A Planar-Gate Graphene Field-Effect Transistor Integrated Portable Platform for Rapid Detection of Colon Cancer-Derived Exosomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
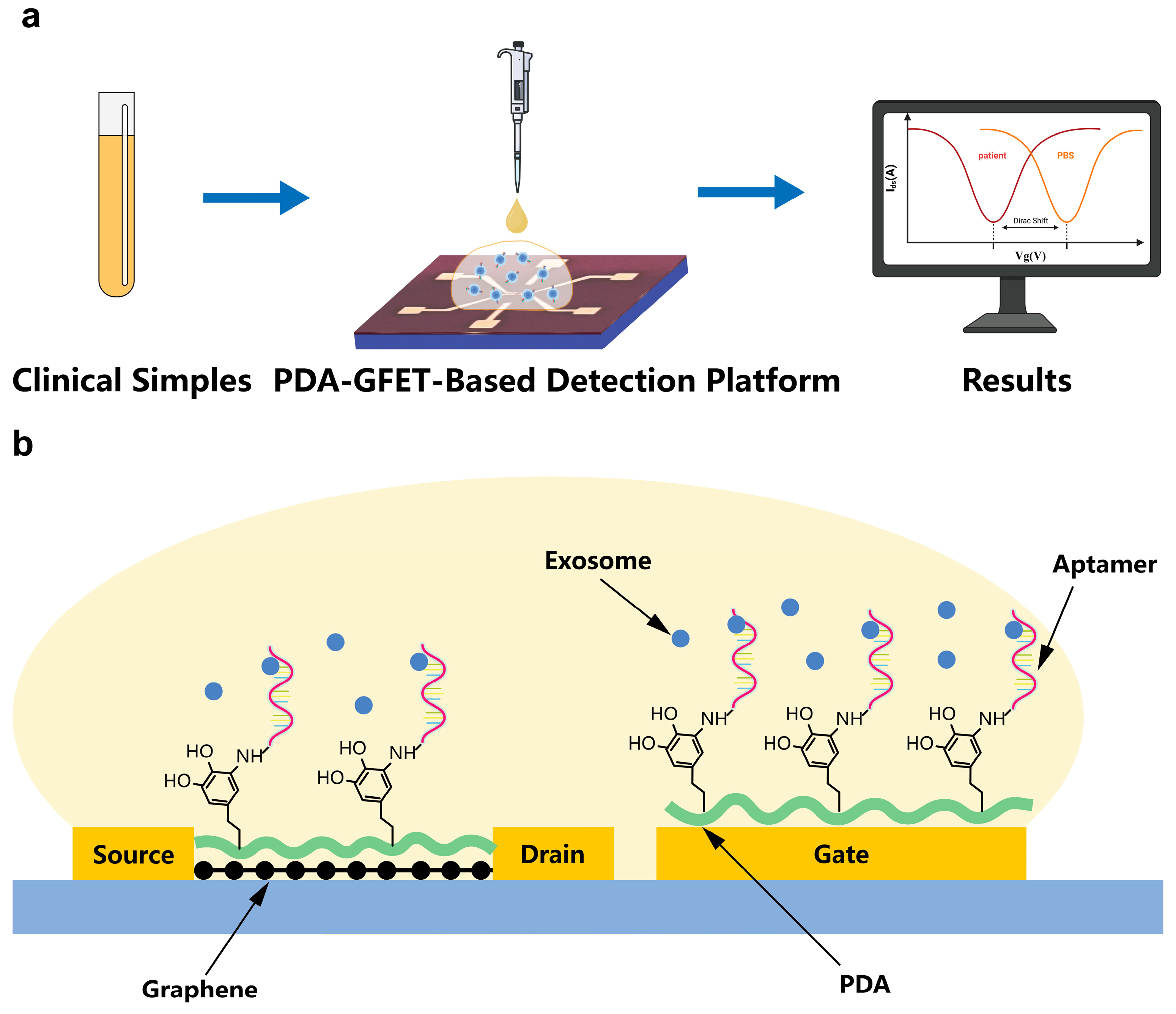
2.2. Design and Fabrication of GFET Biosensor
2.3. Preparation and Functionalization of PDA Self-Assembled Film
2.4. Colon Cancer-Derived Exosome Purification
3. Results
3.1. Characterization of PDA-GFET and Exosomes
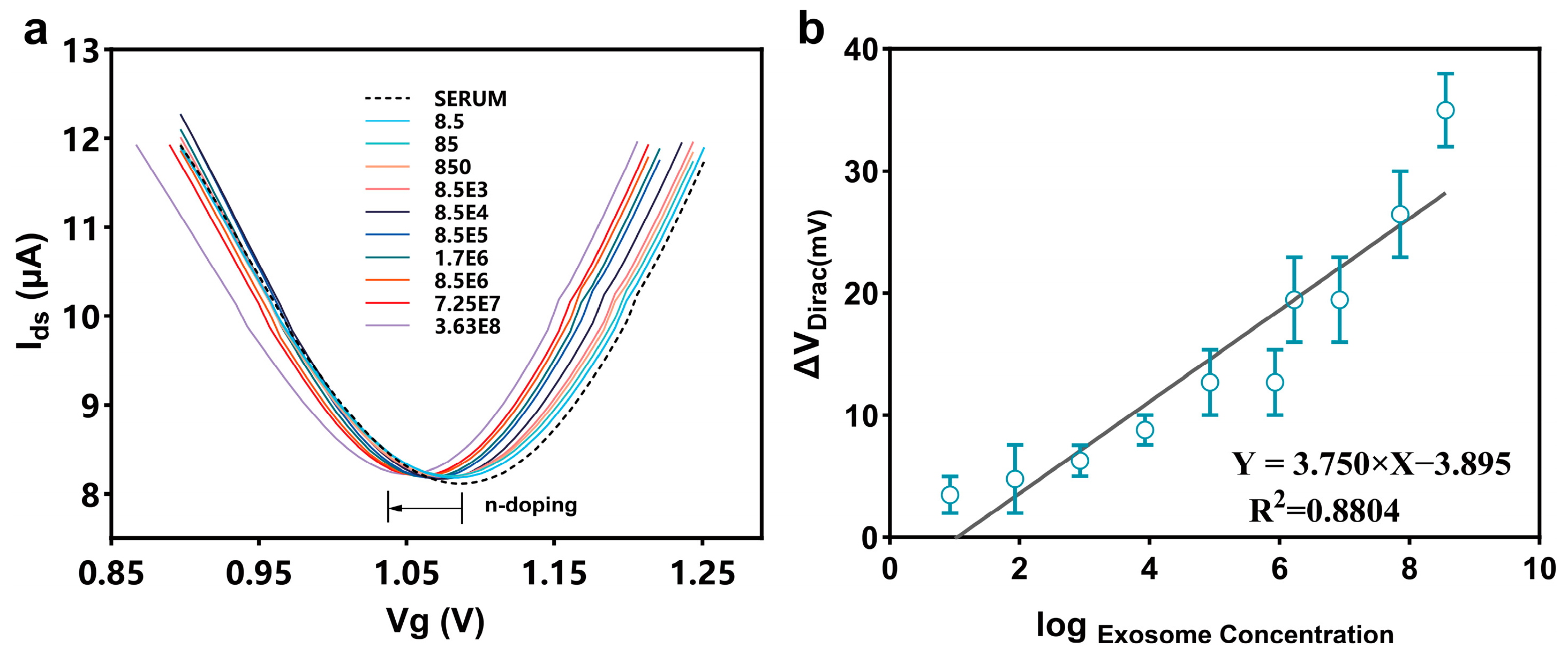
3.2. Detection of Colon Cancer-Derived Exosomes in PBS
3.3. Detection of Colon Cancer-Derived Exosomes in Undiluted Serum
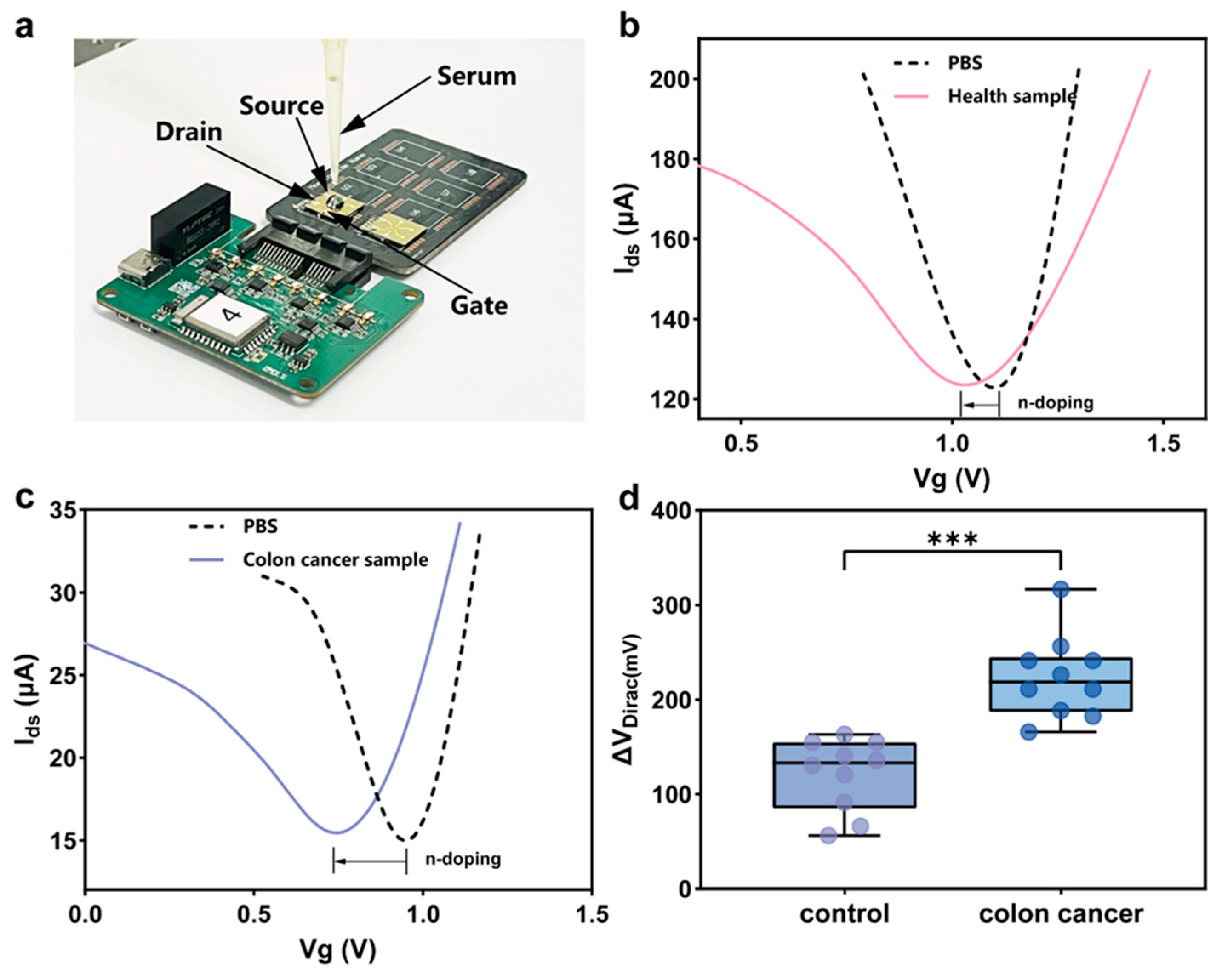
3.4. Integrated Portable Platform for Clinical Sample Detection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDA | Polydopamine |
| GFET | Graphene-based Field-Effect Transistor |
| EpCAM | Epithelial Cell Adhesion Molecule |
| PMMA | Polymethyl Methacrylate |
| NTA | Nanoparticle tracking analysis |
| Tris-HCl | Tris(hydroxymethyl)aminomethane hydrochloride |
References
- Labianca, R.; Beretta, G.D.; Kildani, B.; Milesi, L.; Merlin, F.; Mosconi, S.; Pessi, M.A.; Prochilo, T.; Quadri, A.; Gatta, G. Colon cancer. Crit. Rev. Oncol./Hematol. 2010, 74, 106–133. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, S.D.; Dawson, D.M.; Willis, J.; Willson, J.K. Focus on colon cancer. Cancer Cell 2002, 1, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Duan, B.; Zhao, Y.; Bai, J.; Wang, J.; Duan, X.; Luo, X.; Zhang, R.; Pu, Y.; Kou, M.; Lei, J. Colorectal Cancer: An Overview; Exon Publications: Brisbane, Australia, 2022; pp. 1–12. [Google Scholar]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M. Colon cancer: A clinician’s perspective in 2019. Gastroenterol. Res. 2020, 13, 1. [Google Scholar] [CrossRef]
- Ward, D.; Suggett, N.; Cheng, Y.; Wei, W.; Johnson, H.; Billingham, L.; Ismail, T.; Wakelam, M.; Johnson, P.; Martin, A. Identification of serum biomarkers for colon cancer by proteomic analysis. Br. J. Cancer 2006, 94, 1898–1905. [Google Scholar] [CrossRef]
- Chand, M.; Keller, D.; Joshi, H.; Devoto, L.; Rodriguez-Justo, M.; Cohen, R. Feasibility of fluorescence lymph node imaging in colon cancer: FLICC. Tech. Coloproctol. 2018, 22, 271–277. [Google Scholar] [CrossRef]
- Qi, H.; Liu, C.; Long, L.; Ren, Y.; Zhang, S.; Chang, X.; Qian, X.; Jia, H.; Zhao, J.; Sun, J. Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano 2016, 10, 3323–3333. [Google Scholar] [CrossRef]
- Zhou, H.; Yuen, P.S.; Pisitkun, T.; Gonzales, P.A.; Yasuda, H.; Dear, J.W.; Gross, P.; Knepper, M.A.; Star, R.A. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006, 69, 1471–1476. [Google Scholar] [CrossRef]
- Michael, A.; Bajracharya, S.D.; Yuen, P.S.; Zhou, H.; Star, R.A.; Illei, G.G.; Alevizos, I. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010, 16, 34–38. [Google Scholar] [CrossRef]
- Salehi, M.; Sharifi, M. Exosomal miRNAs as novel cancer biomarkers: Challenges and opportunities. J. Cell. Physiol. 2018, 233, 6370–6380. [Google Scholar] [CrossRef]
- Nedaeinia, R.; Manian, M.; Jazayeri, M.; Ranjbar, M.; Salehi, R.; Sharifi, M.; Mohaghegh, F.; Goli, M.; Jahednia, S.; Avan, A. Circulating exosomes and exosomal microRNAs as biomarkers in gastrointestinal cancer. Cancer Gene Ther. 2017, 24, 48–56. [Google Scholar] [CrossRef]
- Ruiz-López, L.; Blancas, I.; Garrido, J.M.; Mut-Salud, N.; Moya-Jódar, M.; Osuna, A.; Rodríguez-Serrano, F. The role of exosomes on colorectal cancer: A review. J. Gastroenterol. Hepatol. 2018, 33, 792–799. [Google Scholar] [CrossRef]
- Lafitte, M.; Lecointre, C.; Roche, S. Roles of exosomes in metastatic colorectal cancer. Am. J. Physiol.-Cell Physiol. 2019, 317, C869–C880. [Google Scholar] [CrossRef]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Mathivanan, S.; Ji, H.; Simpson, R.J. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol. Cell. Proteom. 2013, 12, 587–598. [Google Scholar] [CrossRef]
- Wang, Z.; Von Au, A.; Schnölzer, M.; Hackert, T.; Zöller, M. CD44v6-competent tumor exosomes promote motility, invasion and cancer-initiating cell marker expression in pancreatic and colorectal cancer cells. Oncotarget 2016, 7, 55409. [Google Scholar] [CrossRef]
- Kang, M.; Kim, S.; Ko, J. Roles of CD133 in microvesicle formation and oncoprotein trafficking in colon cancer. FASEB J. 2019, 33, 4248–4260. [Google Scholar] [CrossRef]
- Ostenfeld, M.S.; Jensen, S.G.; Jeppesen, D.K.; Christensen, L.-L.; Thorsen, S.B.; Stenvang, J.; Hvam, M.L.; Thomsen, A.; Mouritzen, P.; Rasmussen, M.H. miRNA profiling of circulating EpCAM+ extracellular vesicles: Promising biomarkers of colorectal cancer. J. Extracell. Vesicles 2016, 5, 31488. [Google Scholar] [CrossRef]
- Lee, C.-C.; Yu, C.-J.; Panda, S.S.; Chen, K.-C.; Liang, K.-H.; Huang, W.-C.; Wang, Y.-S.; Ho, P.-C.; Wu, H.-C. Epithelial cell adhesion molecule (EpCAM) regulates HGFR signaling to promote colon cancer progression and metastasis. J. Transl. Med. 2023, 21, 530. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Jiang, L.; Van Geest, E.P.; Lima, L.M.; Schneider, G.F. Sensing at the surface of graphene field-effect transistors. Adv. Mater. 2017, 29, 1603610. [Google Scholar] [CrossRef]
- Green, N.S.; Norton, M.L. Interactions of DNA with graphene and sensing applications of graphene field-effect transistor devices: A review. Anal. Chim. Acta 2015, 853, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Zhan, B.; Li, C.; Yang, J.; Jenkins, G.; Huang, W.; Dong, X. Graphene field-effect transistor and its application for electronic sensing. Small 2014, 10, 4042–4065. [Google Scholar] [CrossRef]
- Darmostuk, M.; Rimpelova, S.; Gbelcova, H.; Ruml, T. Current approaches in SELEX: An update to aptamer selection technology. Biotechnol. Adv. 2015, 33, 1141–1161. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Z.; Yu, Y.; Wang, M.; Li, J.; Zhang, Z.; Liu, J.; Wu, X.; Lu, A.; Zhang, G.; Zhang, B. Recent advances in SELEX technology and aptamer applications in biomedicine. Int. J. Mol. Sci. 2017, 18, 2142. [Google Scholar] [CrossRef]
- Jin, D.; Yang, F.; Zhang, Y.; Liu, L.; Zhou, Y.; Wang, F.; Zhang, G.-J. ExoAPP: Exosome-oriented, aptamer nanoprobe-enabled surface proteins profiling and detection. Anal. Chem. 2018, 90, 14402–14411. [Google Scholar] [CrossRef]
- Song, Z.; Mao, J.; Barrero, R.A.; Wang, P.; Zhang, F.; Wang, T. Development of a CD63 aptamer for efficient cancer immunochemistry and immunoaffinity-based exosome isolation. Molecules 2020, 25, 5585. [Google Scholar] [CrossRef]
- Lu, N.; Wang, L.; Li, L.; Liu, M. A review for compact model of graphene field-effect transistors. Chin. Phys. B 2017, 26, 036804. [Google Scholar] [CrossRef]
- Khan, N.I.; Song, E. Detection of an IL-6 biomarker using a GFET platform developed with a facile organic solvent-free aptamer immobilization approach. Sensors 2021, 21, 1335. [Google Scholar] [CrossRef]
- Li, J.; Wijaya, L.N.A.; Jang, D.W.; Hu, Y.; You, J.; Cai, Y.; Gao, Z.; Mi, Y.; Luo, Z. 2D Materials-Based Field-Effect Transistor Biosensors for Healthcare. Small 2024, 2408961. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Han, Z.; An, F.; Gong, X.; Zhao, C.; Zheng, W.; Mei, L.; Zhou, Q. Aptamer-based biosensors for the diagnosis of sepsis. J. Nanobiotechnol. 2021, 19, 216. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, E.; Fenoy, G.E.; Cantillo, A.L.; Allegretto, J.A.; Scotto, J.; Piccinini, J.M.; Marmisollé, W.A.; Azzaroni, O. Biofunctionalization of graphene-based FET sensors through heterobifunctional nanoscaffolds: Technology validation toward rapid COVID-19 diagnostics and monitoring. Adv. Mater. Interfaces 2022, 9, 2102526. [Google Scholar] [CrossRef]
- Zhang, X.; Jing, Q.; Ao, S.; Schneider, G.F.; Kireev, D.; Zhang, Z.; Fu, W. Ultrasensitive field-effect biosensors enabled by the unique electronic properties of graphene. Small 2020, 16, 1902820. [Google Scholar] [CrossRef]
- Dai, C.; Kong, D.; Chen, C.; Liu, Y.; Wei, D. Graphene transistors for in vitro detection of health biomarkers. Adv. Funct. Mater. 2023, 33, 2301948. [Google Scholar] [CrossRef]
- Hao, R.; Liu, L.; Yuan, J.; Wu, L.; Lei, S. Recent advances in field effect transistor biosensors: Designing strategies and applications for sensitive assay. Biosensors 2023, 13, 426. [Google Scholar] [CrossRef]
- Wang, C.; Cui, X.; Li, Y.; Li, H.; Huang, L.; Bi, J.; Luo, J.; Ma, L.Q.; Zhou, W.; Cao, Y. A label-free and portable graphene FET aptasensor for children blood lead detection. Sci. Rep. 2016, 6, 21711. [Google Scholar] [CrossRef]
- Sadighbayan, D.; Hasanzadeh, M.; Ghafar-Zadeh, E. Biosensing based on field-effect transistors (FET): Recent progress and challenges. TrAC Trends Anal. Chem. 2020, 133, 116067. [Google Scholar] [CrossRef]
- Huang, C.; Hao, Z.; Wang, Z.; Zhao, X.; Wang, H.; Li, F.; Liu, S.; Pan, Y. A fully integrated graphene-polymer field-effect transistor biosensing device for on-site detection of glucose in human urine. Mater. Today Chem. 2022, 23, 100635. [Google Scholar] [CrossRef]
- Wang, Z.; Hao, Z.; Yu, S.; De Moraes, C.G.; Suh, L.H.; Zhao, X.; Lin, Q. An ultraflexible and stretchable aptameric graphene nanosensor for biomarker detection and monitoring. Adv. Funct. Mater. 2019, 29, 1905202. [Google Scholar] [CrossRef]
- Hao, Z.; Zhu, Y.; Wang, X.; Rotti, P.G.; Dimarco, C.; Tyler, S.R.; Zhao, X.; Engelhardt, J.F.; Hone, J.; Lin, Q. Real-time monitoring of insulin using a graphene field-effect transistor aptameric nanosensor. ACS Appl. Mater. Interfaces 2017, 9, 27504–27511. [Google Scholar] [CrossRef]
- Huang, C.; Hao, Z.; Qi, T.; Pan, Y.; Zhao, X. An integrated flexible and reusable graphene field effect transistor nanosensor for monitoring glucose. J. Mater. 2020, 6, 308–314. [Google Scholar] [CrossRef]
- Lee, H.A.; Park, E.; Lee, H. Polydopamine and its derivative surface chemistry in material science: A focused review for studies at KAIST. Adv. Mater. 2020, 32, 1907505. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Huang, C.-J. Functionalization of polydopamine via the aza-michael reaction for antimicrobial interfaces. Langmuir 2016, 32, 5019–5028. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, X.; Shi, H.; Wu, L.; Qian, H.; Xu, W. Exosomes in cancer: Small particle, big player. J. Hematol. Oncol. 2015, 8, 83. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- Brinton, L.T.; Sloane, H.S.; Kester, M.; Kelly, K.A. Formation and role of exosomes in cancer. Cell. Mol. Life Sci. 2015, 72, 659–671. [Google Scholar] [CrossRef]
- Liu, X.; Long, Y.-Z.; Liao, L.; Duan, X.; Fan, Z. Large-scale integration of semiconductor nanowires for high-performance flexible electronics. ACS Nano 2012, 6, 1888–1900. [Google Scholar] [CrossRef]
- Chen, Y.; Kong, D.; Qiu, L.; Wu, Y.; Dai, C.; Luo, S.; Huang, Z.; Lin, Q.; Chen, H.; Xie, S. Artificial nucleotide aptamer-based field-effect transistor for ultrasensitive detection of hepatoma exosomes. Anal. Chem. 2022, 95, 1446–1453. [Google Scholar] [CrossRef]
- Yin, T.; Xu, L.; Gil, B.; Merali, N.; Sokolikova, M.S.; Gaboriau, D.C.; Liu, D.S.; Muhammad Mustafa, A.N.; Alodan, S.; Chen, M. Graphene sensor arrays for rapid and accurate detection of pancreatic cancer exosomes in patients’ blood plasma samples. ACS Nano 2023, 17, 14619–14631. [Google Scholar] [CrossRef]
- Doldán, X.; Fagúndez, P.; Cayota, A.; Laíz, J.; Tosar, J.P. Electrochemical sandwich immunosensor for determination of exosomes based on surface marker-mediated signal amplification. Anal. Chem. 2016, 88, 10466–10473. [Google Scholar] [CrossRef]
- Liu, X.; Gao, X.; Yang, L.; Zhao, Y.; Li, F. Metal–organic framework-functionalized paper-based electrochemical biosensor for ultrasensitive exosome assay. Anal. Chem. 2021, 93, 11792–11799. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, M.; Wang, L.; Yan, A.; He, W.; Chen, M.; Lan, J.; Xu, J.; Guan, L.; Chen, J. A visible and colorimetric aptasensor based on DNA-capped single-walled carbon nanotubes for detection of exosomes. Biosens. Bioelectron. 2017, 92, 8–15. [Google Scholar] [CrossRef]
- Xu, L.; Chopdat, R.; Li, D.; Al-Jamal, K.T. Development of a simple, sensitive and selective colorimetric aptasensor for the detection of cancer-derived exosomes. Biosens. Bioelectron. 2020, 169, 112576. [Google Scholar] [CrossRef]
- Hajian, R.; Decastro, J.; Parkinson, J.; Kane, A.; Camelo, A.F.R.; Chou, P.P.; Yang, J.; Wong, N.; Hernandez, E.D.O.; Goldsmith, B. Rapid and Electronic Identification and Quantification of Age-Specific Circulating Exosomes via Biologically Activated Graphene Transistors. Adv. Biol. 2021, 5, 2000594. [Google Scholar] [CrossRef]


| Sensing Method | Probe | Test Time | LOD | Reference |
|---|---|---|---|---|
| Electrochemical biosensor | CD9 antibody | N/A | 2 × 105 exosomes/mL | [52] |
| Electrochemical biosensor | CD63 aptamer | 80 min | 5 × 103 exosomes/mL | [53] |
| colorimetric aptasensor | CD63 aptamer | 40 min | 5.2 × 105 exosomes/mL | [54] |
| colorimetric aptasensor | CD63 aptamer | 10 min | 7.7 × 103 exosomes/mL | [55] |
| FET biosensor | CD63 antibody | 30 min | 2 × 104 exosomes/mL | [56] |
| GFET biosensor | EpCAM aptamer | 10 min | 112 exosomes/mL | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhang, L.; Huang, Y.; Wang, Z.; Ren, Z. A Planar-Gate Graphene Field-Effect Transistor Integrated Portable Platform for Rapid Detection of Colon Cancer-Derived Exosomes. Biosensors 2025, 15, 207. https://doi.org/10.3390/bios15040207
Zhang Z, Zhang L, Huang Y, Wang Z, Ren Z. A Planar-Gate Graphene Field-Effect Transistor Integrated Portable Platform for Rapid Detection of Colon Cancer-Derived Exosomes. Biosensors. 2025; 15(4):207. https://doi.org/10.3390/bios15040207
Chicago/Turabian StyleZhang, Zaiyu, Luyang Zhang, Yuting Huang, Ziran Wang, and Zhongjing Ren. 2025. "A Planar-Gate Graphene Field-Effect Transistor Integrated Portable Platform for Rapid Detection of Colon Cancer-Derived Exosomes" Biosensors 15, no. 4: 207. https://doi.org/10.3390/bios15040207
APA StyleZhang, Z., Zhang, L., Huang, Y., Wang, Z., & Ren, Z. (2025). A Planar-Gate Graphene Field-Effect Transistor Integrated Portable Platform for Rapid Detection of Colon Cancer-Derived Exosomes. Biosensors, 15(4), 207. https://doi.org/10.3390/bios15040207







