permeability may affect assay reproducibility, particularly in quantitative detection biosensors [204].
On the other hand, thermoplastics such as polymethyl methacrylate (PMMA) and polycarbonate (PC) offer greater mechanical stability and lower biomolecule absorption, making them more suitable for precise analytical applications. PMMA stands out for its transparency, lightness, and ease of integration with optical methods, while PC is highly impact-resistant, making it an ideal choice for portable environmental monitoring devices [205]. Additionally, materials such as polystyrene (PS) and cyclic olefin copolymer (COC) are attractive options due to their low cost, ease of molding, and chemical resistance, making them ideal for disposable biosensors and high-throughput analysis [199,205].
An emerging approach in microfluidics is the use of paper-based microfluidic devices (μPADs), which enable passive fluid flow through capillary action, eliminating the need for external pumps [206]. These devices have proven particularly useful for point-of-care detection, such as rapid pathogen identification and environmental monitoring. The integration of electrochemical and colorimetric biosensors into μPADs has led to the development of low-cost, highly sensitive disposable devices suitable for field applications [206,207].
Beyond material selection, fabrication techniques play a key role in the precision, reproducibility, and scalability of microfluidic biosensors. Soft lithography remains one of the most used techniques for PDMS devices, allowing for the high-resolution fabrication of three-dimensional microchannels. However, the low mechanical strength of PDMS and its challenges in large-scale production have driven the development of alternative techniques such as injection molding and micromachining, especially for PMMA, PC, and COC-based devices, which offer greater robustness and reproducibility in mass production [208,209].
Laser-based techniques, such as engraving and ablation, enable the direct fabrication of microchannels on thermoplastics, reducing costs and eliminating the need for molds while maintaining high precision [210]. Laser direct writing uses photopolymerization to create fluidic patterns with minimal contamination, while CO₂ laser micromachining optimizes surface quality and dimensional accuracy in materials like PMMA [211]. Additionally, the use of chemical processes such as inkjet printing facilitates the high-resolution deposition of biomolecules, facilitating the fabrication of biosensors on paper and flexible surfaces. However, reactant adhesion can present a challenge in these devices, requiring the development of improved biomolecule fixation techniques [204].
Advancements in material selection and fabrication techniques have significantly driven the development of high-performance microfluidic biosensors, with applications in pathogen detection, environmental monitoring, and clinical diagnostics. The combination of materials such as PMMA and PDMS with optimized manufacturing processes, such as injection molding and laser engraving, has led to the creation of more efficient and reproducible devices. However, challenges remain, such as long-term mechanical stability, adhesion optimization in biosensors, and reducing production costs [212]. As research in this field progresses, the integration of new manufacturing strategies and the use of innovative materials such as polyimide and stainless steel could enhance the functionality and scalability of these devices in real-world applications [213].
5.2. Applications of Microfluidics: From Lab to Real-World Samples
Beyond manufacturing, microfluidic devices significantly enhance biosensor performance in water quality monitoring by enabling precise fluid control, reducing sample volume, and improving reaction efficiency. These advantages are crucial for real-time contaminant detection, where achieving high sensitivity with minimal sample and reagent consumption is essential. This is particularly evident in various advanced analytical techniques, as illustrated by SWA (Figure 7b), electrochemistry (Figure 7c), and SERS (Figure 7d).
Multiple studies were conducted in synthetic water matrices, ensuring controlled laboratory results. One example is the study by Nissen et al. [214], who integrated water-filled antiresonant hollow fibers into a microfluidic system for UV spectroscopy. Their approach significantly enhances (100-fold longer) light–matter interactions, improving the detection of pharmaceutical residues like sulfamethoxazole and sodium salicylate at 0.1 μM and 0.4 μM concentrations, respectively. By reducing the sample volume by 99.9%, this technique exemplifies how microfluidic integration improves efficiency in environmental analysis, making it ideal for water quality monitoring. Moreover, Burtsev et al. [215] developed a SERS-based system to trace ibuprofen in water, achieving a detection limit of 10−2 μM. By combining an optimized mixer with functionalized gold nanoparticles, the system effectively captures the analyte without requiring prior sample preparation, offering high sensitivity even in complex wastewater matrices.
In the field of pathogen detection, microfluidic biosensors enable rapid, automated, and highly specific quantification, which is particularly valuable in food safety and environmental applications. A clear example of this is the work by Tsougeni et al. [201], who developed a lab-on-a-chip system that integrates immunological capture, chemical lysis, isothermal amplification, and acoustic detection for the identification of pathogen such as Salmonella, Listeria, and E. coli. By optimizing microfluidic flow control, the system enhanced reaction kinetics, reducing the analysis time by five-fold compared to conventional methods, achieving a detection limit of 1–5 CFU per 25 mL within 4.5 h. Additionally, Man et al. [216] presented a portable colorimetric biosensor based on a microchip for detecting Salmonella in fresh produce. Using functionalized polystyrene nanoparticles and an innovative valve, this device achieves detection within 45 min at a limit of 60 CFU/mL. The results are captured via a mobile app, offering accessibility and cost-effectiveness. Similarly, Jiang et al. [217] proposed a dual RCA-based microfluidic platform to detect E. coli O157:H7. By enhancing sensitivity with repeated aptamers and signal amplification, the system achieves detection limits of 80 cells/mL, amplifying signals up to 250-fold. For the same pathogen, Zheng et al. [218] introduced a colorimetric biosensor combining Au nanoparticles with a microfluidic chip to the analysis of chicken samples. This system combines portability and sensitivity, achieving detection within 50 min at a limit of 50 CFU/mL, making it ideal for field-based food safety applications.
Although most studies focus on the detection of pathogens, there are also applications in the environmental field, as demonstrated by the work of Ben Messaoud et al. [88]. As highlighted in Section 2.2, this study introduces an innovative, portable, label-free, and ultrasensitive electrochemical immunosensor designed for the detection of A. salmonicida in seawater. Also, the main innovation of their work lies in the use of a fluidic-integrated electrochemical-cell chip, which features independent chambers housing three electrochemical cells. This not only allows for a compact and portable design but also enhances the capability for simultaneous and reproducible analysis. Furthermore, the sensor offers a wide linear working range, spanning from 1 to 107 CFU/mL, and an extremely low LOD of 1 CFU/mL.
Nevertheless, integrating microfluidics with biosensors has enabled the precise detection of contaminants in real water samples, facilitating environmental monitoring and real-time contaminant identification [219]. A notable example is the study by Doostmohammadi et al. [220], who developed a portable microfluidic biosensor based on cell-imprinted polymers (CIPs) for detecting bacteria in pond water. The device, integrated into a 3D-printed platform, utilized magnetically functionalized CIP microparticles within a magnetophoretic microfluidic system. Tests performed in pond water revealed an E. coli concentration of 2 × 106 CFU/mL, closely matching results obtained in a central laboratory (2.33 × 106 CFU/mL). However, the non-specific bacteria presence, including Salmonella and Sarcina, compromised the sensor’s selectivity, highlighting the need for optimization in more complex aquatic matrices.
In a similar approach, Akhtarian et al. [221] developed a microfluidic biosensor based on EIS, incorporating CIPs for E. coli detection in water. This sensor featured stainless steel microthreads functionalized with CIPs within a PDMS microchannel, enabling rapid and cost-effective detection in environmental samples. When tested in real water, the sensor successfully differentiated E. coli concentrations ranging from 102 to 107 CFU/mL, with a detection limit of 2 × 102 CFU/mL. However, ensuring sensor stability under varying environmental conditions remains a challenge, requiring further optimization to enhance robustness in untreated water matrices.
Further advancing this technology, Akhtarian et al. [222] also designed a conductometric sensor utilizing microthreads coated with CIPs for bacterial detection in real water samples. The sensor exhibited a quantifiable response between 104 and 107 CFU/mL of E. coli, with a sensitivity of 7.35 µS per CFU/mL. Tests conducted in pond water revealed a decrease in electrical resistance following bacterial capture, enabling effective quantification. Notably, in this case, the presence of other bacteria such as Sarcina and Listeria did not significantly impact sensor specificity, suggesting that CIP technology could be a promising approach for the selective detection of pathogens in water.
However, microfluidics is not only used for pathogen quantification but also plays a crucial role in the detection of pharmaceutical contaminants and heavy metals, as demonstrated by Hidi et al. [199] and He et al. [223], respectively. Hidi et al. [199] introduced a lab-on-a-chip platform integrated with SERS for ciprofloxacin detection in river water. By optimizing the pH to 11, the chemical affinity between ciprofloxacin and silver nanoparticles was enhanced, significantly improving the SERS signal. The system achieved a detection range of 0.7–10 μM with a detection limit below 0.74 μM, which corresponds to the minimum inhibitory concentration for relevant bacterial strains. Similarly, He et al. [223] developed a microfluidic biosensor based on SERS for the ultrasensitive detection of uranyl ions in drinking and river water. This sensor utilized ZnO-Ag hybrids modified with aptamers, allowing highly specific detection with a detection limit as low as 7.2 × 10⁻7 μM, five orders of magnitude below the maximum level permitted by the US Environmental Protection Agency. When tested in river and tap water, the sensor demonstrated effective analyte recovery, although interferences from other metal ions, such as Pb2⁺, Hg2⁺, and Cu2⁺, were detected, potentially compromising measurement accuracy in complex environmental samples.
This challenge in selective detection was also observed in the studies by Doostmohammadi et al. and Akhtarian et al. [220,222], where the presence of non-specific bacteria such as Salmonella, Sarcina, and Listeria impacted sensor specificity. These findings underscore the importance of optimizing selectivity in real water matrices to ensure precise measurements across varying environmental conditions.
The relevance of microfluidics in the quantification of contaminants in real water samples is highlighted and demonstrated the impact of different detection strategies on biosensor precision and sensitivity. The presence of interferences in environmental samples remains a major challenge, emphasizing the need for more selective and robust technologies for field applications. As these devices continue to evolve, their integration with artificial intelligence and automated analysis could significantly enhance performance in environmental monitoring and water safety applications.
5.3. Integration of Artificial Intelligence in Microfluidics
Significant advancements in artificial intelligence (AI) have enabled its integration into various technological and scientific fields, including water quality monitoring. AI branch machine learning (ML) and deep learning (DL) have arisen as ideal tools for the design of microfluidic systems based on enhancing the automation, speed, and precision of the related tasks [224]. ML aims to create models from sample data in terms of task and performance metrics, and DL is an ML approach based on complex neural networks to extract the inherent patterns and features from enormous databases.
The incorporation of AI to microfluidic devices holds great potential for advancing the next-generation monitoring systems to attain time-efficient setups and easy operation. This has led to the development of “intelligent microfluidics”, where ML algorithms optimize the design, operation, and analysis of microfluidic biosensors used for environmental applications. By leveraging AI, traditional limitations such as manual calibration, experimental variability, and human intervention in water analysis have been mitigated, enhancing detection speed, accuracy, and automation [225,226,227].
AI-driven microfluidic biosensors have demonstrated remarkable improvements in contaminant detection, particularly in the real-time analysis of heavy metals, pesticides, pharmaceutical residues, and microbial pathogens in surface water, drinking water, and industrial wastewater. A key example is flow control within microchannels, where neural networks dynamically adjust parameters to maintain stability in droplet-based sensing platforms, ensuring optimal reagent mixing and sample processing [225,228]. In biosensing applications, computer vision algorithms facilitate the automated detection of pollutants, including lead (Pb2⁺), mercury (Hg2⁺), and nitrates, through AI-enhanced optical and electrochemical sensors, significantly reducing the detection time and increasing precision. The optimization of microfluidic chip design through AI has enabled the development of more efficient and scalable water analysis systems, minimizing reagent consumption and improving assay reproducibility. Predictive models are now capable of estimating flow dynamics, reaction efficiency, and sensor performance under varying environmental conditions, ensuring robust operation in complex water matrices. Additionally, DL-based algorithms have been employed for the automated classification of microbial contaminants, achieving an accuracy of over 96% in pathogen identification, crucial for preventing waterborne diseases [228].
Another key advancement is the development of portable AI-powered microfluidic devices for on-site water testing, reducing the dependency on centralized laboratories. These systems leverage real-time data processing to analyze water samples instantly, providing rapid alerts for contamination events. For instance, AI-integrated microfluidic sensors have been deployed for the early detection of harmful algal blooms, monitoring toxin concentrations and predicting outbreaks before they escalate. Similarly, AI-enhanced electrochemical biosensors detect antibiotics and endocrine-disrupting chemicals in wastewater, enabling faster decision making in water treatment facilities [224].
Despite these advances, challenges persist, particularly the requirement for extensive datasets to train AI models, ensuring compatibility across different platforms, and addressing sensor fouling and biofouling issues in real-world water samples. Additionally, material selection plays a crucial role in improving microfluidic biosensor performance, as biocompatibility, chemical resistance, and sensor stability must be considered for long-term environmental applications. While PDMS, PMMA, and thermoplastic-based biosensors have shown promise in lab conditions, further research is needed to enhance their durability and adaptability for field applications [225,229].
In summary, these advances in microfluidic devices enhance sensitivity, reduce analysis time, and enable portable, cost-effective solutions for environmental monitoring. However, for widespread adoption, it is essential to validate these systems using real water samples, ensuring their reliability in detecting trace contaminants such as heavy metals, pesticides, and pharmaceutical pollutants. With ongoing improvements in material engineering, AI integration, and reproducibility, microfluidic biosensors are poised to become key tools in water quality monitoring and beyond.
6. New Trend in Biosensors: Molecularly Imprinted Polymers
Molecularly imprinted polymers (MIPs) are synthetic polymers designed to exhibit highly specific recognition capabilities for a target molecule. These materials are produced by polymerizing functional monomers around a template molecule, which is then removed to leave behind cavities complementary in shape, size, and chemical properties to the template [230]. Figure 8 illustrates the formation process combining MIPs with aptamers (Apta-MIPs), highlighting the key steps in their fabrication. This innovative approach has been increasingly adopted in recent years to address challenges in the detection of contaminants such as pharmaceuticals, pesticides, and pathogens. MIPs have emerged as a powerful alternative to traditional detection methods, offering advantages like stability, reusability, and high selectivity even in complex sample matrices [231,232].
Several studies highlight the growing relevance of MIPs in environmental, food safety, and medical applications. Rocha et al. [233] demonstrated the effectiveness of an electrochemical MIP-based sensor for the selective detection of tetrodotoxin in seafood samples, achieving low detection limits and excellent reproducibility in complex matrices. Similarly, Wang et al. [234] showcased the use of MIPs for the selective detection of tetracycline residues in environmental and biological samples, emphasizing their potential to address the persistence of antibiotic contaminants in ecosystems. In addition, Minhas et al. [235] demonstrated the utility of MIPs in the efficient extraction of bioactive compounds, such as methyl gallate from plant extracts, improving selectivity and streamlining the extraction process for complex matrices.
Electrochemical sensors based on MIPs further illustrate their versatility. Shalapy et al. [236] developed a novel MIP sensor for detecting indigo carmine dye in food samples, achieving ultra-trace sensitivity and exceptional selectivity. By integrating molecular imprinting with advanced sensing platforms, this work highlighted the potential of MIPs for rapid, in situ detection, overcoming the limitations of traditional chromatographic and spectrophotometric methods.
Figure 8.
Schematic representation of the fabrication process of an Apta-MIP electrode. The aptamer is immobilized on the electrode surface, followed by dopamine electropolymerization, forming a molecularly imprinted polymer (MIP). After washing to remove PSA, aptamer-lined imprinted cavities remain, enabling selective recognition, a process that can be monitored using CV. This is an adaptation of Jolly et al. [237], copyright 2016, Elsevier.
Agar et al. [238] introduced an innovative hybrid approach using Apta-MIPs, leveraging the robust properties of MIPs with the enhanced specificity of aptamers. This dual recognition system enabled the multiplexed detection of bacteria such as E. coli and S. aureus in water, achieving high sensitivity and selectivity with detection limits as low as 2 CFU/mL for E. coli. Apta-MIPs not only enhance detection capabilities but also broaden the scope of applications, making them a promising tool for point-of-care diagnostics and water quality monitoring.
In another innovative application, MIPs were combined with fluorescence-based nanomaterials, such as Si-doped biomass-derived carbon dots, to create highly selective nanosensors. Wang et al. [234] demonstrated this by designing a MIP-based nanosensor for the detection of rutin, a bioactive flavonoid, in herbal medicine. This combination leverages the high specificity of MIPs and the optical properties of carbon dots, enabling rapid and accurate detection in complex biological matrices.
Additionally, Surface Molecular Imprinting Technology (SMIP) has emerged as a promising evolution of MIP technology. Unlike traditional bulk imprinting methods, SMIP creates imprinted cavities on the surface of substrates, significantly improving the accessibility and binding efficiency of target molecules. Li et al. [239] highlighted the integration of SMIPs with SERS technology, which combines the selective preconcentration capabilities of SMIPs with the signal amplification of SERS. This hybrid system provides a powerful tool for detecting trace contaminants in complex matrices, offering a unified approach for sample pre-treatment and analysis, especially in fields like environmental monitoring and food safety.
These examples illustrate how MIPs, in their various forms and combinations with other technologies, have transformed contaminant detection. The continued integration of MIPs with cutting-edge techniques such as aptamers, fluorescence-based nanomaterials, and SMIP-SERS platforms is expected to further expand their applications, addressing global challenges in public health, environmental safety, and sustainable development.
7. Challenges and Future Prospects
Throughout this review, the potential of biosensors for environmental monitoring has been highlighted. However, various challenges must be addressed to fully exploit their capabilities. These challenges include issues related to sensitivity, stability, standardization, and the exploration of new technological directions.
One of the main challenges lies in achieving the required sensitivity and specificity to detect trace contaminants in complex environments, such as wastewater. Like other analytical techniques, biosensors often face interferences from organic matter, chemical pollutants, and microbial diversity, which can compromise their accuracy and hinder their application in real-world samples. As discussed in Section 2.1 and Section 2.2, strategies such as signal amplification and the integration of nanomaterials could further enhance detection capabilities by improving specificity and reducing noise. Another significant challenge for biosensors is ensuring their durability and reproducibility, which remain major obstacles to their widespread adoption. Variable environmental conditions, including changes in pH, temperature, and salinity, can degrade biosensor components, leading to inconsistent results. Proposed strategies to mitigate these effects include the use of robust protective materials and coatings, the application of synthetic biology to develop more stable bioreceptors, and the implementation of real-time calibration and self-diagnostic systems to ensure consistent performance.
Future research should prioritize exploring innovative strategies to enhance the functionality and applicability of biosensors. Achieving seamless integration with existing monitoring systems is critical to improving their usability and efficiency in large-scale applications. The development of multiplexed biosensors capable of detecting multiple contaminants simultaneously could significantly increase operational efficiency and broaden their applicability. Furthermore, advancing battery-free biosensor technologies—powered by energy harvesting mechanisms such as piezoelectric or photovoltaic systems—would enable sustainable, long-term deployment in remote or resource-limited areas. Expanding wearable and portable biosensor technologies will also facilitate rapid, on-site monitoring, significantly decreasing the reliance on centralized laboratories. By addressing these challenges and focusing on these future directions, biosensors can reach their full potential as indispensable tools for environmental monitoring.
In summary, overcoming the challenges of sensitivity, stability, seamless integration, expanded functionality, and battery-free operation is crucial for the widespread adoption of biosensors in environmental monitoring. Sensitivity and stability are key to ensuring reliable detection in diverse conditions, while seamless integration and multifunctionality will enhance their adaptability and real-time usability. Battery-free operation further enables sustainable and remote deployments. Additionally, establishing clear and consistent guidelines for standardization is essential to ensure comparability, reproducibility, and regulatory acceptance. By leveraging these advancements, biosensors can become indispensable tools for safeguarding water quality and environmental health.
8. Conclusions
For more than three decades, biosensors have represented a transformative technology with multiple applications spanning medicine, food safety, and environmental monitoring. This review underscores their potential as critical tools for water quality assessment, particularly for detecting emerging contaminants (ECs) and pathogens. By classifying biosensors based on their bioreceptor and transducer, the discussion highlights how these devices are being tailored to address the challenges of monitoring pollutants in complex aquatic environments.
Recent innovations such as SELEX, SAMs, and MIPs have significantly enhanced the sensitivity and selectivity of biosensors. SELEX enables the development of highly specific aptamers, which serve as efficient recognition elements for diverse analytes, while SAMs provide functionalized surfaces that ensure stable biomolecule immobilization and minimize non-specific interactions. Additionally, microfluidic systems have revolutionized biosensor integration by improving detection speed, enabling real-time analysis, and reducing the sample volumes required for testing.
Despite these advancements, challenges such as achieving the required sensitivity in complex wastewater environments, ensuring long-term stability, and addressing regulatory barriers remain. Future research should focus on the development of multiplexed biosensors, the incorporation of artificial intelligence for advanced data analysis, and the creation of sustainable and portable devices.
In conclusion, biosensors are positioned to become indispensable tools for safeguarding public health and protecting environmental integrity. Continued innovation and collaboration across disciplines will be critical to overcoming existing challenges and unlocking their full potential.
Author Contributions
Conceptualization, A.F.-S., N.B.-R. and M.Á.S.; resources, M.Á.S.; writing—original draft preparation, N.B.-R., M.Á.S. and A.F.-S.; writing—review and editing, M.P., E.R., E.G.-R. and M.Á.S.; visualization, N.B.-R., A.F.-S. and M.Á.S.; supervision, M.Á.S.; project administration, M.P. and M.Á.S.; funding acquisition, M.P. and M.Á.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been supported by R&D Project PCI2022-132941 (BiodivRestore Cofund 2020) funded by MICIU/AEI/10.13039/501100011033 and by the European Union Next Generation EU/PRTR. Also, Antía Fdez-Sanromán thanks MICIU/AEI/10.13039/501100011033 and FSE+ for investing in your future for her fellowship (PRE2021-098540).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kummu, M.; Guillaume, J.H.A.; De Moel, H.; Eisner, S.; Flörke, M.; Porkka, M.; Siebert, S.; Veldkamp, T.I.E.; Ward, P.J. The World’s Road to Water Scarcity: Shortage and Stress in the 20th Century and Pathways towards Sustainability. Sci. Rep. 2016, 6, 38495. [Google Scholar] [CrossRef]
- Mofijur, M.; Hasan, M.M.; Ahmed, S.F.; Djavanroodi, F.; Fattah, I.M.R.; Silitonga, A.S.; Kalam, M.A.; Zhou, J.L.; Khan, T.M.Y. Advances in Identifying and Managing Emerging Contaminants in Aquatic Ecosystems: Analytical Approaches, Toxicity Assessment, Transformation Pathways, Environmental Fate, and Remediation Strategies. Environ. Pollut. 2024, 341, 122889. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.Y.; Lim, F.Y.; Loh, W.H.; Ong, S.L.; Hu, J. Emerging Contaminants: An Overview of Recent Trends for Their Treatment and Management Using Light-Driven Processes. Water 2021, 13, 2340. [Google Scholar] [CrossRef]
- Wang, F.; Xiang, L.; Sze-Yin Leung, K.; Elsner, M.; Zhang, Y.; Guo, Y.; Pan, B.; Sun, H.; An, T.; Ying, G.; et al. Emerging Contaminants: A One Health Perspective. Innovation 2024, 5, 100612. [Google Scholar] [CrossRef] [PubMed]
- Flores-Contreras, E.A.; González-González, R.B.; González-González, E.; Melchor-Martínez, E.M.; Parra-Saldívar, R.; Iqbal, H.M.N. Detection of Emerging Pollutants Using Aptamer-Based Biosensors: Recent Advances, Challenges, and Outlook. Biosensors 2022, 12, 1078. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, D.; Nałęcz-Jawecki, G.; Drzewicz, P.; Skowronek, A.; Mianowicz, K.; Strzelecka, A.; Giebułtowicz, J. The Assessment of Environmental Risk Related to the Occurrence of Pharmaceuticals in Bottom Sediments of the Odra River Estuary (SW Baltic Sea). Sci. Total Environ. 2022, 828, 154446. [Google Scholar] [CrossRef]
- Fatta-Kassinos, D.; Meric, S.; Nikolaou, A. Pharmaceutical Residues in Environmental Waters and Wastewater: Current State of Knowledge and Future Research. Anal. Bioanal. Chem. 2011, 399, 251–275. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H. Occurrence and Fate of Emerging Contaminants in Municipal Wastewater Treatment Plants from Different Geographical Regions—A Review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Abramova, A.; Berendonk, T.U.; Coelho, L.P.; Forslund, S.K.; Gschwind, R.; Heikinheimo, A.; Jarquín-Díaz, V.H.; Khan, A.A.; Klümper, U.; et al. Towards Monitoring of Antimicrobial Resistance in the Environment: For What Reasons, How to Implement It, and What Are the Data Needs? Environ. Int. 2023, 178, 108089. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wang, T.; Xu, N.; Lu, T.; Hong, W.; Penuelas, J.; Gillings, M.; Wang, M.; Gao, W.; et al. Assessment of Global Health Risk of Antibiotic Resistance Genes. Nat. Commun. 2022, 13, 1553. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.-F. Antibiotic Resistance in the Environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Dou, Q.; Smalla, K.; Wang, Y.; Johnson, T.A.; Brandt, K.K.; Mei, Z.; Liao, M.; Hashsham, S.A.; Schäffer, A.; et al. Gut Microbiota Research Nexus: One Health Relationship between Human, Animal, and Environmental Resistomes. mLife 2023, 2, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Gupta, A.K.; Ghosal, P.S.; Varma, M. A Review on Hospital Wastewater Treatment: A Special Emphasis on Occurrence and Removal of Pharmaceutically Active Compounds, Resistant Microorganisms, and SARS-CoV-2. J. Environ. Chem. Eng. 2021, 9, 104812. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Engel, B.A.; Reuhs, M.; Simsek, H. Cyanophage Technology in Removal of Cyanobacteria Mediated Harmful Algal Blooms: A Novel and Eco-Friendly Method. Chemosphere 2023, 315, 137769. [Google Scholar] [CrossRef]
- Brooks, B.W.; Lazorchak, J.M.; Howard, M.D.A.; Johnson, M.V.V.; Morton, S.L.; Perkins, D.A.K.; Reavie, E.D.; Scott, G.I.; Smith, S.A.; Steevens, J.A. In Some Places, in Some Cases, and at Some Times, Harmful Algal Blooms Are the Greatest Threat to Inland Water Quality. Environ. Toxicol. Chem. 2017, 36, 1125–1127. [Google Scholar] [CrossRef]
- Hemdan, M.; Ali, M.A.; Doghish, A.S.; Mageed, S.S.A.; Elazab, I.M.; Khalil, M.M.; Mabrouk, M.; Das, D.B.; Amin, A.S. Innovations in Biosensor Technologies for Healthcare Diagnostics and Therapeutic Drug Monitoring: Applications, Recent Progress, and Future Research Challenges. Sensors 2024, 24, 5143. [Google Scholar] [CrossRef]
- Nihemaiti, M.; Icker, M.; Seiwert, B.; Reemtsma, T. Revisiting Disinfection Byproducts with Supercritical Fluid Chromatography-High Resolution-Mass Spectrometry: Identification of Novel Halogenated Sulfonic Acids in Disinfected Drinking Water. Environ. Sci. Technol. 2023, 57, 3527–3537. [Google Scholar] [CrossRef]
- Liu, X.; Verma, G.; Chen, Z.; Hu, B.; Huang, Q.; Yang, H.; Ma, S.; Wang, X. Metal-Organic Framework Nanocrystal-Derived Hollow Porous Materials: Synthetic Strategies and Emerging Applications. Innovation 2022, 3, 100281. [Google Scholar] [CrossRef]
- Sarkar, D.J.; Behera, B.K.; Parida, P.K.; Aralappanavar, V.K.; Mondal, S.; Dei, J.; Das, B.K.; Mukherjee, S.; Pal, S.; Weerathunge, P.; et al. Aptamer-Based NanoBioSensors for Seafood Safety. Biosens. Bioelectron. 2023, 219, 114771. [Google Scholar] [CrossRef]
- Guliy, O.I.; Zaitsev, B.D.; Smirnov, A.V.; Karavaeva, O.A.; Borodina, I.A. Prospects of Acoustic Sensor Systems for Antibiotic Detection. Biosens. Bioelectron. X 2022, 12, 100274. [Google Scholar] [CrossRef]
- Chen, B.; He, J.; Tian, K.; Qu, J.; Hong, L.; Lin, Q.; Yang, K.; Ma, L.; Xu, X. Research Progress on Detection of Pathogens in Medical Wastewater by Electrochemical Biosensors. Molecules 2024, 29, 3534. [Google Scholar] [CrossRef] [PubMed]
- Materon, E.M.; Ibáñez-Redín, G.; Joshi, N.; Gonçalves, D.; Oliveira, O.N.; Faria, R.C. Analytical Detection of Pesticides, Pollutants, and Pharmaceutical Waste in the Environment BT—Nanosensors for Environmental Applications; Kumar Tuteja, S., Arora, D., Dilbaghi, N., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 87–129. ISBN 978-3-030-38101-1. [Google Scholar]
- Justino, C.I.L.; Duarte, A.C.; Rocha-Santos, T.A.P. Recent Progress in Biosensors for Environmental Monitoring: A Review. Sensors 2017, 17, 2918. [Google Scholar] [CrossRef] [PubMed]
- Lino, A.; Cardoso, M.A.; Gonçalves, H.M.R.; Martins-Lopes, P. SARS-CoV-2 Detection Methods. Chemosensors 2022, 10, 221. [Google Scholar] [CrossRef]
- Mummareddy, S.; Pradhan, S.; Narasimhan, A.K.; Natarajan, A. On Demand Biosensors for Early Diagnosis of Cancer and Immune Checkpoints Blockade Therapy Monitoring from Liquid Biopsy. Biosensors 2021, 11, 500. [Google Scholar] [CrossRef]
- Polat, E.O.; Cetin, M.M.; Tabak, A.F.; Güven, E.B.; Uysal, B.Ö.; Arsan, T.; Kabbani, A.; Hamed, H.; Gül, S.B. Transducer Technologies for Biosensors and Their Wearable Applications. Biosensors 2022, 12, 385. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Wang, H. Bioreceptors as the Key Components for Electrochemical Biosensing in Medicine. Cell Rep. Phys. Sci. 2024, 5, 101801. [Google Scholar] [CrossRef]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for Biosensing Applications: A Review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef]
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent Advances in Nanomaterial-Based Biosensors for Antibiotics Detection. Biosens. Bioelectron. 2017, 91, 504–514. [Google Scholar] [CrossRef]
- Rahul, P.K.; Kafley, C.; A Sukumaran, R.; Kummari, S.; VVN, P.K.; Kotagiri, Y.G. Electrochemical Strip Sensor Based on a Graphene-Au-PEDOT Nanocomposite for Detection and Continuous Monitoring of Dacarbazine. ACS Appl. Nano Mater. 2023, 6, 16915–16926. [Google Scholar] [CrossRef]
- Khan, M.Q.; Khan, J.; Alvi, M.A.H.; Nawaz, H.; Fahad, M.; Umar, M. Nanomaterial-Based Sensors for Microbe Detection: A Review. Discov. Nano 2024, 19, 120. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, C.W.; Wang, D.; Wei, N. A Whole-Cell Biosensor for Point-of-Care Detection of Waterborne Bacterial Pathogens. ACS Synth. Biol. 2021, 10, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Tanniche, I.; Behkam, B. Engineered Live Bacteria as Disease Detection and Diagnosis Tools. J. Biol. Eng. 2023, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Fu, L.; Yin, C.; Liu, X.; Karimi-Maleh, H. The Role of Electrochemical Biosensors in SARS-CoV-2 Detection: A Bibliometrics-Based Analysis and Review. RSC Adv. 2022, 12, 22592–22607. [Google Scholar] [CrossRef] [PubMed]
- Maryam, S.; Ul Haq, I.; Yahya, G.; Ul Haq, M.; Algammal, A.M.; Saber, S.; Cavalu, S. COVID-19 Surveillance in Wastewater: An Epidemiological Tool for the Monitoring of SARS-CoV-2. Front. Cell Infect. Microbiol. 2023, 12, 978643. [Google Scholar] [CrossRef]
- Valerio, T.L.; Anastácio, R.; da Silva, S.S.; de Oliveira, C.C.; Vidotti, M. An Overview of Electrochemical Biosensors Used for COVID-19 Detection. Anal. Methods 2024, 16, 2164–2176. [Google Scholar] [CrossRef]
- Martínez-Periñán, E.; Palomares-Albarrán, M.; Toyos-Rodríguez, C.; Mateo-Martí, E.; Pariente, F.; de la Escosura-Muñiz, A.; Gutiérrez-Sánchez, C.; Revenga-Parra, M.; Lorenzo, E. Rapid SARS-CoV-2 Sensing through Oxygen Reduction Reaction Catalysed by Au@Pt/Au Core@shell Nanoparticles. Talanta 2024, 280, 126708. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, Z.; Zhou, J.; Jia, X.; Li, H.; Wang, X.; Chen, Y.; Sun, Z.; He, X.; Li, H.; et al. Nano-Biosensor for SARS-CoV-2/COVID-19 Detection: Methods, Mechanism and Interface Design. RSC Adv. 2023, 13, 17883–17906. [Google Scholar] [CrossRef]
- Malhotra, B.D.; Ali, M.A. Functionalized Carbon Nanomaterials for Biosensors in Nanomaterials in Biosensors: Fundamentals and Applications Nanomaterials for Biosensors; William Andrew Publishing: Norwich, NY, USA, 2018. [Google Scholar]
- Khaksarinejad, R.; Arabpour, Z.; RezaKhani, L.; Parvizpour, F.; Rasmi, Y. Biomarker Based Biosensors: An Opportunity for Diagnosis of COVID-19. Rev. Med. Virol. 2022, 32, e2356. [Google Scholar] [CrossRef]
- Saxena, K.; Chauhan, N.; Jain, U. Advances in Diagnosis of Helicobacter Pylori through Biosensors: Point of Care Devices. Anal. Biochem. 2021, 630, 114325. [Google Scholar] [CrossRef]
- Liu, X.; Dumitrescu, E.; Andreescu, S. Electrochemical Biosensors for Real-Time Monitoring of Reactive Oxygen and Nitrogen Species. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2015; Volume 1200, pp. 301–327. ISBN 9780841231009. [Google Scholar]
- Prabhakar, T.; Giaretta, J.; Zulli, R.; Rath, R.J.; Farajikhah, S.; Talebian, S.; Dehghani, F. Covalent Immobilization: A Review from an Enzyme Perspective. Chem. Eng. J. 2025, 503, 158054. [Google Scholar] [CrossRef]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization Strategies to Develop Enzymatic Biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Ben Amor, I.; Ahmed, S.; Zeghoud, S.; Hemmami, H. Nanocomposites Derived from Chitosan for Biosensor Applications. In Novel Bio-Nanocomposites for Biomedical Applications; Sharma, K., Tiwari, S.K., Kumar, V., Kalia, S., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 203–225. ISBN 978-3-031-69654-1. [Google Scholar]
- Devnani, H.; Sharma, C.; Jain, P. Immobilization Techniques in the Fabrication of Nanomaterial-Based Electrodes for Biosensing. In Nanomaterial-Modified Electrodes: Design and Applications; Khan, A.A.P., Kulkarni, R.M., Omaish Ansari, M., Asiri, A.M., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 135–156. ISBN 978-3-031-67176-0. [Google Scholar]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, L.; Deng, L.; Feng, Z.; Cao, Z.; Yin, Y. An Electrochemical Impedimetric Sensing Platform Based on a Peptide Aptamer Identified by High-Throughput Molecular Docking for Sensitive L-Arginine Detection. Bioelectrochemistry 2021, 137, 107634. [Google Scholar] [CrossRef] [PubMed]
- Kościelniak, P. Unified Principles of Univariate Analytical Calibration. TrAC—Trends Anal. Chem. 2022, 149, 116547. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Shahat, F.M.; Youssef, D.G.; Sayed, F.E.; Elbadry, A.M.M.; Fouad, F.A.; Mohammed, M.S.; Elsaadani, M. Fundamentals of Biosensors. In Handbook of Nanosensors; Ali, G.A.M., Chong, K.F., Makhlouf, A.S.H., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2024; pp. 265–301. ISBN 978-3-031-47180-3. [Google Scholar]
- Kaushal, J.B.; Raut, P.; Kumar, S. Organic Electronics in Biosensing: A Promising Frontier for Medical and Environmental Applications. Biosensors 2023, 13, 976. [Google Scholar] [CrossRef]
- Shanbhag, M.M.; Manasa, G.; Mascarenhas, R.J.; Mondal, K.; Shetti, N.P. Fundamentals of Bio-Electrochemical Sensing. Chem. Eng. J. Adv. 2023, 16, 100516. [Google Scholar] [CrossRef]
- Bucur, B.; Purcarea, C.; Andreescu, S.; Vasilescu, A. Addressing the Selectivity of Enzyme Biosensors: Solutions and Perspectives. Sensors 2021, 21, 3038. [Google Scholar] [CrossRef]
- Fan, Y.F.; Guo, Z.B.; Ge, G.B. Enzyme-Based Biosensors and Their Applications. Biosensors 2023, 13, 476. [Google Scholar] [CrossRef]
- Nigam, V.K.; Shukla, P. Enzyme Based Biosensors for Detection of Environmental Pollutants-A Review. J. Microbiol. Biotechnol. 2015, 25, 1773–1781. [Google Scholar] [CrossRef]
- Fernández-Fernández, M.; Sanromán, M.T.; Moldes, D. Recent Developments and Applications of Immobilized Laccase. Biotechnol. Adv. 2013, 31, 1808–1825. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P.; Gorton, L. Enzyme Based Amperometric Biosensors. Curr. Opin. Electrochem. 2018, 10, 157–173. [Google Scholar] [CrossRef]
- Sivasubramanian, P.D.; Kumar, M.; Chen, C.L.; Kiran-kumar, V.S.; Samuel, M.S.; Chang, J.H. A Review of Metal Nanomaterials-Based Electrochemical Biosensors for Environmental Wastewater Monitoring and Their Remediation. Environ. Nanotechnol. Monit. Manag. 2024, 22, 101023. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Marco, M.P.; Lopez De Alda, M.J.; Barceló, D. Biosensors for Environmental Applications: Future Development Trends. Pure Appl. Chem. 2004, 76, 723–752. [Google Scholar] [CrossRef]
- La Rosa, C.; Pariente, F.; Hernández, L.; Lorenzo, E. Determination of Organophosphorus and Carbamic Pesticides with an Acetylcholinesterase Amperometric Biosensor Using 4-Aminophenyl Acetate as Substrate. Anal. Chim. Acta 1994, 295, 273–282. [Google Scholar] [CrossRef]
- Abad, J.M.; Pariente, F.; Hernández, L.; Abruña, H.D.; Lorenzo, E. Determination of Organophosphorus and Carbamate Pesticides Using a Piezoelectric Biosensor. Anal. Chem. 1998, 70, 2848–2855. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device Integration of Electrochemical Biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef]
- Mool-am-kha, P.; Phetduang, S.; Phongsanam, N.; Surawanitkun, C.; Ngamdee, K.; Ngeontae, W. A Fluorescence Biosensor for Organophosphorus Pesticide Detection with a Portable Fluorescence Device-Based Smartphone. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 327, 125330. [Google Scholar] [CrossRef]
- Miglione, A.; Raucci, A.; Mancini, M.; Gioia, V.; Frugis, A.; Cinti, S. An Electrochemical Biosensor for On-Glove Application: Organophosphorus Pesticide Detection Directly on Fruit Peels. Talanta 2025, 283, 127093. [Google Scholar] [CrossRef]
- Kasuno, M.; Yamagata, Y.; Kawada, T.; Kayano, S.; Furumoto, T. Photoinduced Electron Transfer from Spinach Chloroplasts to Carbon Paste Electrodes through an Exogenous Electron Acceptor. Int. J. Electrochem. Sci. 2023, 18, 100265. [Google Scholar] [CrossRef]
- Koblížek, M.; Malý, J.; Masojídek, J.; Komenda, J.; Kučera, T.; Giardi, M.T.; Mattoo, A.K.; Pilloton, R. A Biosensor for the Detection of Triazine and Phenylurea Herbicides Designed Using Photosystem II Coupled to a Screen-Printed Electrode. Biotechnol. Bioeng. 2002, 78, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.F.; Li, M.G.; Gao, Y.C.; Fang, B. Amperometric Sensor Used for Determination of Thiocyanate with a Silver Nanoparticles Modified Electrode. Sensors 2004, 4, 147–155. [Google Scholar] [CrossRef]
- Malitesta, C.; Guascito, M.R. Heavy Metal Determination by Biosensors Based on Enzyme Immobilised by Electropolymerisation. Biosens. Bioelectron. 2005, 20, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Mir, T.u.G.; Wani, A.K.; Akhtar, N.; Katoch, V.; Shukla, S.; Kadam, U.S.; Hong, J.C. Advancing Biological Investigations Using Portable Sensors for Detection of Sensitive Samples. Heliyon 2023, 9, e22679. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Yang, M.; Guo, M.; Wu, Z.; Shen, G. Inhibitive Determination of Mercury Ion Using a Renewable Urea Biosensor Based on Self-Assembled Gold Nanoparticles. Sens. Actuators B Chem. 2006, 114, 1–8. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, M.; Singh, G. Recent Advancementsin Urea Biosensors for Biomedical Applications. IET Nanobiotechnol. 2021, 15, 358–379. [Google Scholar] [CrossRef]
- Malik, S.; Singh, J.; Goyat, R.; Saharan, Y.; Chaudhry, V.; Umar, A.; Ibrahim, A.A.; Akbar, S.; Ameen, S.; Baskoutas, S. Nanomaterials-Based Biosensor and Their Applications: A Review. Heliyon 2023, 9, e19929. [Google Scholar] [CrossRef]
- Kurup, C.P.; Ahmed, M.U. Nanozymes towards Personalized Diagnostics: A Recent Progress in Biosensing. Biosensors 2023, 13, 461. [Google Scholar] [CrossRef]
- Purcarea, C.; Ruginescu, R.; Banciu, R.M.; Vasilescu, A. Extremozyme-Based Biosensors for Environmental Pollution Monitoring: Recent Developments. Biosensors 2024, 14, 143. [Google Scholar] [CrossRef]
- Wen, W.; Yan, X.; Zhu, C.; Du, D.; Lin, Y. Recent Advances in Electrochemical Immunosensors. Anal. Chem. 2017, 89, 138–156. [Google Scholar] [CrossRef]
- Junior, B.B.; Rossignatti, B.C.; Mello, H.J.N.P.D.; Guidelli, É.J.; Mulato, M. Gold-Enhanced Aptasensors for Highly Sensitive Dengue Detection: A Cost-Effective Approach. Surf. Interfaces 2024, 55, 105282. [Google Scholar] [CrossRef]
- Sakalauskiene, L.; Popov, A.; Kausaite-Minkstimiene, A.; Ramanavicius, A.; Ramanaviciene, A. The Impact of Glucose Oxidase Immobilization on Dendritic Gold Nanostructures on the Performance of Glucose Biosensors. Biosensors 2022, 12, 320. [Google Scholar] [CrossRef]
- Drobysh, M.; Liustrovaite, V.; Baradoke, A.; Viter, R.; Chen, C.F.; Ramanavicius, A.; Ramanaviciene, A. Determination of RSpike Protein by Specific Antibodies with Screen-Printed Carbon Electrode Modified by Electrodeposited Gold Nanostructures. Biosensors 2022, 12, 593. [Google Scholar] [CrossRef] [PubMed]
- Grzibovskis, R.; Aizstrauts, A.; Pidluzhna, A.; Marcinskas, M.; Magomedov, A.; Karazhanov, S.; Malinauskas, T.; Getautis, V.; Vembris, A. Energy-Level Interpretation of Carbazole Derivatives in Self-Assembling Monolayer. Molecules 2024, 29, 1910. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Tran, H.V.; Lee, T.R. Self-Assembled Monolayer Coatings on Gold and Silica Surfaces for Antifouling Applications: A Review. Coatings 2022, 12, 1462. [Google Scholar] [CrossRef]
- Butterworth, A.; Blues, E.; Williamson, P.; Cardona, M.; Gray, L.; Corrigan, D.K. SAM Composition and Electrode Roughness Affect Performance of a DNA Biosensor for Antibiotic Resistance. Biosensors 2019, 9, 22. [Google Scholar] [CrossRef]
- Sami, A.S.; Barani, S.S.I.; Rashid, R.F.; Zeebaree, S.Y.S.; Zeebaree, A.Y.S.; Zebari, O.I.H.; Ali, A.M.; Ilyas, K.S.; Biso, F.H.; Issa, M.M. Novel Biosensor for Highly Sensitive Detection of Serum Albumin in Artificial Human Urine Using CuNPs@AG. Sens. Biosensing Res. 2024, 43, 100633. [Google Scholar] [CrossRef]
- Lundin, P.M.; Fiser, B.L.; Blackledge, M.S.; Pickett, H.L.; Copeland, A.L. Functionalized Self-Assembled Monolayers: Versatile Strategies to Combat Bacterial Biofilm Formation. Pharmaceutics 2022, 14, 1613. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, Y.; Fan, X.; Zhou, J.; Lv, Y.; Xia, N. Electrochemical Immunosensors Based on the Solubility Difference of Electroactive Probe and the Dual Signal Amplification of Nanocarrier plus Redox Cycling. Bioelectrochemistry 2025, 162, 108858. [Google Scholar] [CrossRef]
- Ben Messaoud, N.; Barreiros dos Santos, M.; Trocado, V.; Nogueira-Silva, C.; Queirós, R. A Novel Label-Free Electrochemical Immunosensor for Detection of Surfactant Protein B in Amniotic Fluid. Talanta 2023, 251, 123744. [Google Scholar] [CrossRef]
- Ferreira, M.D.P.; Yamada-Ogatta, S.F.; Teixeira Tarley, C.R. Electrochemical and Bioelectrochemical Sensing Platforms for Diagnostics of COVID-19. Biosensors 2023, 13, 336. [Google Scholar] [CrossRef] [PubMed]
- Ben Messaoud, N.; dos Santos, M.B.; Vieira, A.; Garrido-Maestu, A.; Espiña, B.; Queirós, R.B. A Novel Portable Label-Free Electrochemical Immunosensor for Ultrasensitive Detection of Aeromonas Salmonicida in Aquaculture Seawater. Anal. Bioanal. Chem. 2022, 414, 6591–6600. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.; Antonacci, A.; Moccia, M.; Frisulli, V.; De Felice, M.; De Falco, M.; Scognamiglio, V. CRISPR-Cas Assisted Diagnostics: A Broad Application Biosensing Approach. TrAC—Trends Anal. Chem. 2023, 162, 117028. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Huang, Y.; Du, Y.; Zhang, Y.; Cui, Y.; Kong, D.M. Development of the DNA-Based Biosensors for High Performance in Detection of Molecular Biomarkers: More Rapid, Sensitive, and Universal. Biosens. Bioelectron. 2022, 197, 113739. [Google Scholar] [CrossRef]
- Gavrilaș, S.; Ursachi, C.Ș.; Perța-Crișan, S.; Munteanu, F.D. Recent Trends in Biosensors for Environmental Quality Monitoring. Sensors 2022, 22, 1513. [Google Scholar] [CrossRef]
- Zhou, W.; Saran, R.; Liu, J. Metal Sensing by DNA. Chem. Rev. 2017, 117, 8272–8325. [Google Scholar] [CrossRef]
- Bi, S.; Yue, S.; Zhang, S. Hybridization Chain Reaction: A Versatile Molecular Tool for Biosensing, Bioimaging, and Biomedicine. Chem. Soc. Rev. 2017, 46, 4281–4298. [Google Scholar] [CrossRef]
- Voon, C.H.; Yusop, N.M.; Khor, S.M. The State-of-the-Art in Bioluminescent Whole-Cell Biosensor Technology for Detecting Various Organic Compounds in Oil and Grease Content in Wastewater: From the Lab to the Field. Talanta 2022, 241, 123271. [Google Scholar] [CrossRef]
- Atkinson, J.T.; Su, L.; Zhang, X.; Bennett, G.N.; Silberg, J.J.; Ajo-Franklin, C.M. Real-Time Bioelectronic Sensing of Environmental Contaminants. Nature 2022, 611, 548–553. [Google Scholar] [CrossRef]
- Gui, Q.; Lawson, T.; Shan, S.; Yan, L.; Liu, Y. The Application of Whole Cell-Based Biosensors for Use in Environmental Analysis and in Medical Diagnostics. Sensors 2017, 17, 1623. [Google Scholar] [CrossRef]
- Dey, S.; Baba, S.A.; Bhatt, A.; Dhyani, R.; Navani, N.K. Transcription Factor Based Whole-Cell Biosensor for Specific and Sensitive Detection of Sodium Dodecyl Sulfate. Biosens. Bioelectron. 2020, 170, 112659. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Hu, J.; Lu, F. Aptamers Used for Biosensors and Targeted Therapy. Biomed. Pharmacother. 2020, 132, 110902. [Google Scholar] [CrossRef]
- Léguillier, V.; Heddi, B.; Vidic, J. Recent Advances in Aptamer-Based Biosensors for Bacterial Detection. Biosensors 2024, 14, 210. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lu, C.; Lin, X.; Kang, L.; Duan, N.; Wang, Z.; Liu, C.; Wu, S. A Portable Microfluidic Chip-Based Fluorescent and Colorimetric Aptasensor Combining Recombinase Polymerase Amplification for Bovine Pregnancy-Associated Glycoproteins Detection. Biosens. Bioelectron. 2025, 270, 116981. [Google Scholar] [CrossRef]
- Shaukat, A.; Chrouda, A.; Sadaf, S.; Alhamlan, F.; Eissa, S.; Zourob, M. Cell-SELEX for Aptamer Discovery and Its Utilization in Constructing Electrochemical Biosensor for Rapid and Highly Sensitive Detection of Legionella Pneumophila Serogroup 1. Sci. Rep. 2024, 14, 14132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ning, Z.; Zhang, Y.; Lin, X.; Duan, N.; Wang, Z.; Wu, S. Construction of a Microfluidic SELEX Platform for Efficient Screening of Advanced Glycation End Products Aptamer. Biosens. Bioelectron. 2025, 271, 117038. [Google Scholar] [CrossRef]
- Liu, L.; Han, Z.; An, F.; Gong, X.; Zhao, C.; Zheng, W.; Mei, L.; Zhou, Q. Aptamer-Based Biosensors for the Diagnosis of Sepsis. J. Nanobiotechnol. 2021, 19, 216. [Google Scholar] [CrossRef]
- Zandieh, M.; Luo, X.; Zhao, Y.; Feng, C.; Liu, J. Selection of Plastic-Binding DNA Aptamers for Microplastics Detection. Angew. Chem. Int. Ed. 2024, 64, e202421438. [Google Scholar] [CrossRef]
- Feng, T.; Huang, Y.; Liu, X.; Yan, S. Sensitive and Selective Fluorescence Detection of Acetamiprid Using Oxidized Single-Walled Carbon Nanohorns and Cryonase-Assisted Signal Amplification. J. Photochem. Photobiol. A Chem. 2025, 459, 116025. [Google Scholar] [CrossRef]
- Rai, A.; Ferrão, R.; Palma, P.; Patricio, T.; Parreira, P.; Anes, E.; Tonda-Turo, C.; Martins, M.C.L.; Alves, N.; Ferreira, L. Antimicrobial Peptide-Based Materials: Opportunities and Challenges. J. Mater. Chem. B 2022, 10, 2384–2429. [Google Scholar] [CrossRef]
- Gupta, S.; Kakkar, V. DARPin Based GMR Biosensor for the Detection of ESAT-6 Tuberculosis Protein. Tuberculosis 2019, 118, 101852. [Google Scholar] [CrossRef] [PubMed]
- Cieplak, M.; Kutner, W. Artificial Biosensors: How Can Molecular Imprinting Mimic Biorecognition? Trends Biotechnol. 2016, 34, 922–941. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C.; Kaplan, S.; Matthews, E.C.; Edwards, P.K.; Helmsworth, J.A. Monitor and Control of Blood Oxygen Tension and Ph During Total Body Perfusion. J. Thorac. Surg. 1958, 36, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C., Jr.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Turner, A.P.F. Biosensors: Fundamentals and Applications—Historic Book Now Open Access. Biosens. Bioelectron. 2015, 65, A1. [Google Scholar] [CrossRef]
- Newman, J.D.; Setford, S.J. Enzymatic Biosensors. Mol. Biotechnol. 2006, 32, 249–268. [Google Scholar] [CrossRef]
- Kizilkurtlu, A.A.; Demirbas, E.; Agel, H.E. Electrochemical Aptasensors for Pathogenic Detection toward Point-of-Care Diagnostics. Biotechnol. Appl. Biochem. 2023, 70, 1460–1479. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Wu, L.; Huang, Y.; Zhang, Y.; Zhu, M.; Wang, Y.; Zhu, Z.; Yang, C. Trends in Miniaturized Biosensors for Point-of-Care Testing. TrAC—Trends Anal. Chem. 2020, 122, 115701. [Google Scholar] [CrossRef]
- Ainla, A.; Mousavi, M.P.S.; Tsaloglou, M.N.; Redston, J.; Bell, J.G.; Fernández-Abedul, M.T.; Whitesides, G.M. Open-Source Potentiostat for Wireless Electrochemical Detection with Smartphones. Anal. Chem. 2018, 90, 6240–6246. [Google Scholar] [CrossRef]
- Schachinger, F.; Chang, H.; Scheiblbrandner, S.; Ludwig, R. Amperometric Biosensors Based on Direct Electron Transfer Enzymes. Molecules 2021, 26, 4525. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, T.; Rong, S.; Zeng, D.; Yu, H.; Zhang, Z.; Chang, D.; Pan, H. A Sensitive Amperometric AChE-Biosensor for Organophosphate Pesticides Detection Based on Conjugated Polymer and Ag-RGO-NH 2 Nanocomposite. Bioelectrochemistry 2019, 127, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, X.; Liu, G.; Yang, L.; Gao, F. Core-Shell ZnO@CoO Nitrogen Doped Nano-Composites as Highly Sensitive Electrochemical Sensor for Organophosphate Pesticides Detection. Anal. Biochem. 2024, 686, 115422. [Google Scholar] [CrossRef] [PubMed]
- Kızılkurtlu, A.A.; Agel, E.; Şenocak, A.; Demirbas, E. Development of CoFe2O4/SWCNT Modified Gold Nanoparticle Coated LAMP Incorporated Electrochemical DNA Biosensor for Salmonella in Contaminated Water Filtered Samples. Microchem. J. 2025, 208, 112412. [Google Scholar] [CrossRef]
- Dabhade, A.H.; Paramasivan, B.; Kumawat, A.S.; Saha, B. Miniature Lab-Made Electrochemical Biosensor: A Promising Sensing Kit for Rapid Detection of E. Coli in Water, Urine and Milk. Talanta 2025, 285, 127306. [Google Scholar] [CrossRef]
- Kumar, M.S.; Nandeshwar, R.; Lad, S.B.; Megha, K.; Mangat, M.; Butterworth, A.; Knapp, C.W.; Knapp, M.; Hoskisson, P.A.; Corrigan, D.K.; et al. Electrochemical Sensing of SARS-CoV-2 Amplicons with PCB Electrodes. Sens. Actuators B Chem. 2021, 343, 130169. [Google Scholar] [CrossRef]
- Del Torno-De Román, L.; Asunción Alonso-Lomillo, M.; Domínguez-Renedo, O.; Julia Arcos-Martínez, M. Tyrosinase Based Biosensor for the Electrochemical Determination of Sulfamethoxazole. Sens. Actuators B Chem. 2016, 227, 48–53. [Google Scholar] [CrossRef]
- Wang, X.; Mohammadzadehsaliani, S.; Vafaei, S.; Ahmadi, L.; Iqbal, A.; Alreda, B.A.; Talib Al-Naqeeb, B.Z.; Kheradjoo, H. Synthesis and Electrochemical Study of Enzymatic Graphene Oxide-Based Nanocomposite as Stable Biosensor for Determination of Bevacizumab as a Medicine in Colorectal Cancer in Human Serum and Wastewater Fluids. Chemosphere 2023, 336, 139012. [Google Scholar] [CrossRef]
- Al borhani, W.; Rhouati, A.; Cialla-May, D.; Popp, J.; Zourob, M. Multiplex Electrochemical Aptasensor for the Simultaneous Detection of Linomycin and Neomycin Antibiotics. Talanta 2025, 282, 126922. [Google Scholar] [CrossRef]
- Yue, P.; Ruiyi, L.; Xie, W.; Yirui, S.; Zaijun, L. Electrochemical Biosensor Based on Au5Ir@graphene Quantum Dot Nanocomposite and DNA Walker for Detection of Atrazine in Environmental Water with Extremely High Sensitivity and Selectivity. Sens. Actuators B Chem. 2025, 423, 136873. [Google Scholar] [CrossRef]
- Thet Tun, W.S.; Saenchoopa, A.; Daduang, S.; Daduang, J.; Kulchat, S.; Patramanon, R. Electrochemical Biosensor Based on Cellulose Nanofibers/Graphene Oxide and Acetylcholinesterase for the Detection of Chlorpyrifos Pesticide in Water and Fruit Juice. RSC Adv. 2023, 13, 9603–9614. [Google Scholar] [CrossRef]
- Suwannachat, J.; Saenchoopa, A.; Tun, W.S.T.; Patramanon, R.; Daduang, S.; Daduang, J.; Kulchat, S. An Electrochemical AChE-Based Biosensor for Organophosphate Pesticides Using a Modified CuNWs/RGO Nanocomposite on a Screen-Printed Carbon Electrode. Food Chem. 2024, 434, 137431. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cheong, L.; Li, Y.; Lou, Y.; Fu, S. Sensitive and Rapid Electrochemical Detection of Nitroaromatic Pesticides by the Ordered Macro-Microporous ZIF-8 Immobilized Lipase-Based Sensor. Microchem. J. 2023, 193, 109007. [Google Scholar] [CrossRef]
- Gurban, A.M.; Rotariu, L.; Baibarac, M.; Baltog, I.; Bala, C. Sensitive Detection of Endocrine Disrupters Using Ionic Liquid—Single Walled Carbon Nanotubes Modified Screen-Printed Based Biosensors. Talanta 2011, 85, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhao, G.; Shi, H.; Liu, M. A Simple and Label-Free Aptasensor Based on Nickel Hexacyanoferrate Nanoparticles as Signal Probe for Highly Sensitive Detection of 17β-Estradiol. Biosens. Bioelectron. 2015, 68, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Nomngongo, P.N.; Catherine Ngila, J.; Msagati, T.A.M.; Gumbi, B.P.; Iwuoha, E.I. Determination of Selected Persistent Organic Pollutants in Wastewater from Landfill Leachates, Using an Amperometric Biosensor. Phys. Chem. Earth 2012, 50–52, 252–261. [Google Scholar] [CrossRef]
- Sundeep, D.; Varadharaj, E.K.; Umadevi, K.; Jhansi, R. Review—Role of Nanomaterials in Screenprinted Electrochemical Biosensors for Detection of COVID-19 and for Post-Covid Syndromes. ECS Adv. 2023, 2, 016502. [Google Scholar] [CrossRef]
- Razmi, N.; Hasanzadeh, M.; Willander, M.; Nur, O. Recent Progress on the Electrochemical Biosensing of Escherichia coli O157:H7: Material and Methods Overview. Biosensors 2020, 10, 54. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent Advances in Electrochemical Biosensors: Applications, Challenges, and Future Scope. Biosensors 2021, 11, 336. [Google Scholar] [CrossRef]
- Coppedè, N.; Giannetto, M.; Villani, M.; Lucchini, V.; Battista, E.; Careri, M.; Zappettini, A. Ion Selective Textile Organic Electrochemical Transistor for Wearable Sweat Monitoring. Org. Electron. 2020, 78, 105579. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Orooji, Y.; Karimi, F.; Alizadeh, M.; Baghayeri, M.; Rouhi, J.; Tajik, S.; Beitollahi, H.; Agarwal, S.; Gupta, V.K.; et al. A Critical Review on the Use of Potentiometric Based Biosensors for Biomarkers Detection. Biosens. Bioelectron. 2021, 184, 113252. [Google Scholar] [CrossRef]
- Shaibani, P.M.; Etayash, H.; Jiang, K.; Sohrabi, A.; Hassanpourfard, M.; Naicker, S.; Sadrzadeh, M.; Thundat, T. Portable Nanofiber-Light Addressable Potentiometric Sensor for Rapid Escherichia coli Detection in Orange Juice. ACS Sens. 2018, 3, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jiang, T.; Qin, W. Potentiometric Aptasensing of Escherichia coli Based on Electrogenerated Chemiluminescence as a Highly Sensitive Readout. Biosens. Bioelectron. 2022, 200, 113923. [Google Scholar] [CrossRef] [PubMed]
- Antonacci, A.; Arduini, F.; Attaallah, R.; Amine, A.; Giardi, M.T.; Scognamiglio, V. A Proof-of-Concept Electrochemical Cytosensor Based on Chlamydomonas Reinhardtii Functionalized Carbon Black Screen-Printed Electrodes: Detection of Escherichia coli in Wastewater as a Case Study. Biosensors 2022, 12, 401. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fu, Y.; Fang, W.; Li, Y. Electrochemical Impedance Immunosensor Based on Self-Assembled Monolayers for Rapid Detection of Escherichia coli O157:H7 with Signal Amplification Using Lectin. Sensors 2015, 15, 19212–19224. [Google Scholar] [CrossRef]
- Perumal, V.; Hashim, U. Advances in Biosensors: Principle, Architecture and Applications. J. Appl. Biomed. 2014, 12, 1–15. [Google Scholar] [CrossRef]
- Barreiros dos Santos, M.; Azevedo, S.; Agusil, J.P.; Prieto-Simón, B.; Sporer, C.; Torrents, E.; Juárez, A.; Teixeira, V.; Samitier, J. Label-Free ITO-Based Immunosensor for the Detection of Very Low Concentrations of Pathogenic Bacteria. Bioelectrochemistry 2015, 101, 146–152. [Google Scholar] [CrossRef]
- Yao, L.; Wang, L.; Huang, F.; Cai, G.; Xi, X.; Lin, J. A Microfluidic Impedance Biosensor Based on Immunomagnetic Separation and Urease Catalysis for Continuous-Flow Detection of E. coli O157:H7. Sens. Actuators B Chem. 2018, 259, 1013–1021. [Google Scholar] [CrossRef]
- Cimafonte, M.; Fulgione, A.; Gaglione, R.; Papaianni, M.; Capparelli, R.; Arciello, A.; Censi, S.B.; Borriello, G.; Velotta, R.; Ventura, B. Della Screen Printed Based Impedimetric Immunosensor for Rapid Detection of Escherichia coli in Drinking Water. Sensors 2020, 20, 274. [Google Scholar] [CrossRef]
- Zare, A.R.; Ensafi, A.A.; Rezaei, B. An Impedimetric Biosensor Based on Poly(l-Lysine)-Decorated Multiwall Carbon Nanotubes for the Determination of Diazinon in Water and Fruits. J. Iran. Chem. Soc. 2019, 16, 2777–2785. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Fallahi Nezhad, F.; Akmal, M.H.; Althomali, R.H.; Sharma, N.; Rahmanian, V.; Azhdari, R.; Gholami, A.; Rahman, M.M.; Chiang, W.H. Recent Advances and Synergistic Effect of Bioactive Zeolite Imidazolate Frameworks (ZIFs) for Biosensing Applications. Talanta 2024, 275, 126097. [Google Scholar] [CrossRef] [PubMed]
- Berketa, K.; Saiapina, O.; Fayura, L.; Sibirny, A.; Dzyadevych, S.; Soldatkin, O. Novel Highly Sensitive Conductometric Biosensor Based on Arginine Deiminase from Mycoplasma Hominis for Determination of Arginine. Sens. Actuators B Chem. 2022, 367, 132023. [Google Scholar] [CrossRef]
- Uda, M.N.A.; Dafhalla, A.K.Y.; Dhahi, T.S.; Adam, T.; Gopinath, S.C.B.; Uda, M.N.A.; Mohammed, M.; Parmin, N.A.; Ibrahim, N.H.; Hashim, U. Conductometric Immunosensor for Specific Escherichia coli O157:H7 Detection on Chemically Funcationalizaed Interdigitated Aptasensor. Heliyon 2024, 10, e26988. [Google Scholar] [CrossRef]
- Hosseini, S.N.; Das, P.S.; Lazarjan, V.K.; Gagnon-Turcotte, G.; Bouzid, K.; Gosselin, B. Recent Advances in CMOS Electrochemical Biosensor Design for Microbial Monitoring: Review and Design Methodology. IEEE Trans. Biomed. Circuits Syst. 2023, 17, 202–228. [Google Scholar] [CrossRef]
- Yoo, J.; Jeong, H.; Park, S.K.; Park, S.; Lee, J.S. Interdigitated Electrode Biosensor Based on Plasma-Deposited Tio2 Nanoparticles for Detecting Dna. Biosensors 2021, 11, 212. [Google Scholar] [CrossRef]
- Chio, W.-I.K.; Xie, H.; Zhang, Y.; Lan, Y.; Lee, T.-C. SERS Biosensors Based on Cucurbituril-Mediated Nanoaggregates for Wastewater-Based Epidemiology. TrAC Trends Anal. Chem. 2022, 146, 116485. [Google Scholar] [CrossRef]
- Mao, K.; Yang, Z.; Zhang, H.; Li, X.; Cooper, J.M. Paper-Based Nanosensors to Evaluate Community-Wide Illicit Drug Use for Wastewater-Based Epidemiology. Water Res. 2021, 189, 116559. [Google Scholar] [CrossRef]
- Zhao, B.; Yang, T.; Qu, Y.; Mills, A.J.; Zhang, G.; He, L. Rapid Capture and SERS Detection of Triclosan Using a Silver Nanoparticle Core—Protein Satellite Substrate. Sci. Total Environ. 2020, 716, 137097. [Google Scholar] [CrossRef]
- Zhu, A.; Ali, S.; Wang, Z.; Jiao, T.; Ouyang, Q.; Chen, Q. Polydimethylsiloxane Assisted Surface-Enhanced Raman Spectroscopy for Staphylococcus Aureus Detection Based on Aptamer Recognition. Microchem. J. 2023, 193, 109172. [Google Scholar] [CrossRef]
- Herrera-Domínguez, M.; Lambert, A.S.; Morales-Luna, G.; Pisano, E.; Aguilar-Hernandez, I.; Mahlknecht, J.; Cheng, Q.; Ornelas-Soto, N. Development of a Surface Plasmon Resonance Based Immunosensor for Diclofenac Quantification in Water. Chemosphere 2023, 336, 139156. [Google Scholar] [CrossRef]
- Mazouzi, Y.; Miche, A.; Loiseau, A.; Beito, B.; Méthivier, C.; Knopp, D.; Salmain, M.; Boujday, S. Design and Analytical Performances of a Diclofenac Biosensor for Water Resources Monitoring. ACS Sens. 2021, 6, 3485–3493. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Chen, X.; Chen, J.; Zhang, Y.; Wang, X.; Wu, Z.; Zhou, N. In Vitro Selection and Engineering Azithromycin-Specific Aptamers and Construction of a Ratiometric Fluorescent Aptasensor for Sensitive Detection of Azithromycin. Sens. Actuators B Chem. 2024, 411, 135789. [Google Scholar] [CrossRef]
- Nguyen, D.K.; Jang, C.H. Ultrasensitive Colorimetric Detection of Amoxicillin Based on Tris-HCl-Induced Aggregation of Gold Nanoparticles. Anal. Biochem. 2022, 645, 114634. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, Y.; Wang, X.; Zhou, X.; Qiu, Y.; Qin, W.; ShenTu, X.; Wang, S.; Yu, X.; Ye, Z. Dosage-Sensitive and Simultaneous Detection of Multiple Small-Molecule Pollutants in Environmental Water and Agriproducts Using Portable SERS-Based Lateral Flow Immunosensor. Sci. Total Environ. 2024, 912, 169440. [Google Scholar] [CrossRef]
- Tun, W.S.T.; Talodthaisong, C.; Daduang, S.; Daduang, J.; Rongchai, K.; Patramanon, R.; Kulchat, S. A Machine Learning Colorimetric Biosensor Based on Acetylcholinesterase and Silver Nanoparticles for the Detection of Dichlorvos Pesticides. Mater. Chem. Front. 2022, 6, 1487–1498. [Google Scholar] [CrossRef]
- Sunantha, G.; Vasudevan, N. A Method for Detecting Perfluorooctanoic Acid and Perfluorooctane Sulfonate in Water Samples Using Genetically Engineered Bacterial Biosensor. Sci. Total Environ. 2021, 759, 143544. [Google Scholar] [CrossRef]
- Quintanilla-Villanueva, G.E.; Luna-Moreno, D.; Blanco-Gámez, E.A.; Rodríguez-Delgado, J.M.; Villarreal-Chiu, J.F.; Rodríguez-Delgado, M.M. A Novel Enzyme-Based SPR Strategy for Detection of the Antimicrobial Agent Chlorophene. Biosensors 2021, 11, 43. [Google Scholar] [CrossRef]
- Seggio, M.; Arcadio, F.; Cennamo, N.; Zeni, L.; Bossi, A.M. A Plasmonic Gold Nano-Surface Functionalized with the Estrogen Receptor for Fast and Highly Sensitive Detection of Nanoplastics. Talanta 2024, 267, 125211. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, Y.; Hong, S. Quantitatively Sensitive and Resolution-Enhancing Strategies in Optical Signal Conversion of SPR Images Using Extensive Angle-Scanning and Image-Reconstruction. Sens. Actuators B Chem. 2024, 400, 134897. [Google Scholar] [CrossRef]
- Matsunaga, R.; Ujiie, K.; Inagaki, M.; Fernández Pérez, J.; Yasuda, Y.; Mimasu, S.; Soga, S.; Tsumoto, K. High-Throughput Analysis System of Interaction Kinetics for Data-Driven Antibody Design. Sci. Rep. 2023, 13, 19417. [Google Scholar] [CrossRef]
- Herrera-Domínguez, M.; Morales-Luna, G.; Mahlknecht, J.; Cheng, Q.; Aguilar-Hernández, I.; Ornelas-Soto, N. Optical Biosensors and Their Applications for the Detection of Water Pollutants. Biosensors 2023, 13, 370. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Dong, X.; Hamed, E.M.; Jiang, H.; Cao, W.; Yau Li, S.F.; Wang, Z. Ratiometric Fluorescence Aptasensor of Allergen Protein Based on Multivalent Aptamer-Encoded DNA Flowers as Fluorescence Resonance Energy Transfer Platform. Anal. Chem. 2024, 96, 6947–6957. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.A.M.; Chong, K.F.; Makhlouf, A.S.H. Handbook of Nanosensors: Materials and Technological Applications; Springer Nature: Berlin/Heidelberg, Germany, 2024; ISBN 978-303147180-3; 978-303147179-7. [Google Scholar]
- Magar, H.S.; Hassan, R.Y.A. Nanoelectrochemical Biosensors: Principles, Architectures Applications, and Future Directions. In Handbook of Nanosensors: Materials and Technological Applications; Ali, G.A.M., Chong, K.F., Makhlouf, A.S.H., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 1–26. ISBN 978-3-031-16338-8. [Google Scholar]
- Pohanka, M. Overview of Piezoelectric Biosensors, Immunosensors and DNA Sensors and Their Applications. Materials 2018, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Zhang, H.; Pan, Y.; Yang, Z. Biosensors for Wastewater-Based Epidemiology for Monitoring Public Health. Water Res. 2021, 191, 116787. [Google Scholar] [CrossRef]
- Yildirim-Tirgil, N.; Lee, J.; Cho, H.; Lee, H.; Somu, S.; Busnaina, A.; Gu, A.Z. A SWCNT Based Aptasensor System for Antibiotic Oxytetracycline Detection in Water Samples. Anal. Methods 2019, 11, 2692–2699. [Google Scholar] [CrossRef]
- Hamami, M.; Bouaziz, M.; Raouafi, N.; Bendounan, A.; Korri-Youssoufi, H. MoS2/PPy Nanocomposite as a Transducer for Electrochemical Aptasensor of Ampicillin in River Water. Biosensors 2021, 11, 311. [Google Scholar] [CrossRef]
- Wei, X.; Sun, Y.; Luo, Y.; Shu, R.; Lin, H.; Xu, D. Electrochemical Biosensor Bi2O3@Au@Apta Based on Bi2O3 Nanofibers for Sensitive Detection of Ampicillin. Biochem. Eng. J. 2023, 200, 109107. [Google Scholar] [CrossRef]
- Bao, Y.; Cai, K.; Liu, Y.; Li, B. Aptamer-Based Antibiotic Electrochemical Detection Platform Using Portable Plastic Gold Electrode and Multichannel Chip. Chin. J. Chem. 2024, 42, 171–176. [Google Scholar] [CrossRef]
- Reinemann, C.; Freiin von Fritsch, U.; Rudolph, S.; Strehlitz, B. Generation and Characterization of Quinolone-Specific DNA Aptamers Suitable for Water Monitoring. Biosens. Bioelectron. 2016, 77, 1039–1047. [Google Scholar] [CrossRef]
- Asmare, M.M.; Krishnaraj, C.; Radhakrishnan, S.; Kim, B.-S.; Yoon, J.-S.; Yun, S.-I. In Silico Modelling of Ciprofloxacin Specific Aptamer for the Development of High-Performance Biosensor. J. Mol. Graph. Model. 2024, 130, 108787. [Google Scholar] [CrossRef]
- Huang, S.; Gan, N.; Li, T.; Zhou, Y.; Cao, Y.; Dong, Y. Electrochemical Aptasensor for Multi-Antibiotics Detection Based on Endonuclease and Exonuclease Assisted Dual Recycling Amplification Strategy. Talanta 2018, 179, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Bor, G.; Man, E.; Ugurlu, O.; Ceylan, A.E.; Balaban, S.; Durmus, C.; Pinar Gumus, Z.; Evran, S.; Timur, S. In Vitro Selection of Aptamer for Imidacloprid Recognition as Model Analyte and Construction of a Water Analysis Platform. Electroanalysis 2020, 32, 1922–1929. [Google Scholar] [CrossRef]
- Phopin, K.; Ruankham, W.; Dave, J.; Rukkhapiban, P.; Nuttavuttisit, C.; Hongoeb, J.; Zine, N.; Errachid, A.; Tantimongcolwat, T. Portable Label-Free Electrochemical Aptasensor for Sensitive Detection of Paraquat Herbicide Mediated by Oxygen Reduction Reaction. Talanta 2025, 281, 126841. [Google Scholar] [CrossRef] [PubMed]
- Madianos, L.; Tsekenis, G.; Skotadis, E.; Patsiouras, L.; Tsoukalas, D. A Highly Sensitive Impedimetric Aptasensor for the Selective Detection of Acetamiprid and Atrazine Based on Microwires Formed by Platinum Nanoparticles. Biosens. Bioelectron. 2018, 101, 268–274. [Google Scholar] [CrossRef]
- Gaviria-Arroyave, M.I.; Arango, J.P.; Barrientos Urdinola, K.; Cano, J.B.; Peñuela Mesa, G.A. Fluorescent Nanostructured Carbon Dot-Aptasensor for Chlorpyrifos Detection: Elucidating the Interaction Mechanism for an Environmentally Hazardous Pollutant. Anal. Chim. Acta 2023, 1278, 341711. [Google Scholar] [CrossRef]
- Khansili, N.; Rattu, G.; Krishna, P.M. Label-Free Optical Biosensors for Food and Biological Sensor Applications. Sens. Actuators B Chem. 2018, 265, 35–49. [Google Scholar] [CrossRef]
- Irkham, I.; Ibrahim, A.U.; Pwavodi, P.C.; Al-Turjman, F.; Hartati, Y.W. Smart Graphene-Based Electrochemical Nanobiosensor for Clinical Diagnosis: Review. Sensors 2023, 23, 2240. [Google Scholar] [CrossRef]
- Majdinasab, M.; Mitsubayashi, K.; Marty, J.L. Optical and Electrochemical Sensors and Biosensors for the Detection of Quinolones. Trends Biotechnol. 2019, 37, 898–915. [Google Scholar] [CrossRef]
- Talari, F.F.; Bozorg, A.; Faridbod, F.; Vossoughi, M. A Novel Sensitive Aptamer-Based Nanosensor Using RGQDs and MWCNTs for Rapid Detection of Diazinon Pesticide. J. Environ. Chem. Eng. 2021, 9, 104878. [Google Scholar] [CrossRef]
- Hatamluyi, B.; Sadeghzadeh, S.; Rezayi, M.; Tavakoly Sany, S.B. Diazinon Electrochemical Biosensor Mediated by Aptamer and Nanoscale Porous Carbon Derived from ZIF-8. Sens. Actuators B Chem. 2023, 381, 133424. [Google Scholar] [CrossRef]
- Roushani, M.; Shahdost-fard, F. Applicability of AuNPs@N-GQDs Nanocomposite in the Modeling of the Amplified Electrochemical Ibuprofen Aptasensing Assay by Monitoring of Riboflavin. Bioelectrochemistry 2019, 126, 38–47. [Google Scholar] [CrossRef]
- Gharib, G.; Bütün, İ.; Muganlı, Z.; Kozalak, G.; Namlı, İ.; Sarraf, S.S.; Ahmadi, V.E.; Toyran, E.; van Wijnen, A.J.; Koşar, A. Biomedical Applications of Microfluidic Devices: A Review. Biosensors 2022, 12, 1023. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, G.; Samadi Pakchin, P.; Shamloo, A.; Mota, A.; de la Guardia, M.; Omidian, H.; Omidi, Y. Label-Free Electrochemical Microfluidic Biosensors: Futuristic Point-of-Care Analytical Devices for Monitoring Diseases. Microchim. Acta 2022, 189, 252. [Google Scholar] [CrossRef] [PubMed]
- Lonchamps, P.-L.; He, Y.; Wang, K.; Lu, X. Detection of Pathogens in Foods Using Microfluidic “Lab-on-Chip”: A Mini Review. J. Agric. Food Res. 2022, 10, 100430. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Jiao, S.; Li, Y.; Zhou, Y.; Zhang, X.; Maryam, B.; Liu, X. Microfluidic Sensors for the Detection of Emerging Contaminants in Water: A Review. Sci. Total Environ. 2024, 929, 172734. [Google Scholar] [CrossRef]
- Wang, J.; Pumera, M.; Chatrathi, M.P.; Escarpa, A.; Konrad, R.; Griebel, A.; Drner, W.; Lwe, H. Towards Disposable Lab-on-a-Chip: Poly(Methylmethacrylate) Microchip Electrophoresis Device with Electrochemical Detection. Electrophoresis 2002, 23, 596–601. [Google Scholar] [CrossRef]
- Javanifar, R.; Ghorbanpoor, H.; Avci, H. Improved Bacterial Filtration and DNA Retention by a Novel Microfiltration Process Including Composite Membranes. Sep. Purif. Technol. 2025, 357, 130114. [Google Scholar] [CrossRef]
- Saravanakumar, S.M.; Cicek, P.V. Microfluidic Mixing: A Physics-Oriented Review. Micromachines 2023, 14, 1827. [Google Scholar] [CrossRef]
- Li, N.; Shen, M.; Xu, Y. A Portable Microfluidic System for Point-of-Care Detection of Multiple Protein Biomarkers. Micromachines 2021, 12, 347. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Biosensors and Microfluidic Biosensors: From Fabrication to Application. Biosensors 2022, 12, 543. [Google Scholar] [CrossRef]
- AlMashrea, B.A.; Almehdi, A.M.; Damiati, S. Simple Microfluidic Devices for in Situ Detection of Water Contamination: A State-of-Art Review. Front. Bioeng. Biotechnol. 2024, 12, 1355768. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Zhao, W.; Neethirajan, S.; Duffield, T. Microfluidic Biosensor for β-Hydroxybutyrate (ΒHBA) Determination of Subclinical Ketosis Diagnosis. J. Nanobiotechnol. 2015, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Tsougeni, K.; Kaprou, G.; Loukas, C.M.; Papadakis, G.; Hamiot, A.; Eck, M.; Rabus, D.; Kokkoris, G.; Chatzandroulis, S.; Papadopoulos, V.; et al. Lab-on-Chip Platform and Protocol for Rapid Foodborne Pathogen Detection Comprising on-Chip Cell Capture, Lysis, DNA Amplification and Surface-Acoustic-Wave Detection. Sens. Actuators B Chem. 2020, 320, 128345. [Google Scholar] [CrossRef]
- Xie, Y.; Zhi, X.; Su, H.; Wang, K.; Yan, Z.; He, N.; Zhang, J.; Chen, D.; Cui, D. A Novel Electrochemical Microfluidic Chip Combined with Multiple Biomarkers for Early Diagnosis of Gastric Cancer. Nanoscale Res. Lett. 2015, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhang, S.; Liu, Q.; Xu, T.; Zhang, X. Microfluidic-Based Plasmonic Microneedle Biosensor for Uric Acid Ultrasensitive Monitoring. Sens. Actuators B Chem. 2024, 398, 134685. [Google Scholar] [CrossRef]
- Ehzari, H.; Amiri, M.; Hallaj, R.; Sadeghi, M. Rapid, Flexible Fabrication of a Microfluidic Electrochemical Chip Nucleic Acid Target for Selective, Label-Free Detection of Influenza Virus DNA Using Catalytic Redox-Recycling. Anal. Biochem. 2025, 700, 115771. [Google Scholar] [CrossRef]
- Aleklett, K.; Kiers, E.T.; Ohlsson, P.; Shimizu, T.S.; Caldas, V.E.A.; Hammer, E.C. Build Your Own Soil: Exploring Microfluidics to Create Microbial Habitat Structures. ISME J. 2018, 12, 312–319. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, X.; Ye, Q.; Zou, J.; Wan, Q.; Jiang, F.; Cai, Z.; Zhang, J.; Qu, X.; Huang, J.; et al. Isothermal Nucleic Acid Amplification-Based Biosensors: The next Generation Analytical Toolkit for Point-of-Care Assay of Foodborne Pathogens. Trends Food Sci. Technol. 2025, 157, 104882. [Google Scholar] [CrossRef]
- Gale, B.K.; Jafek, A.R.; Lambert, C.J.; Goenner, B.L.; Moghimifam, H.; Nze, U.C.; Kamarapu, S.K. A Review of Current Methods in Microfluidic Device Fabrication and Future Commercialization Prospects. Inventions 2018, 3, 60. [Google Scholar] [CrossRef]
- Zolti, O.; Suganthan, B.; Ramasamy, R.P. Lab-on-a-Chip Electrochemical Biosensors for Foodborne Pathogen Detection: A Review of Common Standards and Recent Progress. Biosensors 2023, 13, 215. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Hanson, R.L.; Almughamsi, H.M.; Pang, C.; Fish, T.R.; Woolley, A.T. Microfluidics: Innovations in Materials and Their Fabrication and Functionalization. Anal. Chem. 2020, 92, 150–168. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Fabrication and Applications of Microfluidic Devices: A Review. Int. J. Mol. Sci. 2021, 22, 2011. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Islam, M.; Gupta, A. Microfluidic Devices for the Detection of Contamination in Water Samples: A Review. Sens. Actuators A Phys. 2022, 347, 113926. [Google Scholar] [CrossRef]
- Scott, S.M.; Ali, Z. Fabrication Methods for Microfluidic Devices: An Overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef]
- Liu, X.; Sun, A.; Brodský, J.; Gablech, I.; Lednický, T.; Vopařilová, P.; Zítka, O.; Zeng, W.; Neužil, P. Microfluidics Chips Fabrication Techniques Comparison. Sci. Rep. 2024, 14, 28793. [Google Scholar] [CrossRef]
- Nissen, M.; Doherty, B.; Hamperl, J.; Kobelke, J.; Weber, K.; Henkel, T.; Schmidt, M.A. UV Absorption Spectroscopy in Water-Filled Antiresonant Hollow Core Fibers for Pharmaceutical Detection. Sensors 2018, 18, 478. [Google Scholar] [CrossRef]
- Burtsev, V.; Erzina, M.; Guselnikova, O.; Miliutina, E.; Kalachyova, Y.; Svorcik, V.; Lyutakov, O. Detection of Trace Amounts of Insoluble Pharmaceuticals in Water by Extraction and SERS Measurements in a Microfluidic Flow Regime. Analyst 2021, 146, 3686–3696. [Google Scholar] [CrossRef]
- Man, Y.; Ban, M.; Li, A.; Jin, X.; Du, Y.; Pan, L. A Microfluidic Colorimetric Biosensor for In-Field Detection of Salmonella in Fresh-Cut Vegetables Using Thiolated Polystyrene Microspheres, Hose-Based Microvalve and Smartphone Imaging APP. Food Chem. 2021, 354, 129578. [Google Scholar] [CrossRef]
- Jiang, Y.; Qiu, Z.; Le, T.; Zou, S.; Cao, X. Developing a Dual-RCA Microfluidic Platform for Sensitive E. coli O157:H7 Whole-Cell Detections. Anal. Chim. Acta 2020, 1127, 79–88. [Google Scholar] [CrossRef]
- Zheng, L.; Cai, G.; Wang, S.; Liao, M.; Li, Y.; Lin, J. A Microfluidic Colorimetric Biosensor for Rapid Detection of Escherichia coli O157:H7 Using Gold Nanoparticle Aggregation and Smart Phone Imaging. Biosens. Bioelectron. 2019, 124–125, 143–149. [Google Scholar] [CrossRef]
- Saez, J.; Catalan-Carrio, R.; Owens, R.M.; Basabe-Desmonts, L.; Benito-Lopez, F. Microfluidics and Materials for Smart Water Monitoring: A Review. Anal. Chim. Acta 2021, 1186, 338392. [Google Scholar] [CrossRef] [PubMed]
- Doostmohammadi, A.; Huang, H.; Naushad, S.; Rezai, P. Portable Cell Imprinted Polymer-Based Microfluidic Sensor for Bacteria Detection in Real Water. Microchem. J. 2024, 206, 111611. [Google Scholar] [CrossRef]
- Akhtarian, S.; Kaur Brar, S.; Rezai, P. Electrochemical Impedance Spectroscopy-Based Microfluidic Biosensor Using Cell-Imprinted Polymers for Bacteria Detection. Biosensors 2024, 14, 445. [Google Scholar] [CrossRef] [PubMed]
- Akhtarian, S.; Doostmohammadi, A.; Archonta, D.-E.; Kraft, G.; Brar, S.K.; Rezai, P. Microfluidic Sensor Based on Cell-Imprinted Polymer-Coated Microwires for Conductometric Detection of Bacteria in Water. Biosensors 2023, 13, 943. [Google Scholar] [CrossRef]
- He, X.; Zhou, X.; Liu, Y.; Wang, X. Ultrasensitive, Recyclable and Portable Microfluidic Surface-Enhanced Raman Scattering (SERS) Biosensor for Uranyl Ions Detection. Sens. Actuators B Chem. 2020, 311, 127676. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.W.; Jeon, H.J. Machine Learning-Driven Innovations in Microfluidics. Biosensors 2024, 14, 613. [Google Scholar] [CrossRef]
- Asadian, E.; Bahramian, F.; Siavashy, S.; Movahedi, S.; Keçili, R.; Hussain, C.M.; Ghorbani-Bidkorpeh, F. A Review on Recent Advances of AI-Integrated Microfluidics for Analytical and Bioanalytical Applications. TrAC Trends Anal. Chem. 2024, 181, 118004. [Google Scholar] [CrossRef]
- Galan, E.A.; Zhao, H.; Wang, X.; Dai, Q.; Huck, W.T.S.; Ma, S. Intelligent Microfluidics: The Convergence of Machine Learning and Microfluidics in Materials Science and Biomedicine. Matter 2020, 3, 1893–1922. [Google Scholar] [CrossRef]
- McIntyre, D.; Lashkaripour, A.; Fordyce, P.; Densmore, D. Machine Learning for Microfluidic Design and Control. Lab. Chip 2022, 22, 2925–2937. [Google Scholar] [CrossRef]
- Praljak, N.; Iram, S.; Goreke, U.; Singh, G.; Hill, A.; Gurkan, U.A.; Hinczewski, M. Integrating Deep Learning with Microfluidics for Biophysical Classification of Sickle Red Blood Cells Adhered to Laminin. PLoS Comput. Biol. 2021, 17, e1008946. [Google Scholar] [CrossRef]
- Antonelli, G.; Filippi, J.; D’Orazio, M.; Curci, G.; Casti, P.; Mencattini, A.; Martinelli, E. Integrating Machine Learning and Biosensors in Microfluidic Devices: A Review. Biosens. Bioelectron. 2024, 263, 116632. [Google Scholar] [CrossRef] [PubMed]
- Masekela, D.; Dlamini, L.N. Molecularly Imprinted and MXene-Based Electrochemical Sensors for Detecting Pharmaceuticals and Toxic Compounds: A Concise Review. J. Environ. Chem. Eng. 2024, 12, 114569. [Google Scholar] [CrossRef]
- Pratiwi, R.; Nur Azizah, P.; Nur Hasanah, A.; Binti Asman, S. Analytical Method for Monitoring Tetracycline Residues in Various Samples: A Focus on Environmental and Health Implications. Microchem. J. 2024, 206, 111408. [Google Scholar] [CrossRef]
- Bahrani, S.; Sadati Behbahani, E.; Ghaedi, M.; Amrollahi Miandeh, Y.; Asfaram, A. Molecular Imprinted Technology Using in Biosensing: A Review. Microchem. J. 2024, 203, 110888. [Google Scholar] [CrossRef]
- Rocha, P.; Rebelo, P.; Pacheco, J.G.; Geraldo, D.; Bento, F.; Leão-Martins, J.M.; Delerue-Matos, C.; Nouws, H.P.A. Electrochemical Molecularly Imprinted Polymer Sensor for Simple and Fast Analysis of Tetrodotoxin in Seafood. Talanta 2025, 282, 127002. [Google Scholar] [CrossRef]
- Wang, X.; Hou, X.; Sun, N.; Wang, Y.; Zhang, Y.; Lv, Y.; Ding, L.; Sun, X. Biowaste-Derived Carbon Dots-Based Molecularly Imprinted Fluorescent Nanosensor for Selective Detection of Rutin. J. Fluoresc. 2024. [Google Scholar] [CrossRef]
- Minhas, M.A.; Shezadi, S.; Andac, M.; Shaikh, H.; Duran, S.; Tarique Moin, S.; Faizi, S.; Bhanger, M.I.; Malik, M.I. Imprinting-Based Smart Syringe: A Fast, Efficient, and Selective Approach for Extraction of Methyl Gallate from Caesalpinia Pulcherrima Extract. J. Ind. Eng. Chem. 2024, 136, 378–390. [Google Scholar] [CrossRef]
- Shalapy, A.E.; Molouk, A.F.S.; Awad, F.S.; Khalifa, M.E.; Abdallah, A.B. Flexible Imprinted Electrochemical Sensing Platform for Ultra-Trace Detection of Indigo Carmine Dye in Coloured Food Additive for Candy and Ice Cream. Inorg. Chem. Commun. 2025, 174, 113951. [Google Scholar] [CrossRef]
- Jolly, P.; Tamboli, V.; Harniman, R.L.; Estrela, P.; Allender, C.J.; Bowen, J.L. Aptamer–MIP Hybrid Receptor for Highly Sensitive Electrochemical Detection of Prostate Specific Antigen. Biosens. Bioelectron. 2016, 75, 188–195. [Google Scholar] [CrossRef]
- Agar, M.; Laabei, M.; Leese, H.S.; Estrela, P. Aptamer-Molecularly Imprinted Polymer Sensors for the Detection of Bacteria in Water. Biosens. Bioelectron. 2025, 272, 117136. [Google Scholar] [CrossRef]
- Li, Y.-T.; Yang, Y.-Y.; Han, S. Surface Molecular Imprinting Technology Integrated SERS Sensing: Emerging Trends and Future Perspectives toward on-Site Hazardous Analysis. TrAC Trends Anal. Chem. 2024, 179, 117866. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).