Research Progress of Electrochemical Biosensors for Diseases Detection in China: A Review
Abstract
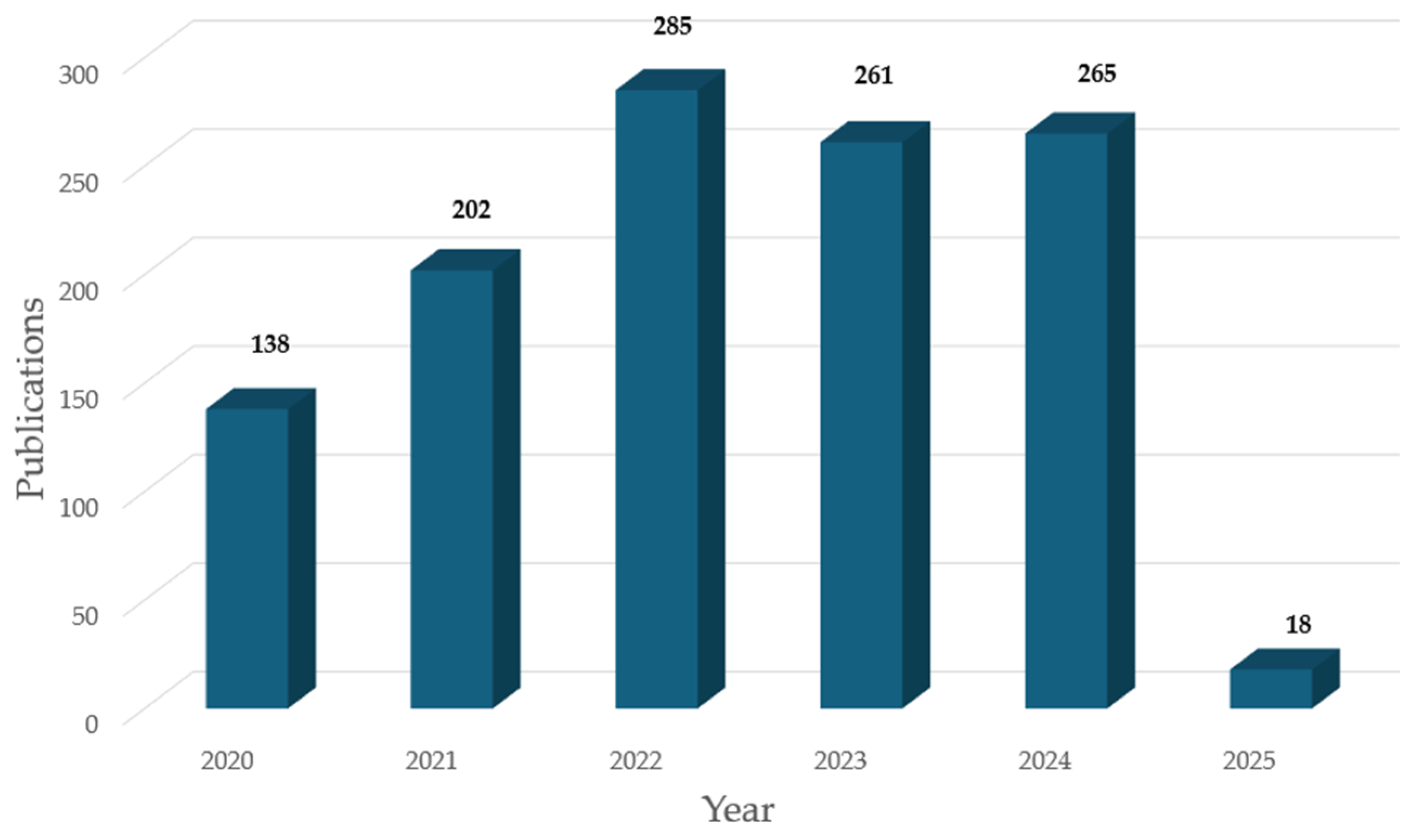
:1. Introduction
2. Electrochemical Biosensors
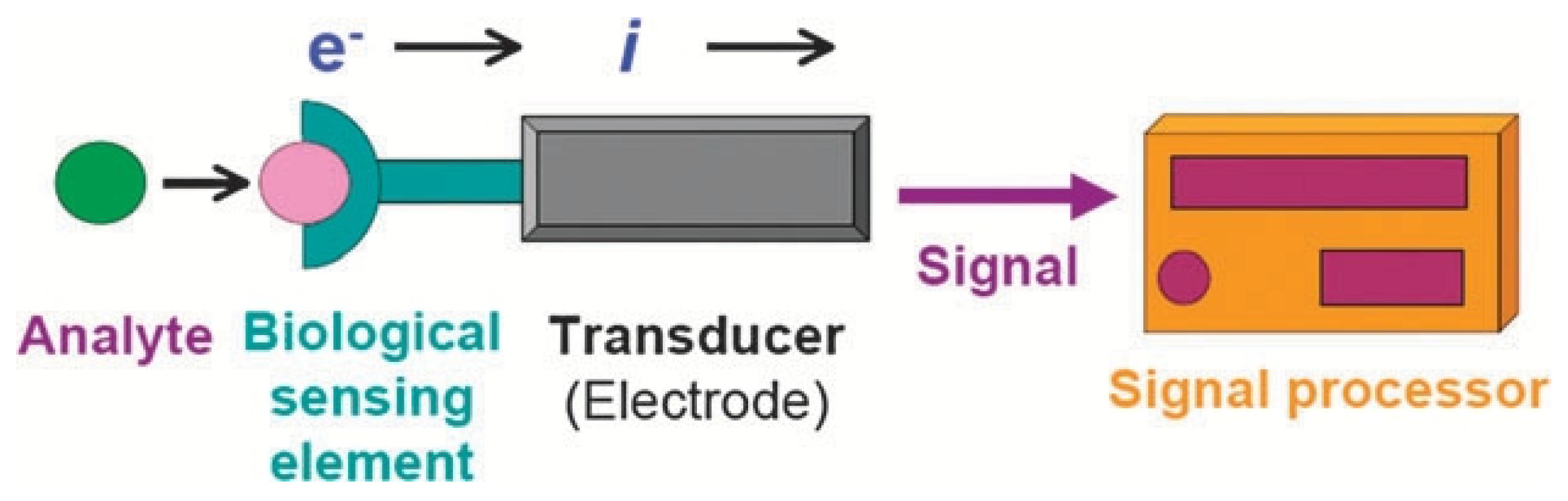
Principle of Electrochemical Biosensors
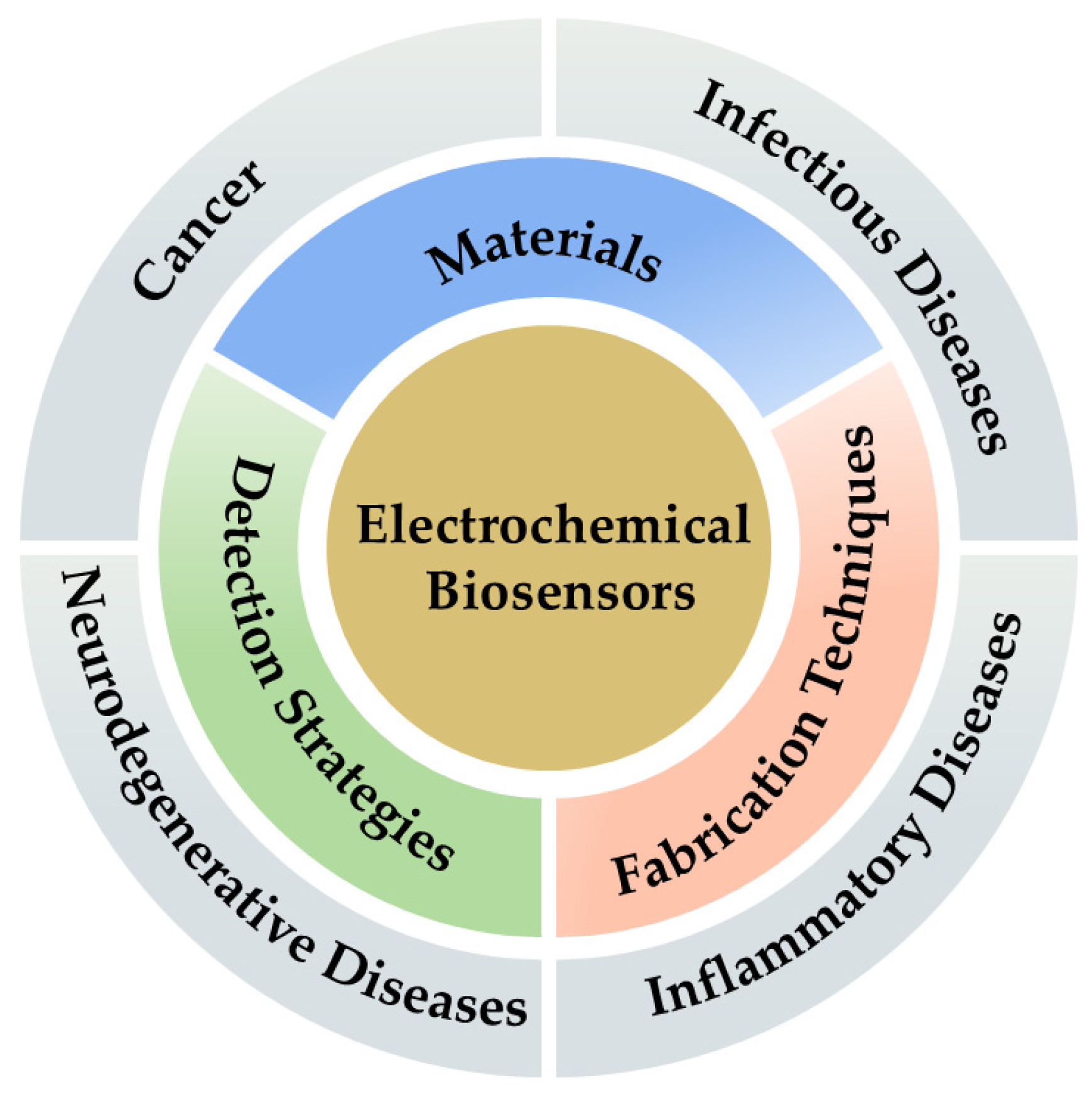
3. Advances in Materials and Fabrication Technologies for Electrochemical Biosensors
3.1. Materials
3.1.1. Noble Metal Nanomaterials
3.1.2. Carbon-Based Nanomaterials
3.1.3. Conductive Polymer-Based Nanomaterials
3.1.4. Porous Materials
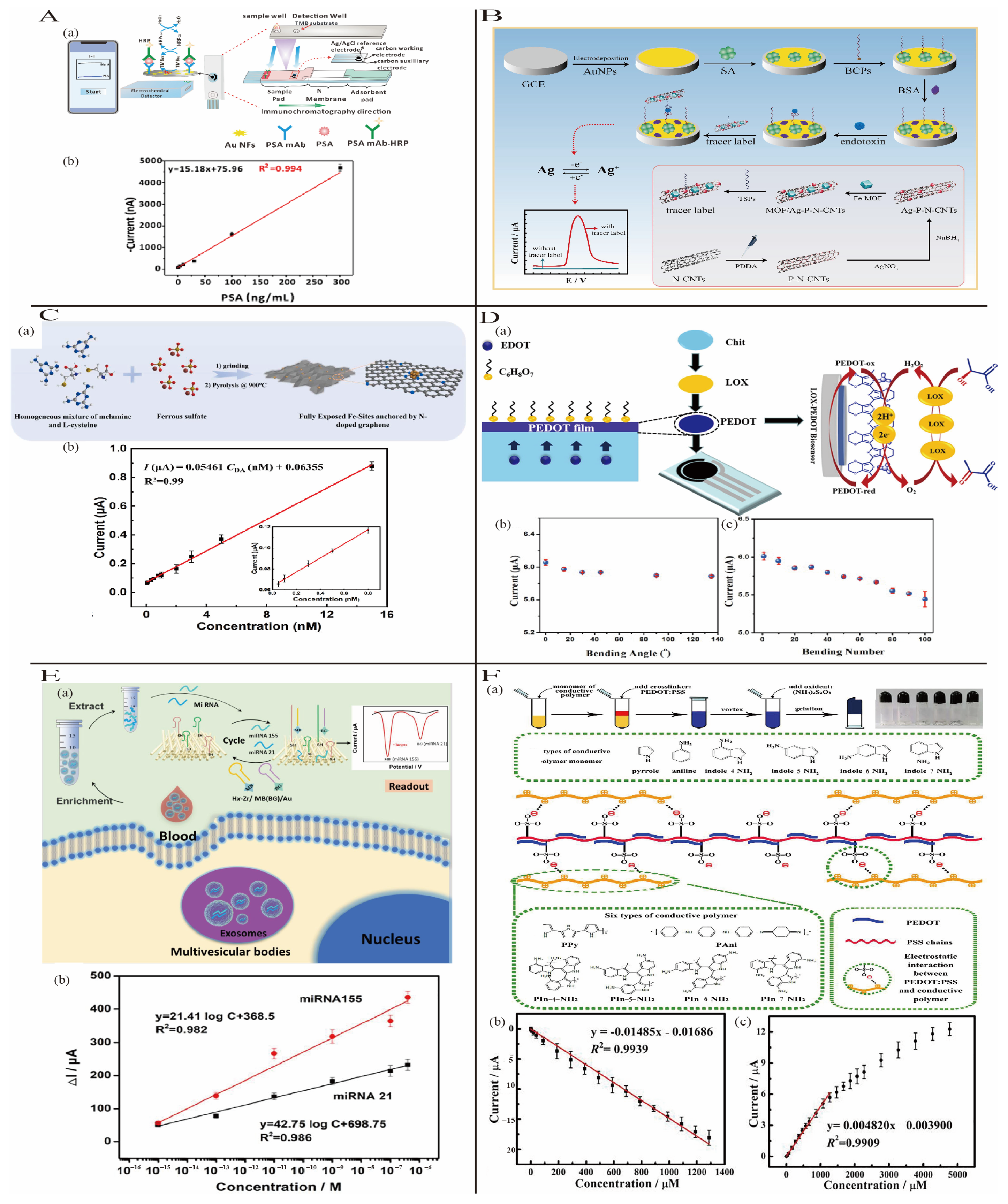
3.1.5. Gel Materials
3.2. Fabrication Techniques for Electrochemical Biosensors
3.2.1. Printing Methods
3.2.2. Vapor Deposition Techniques
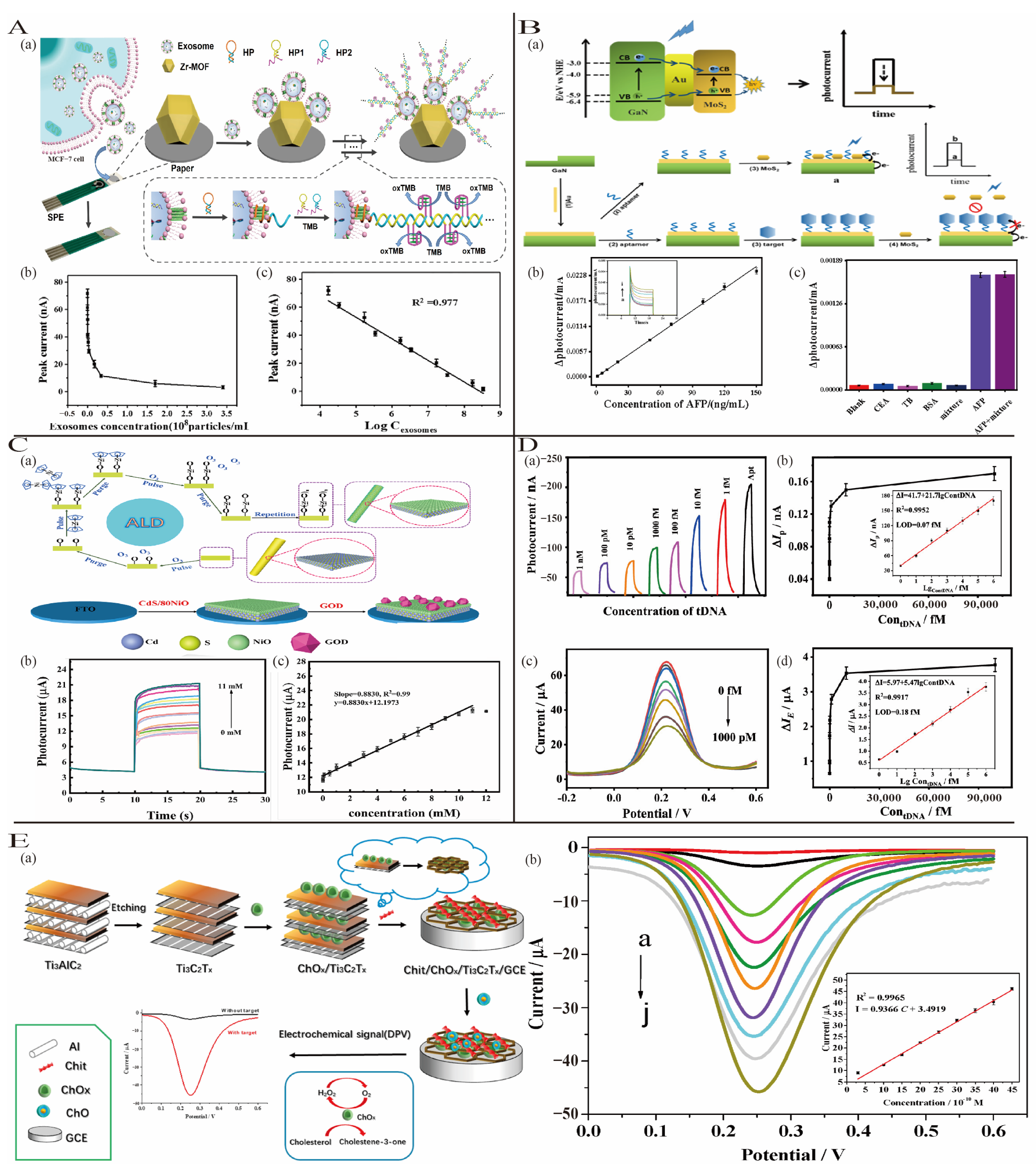
3.2.3. Template Synthesis and Self-Assembly
3.3. Detection Strategies for Enhancing the Performance of Electrochemical Biosensors
3.3.1. Strategy Based on Nucleic Acid
3.3.2. Strategy Based on Enzyme
3.3.3. Strategy Based on Magnetic Nanoparticles
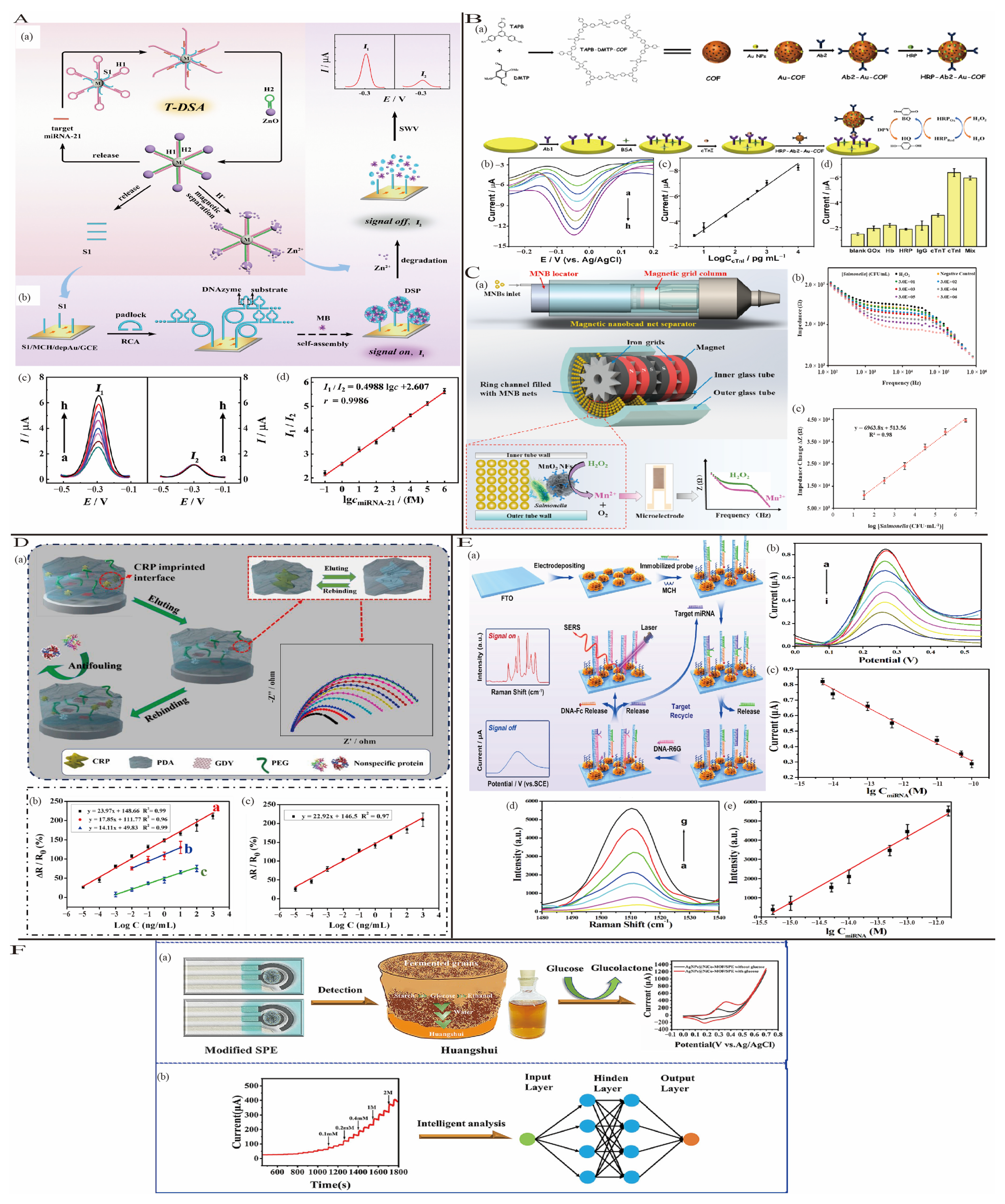
3.3.4. Strategy Based on Molecular Imprinting Technology (MIP)
3.3.5. Strategy Based on Multi-Modal/Signal Synergy
3.3.6. Strategy Based on Machine Learning
4. Electrochemical Biosensors for Diseases Detection
4.1. Cancer
4.1.1. Leukemia
4.1.2. Lung Cancer
4.1.3. Ovarian Cancer
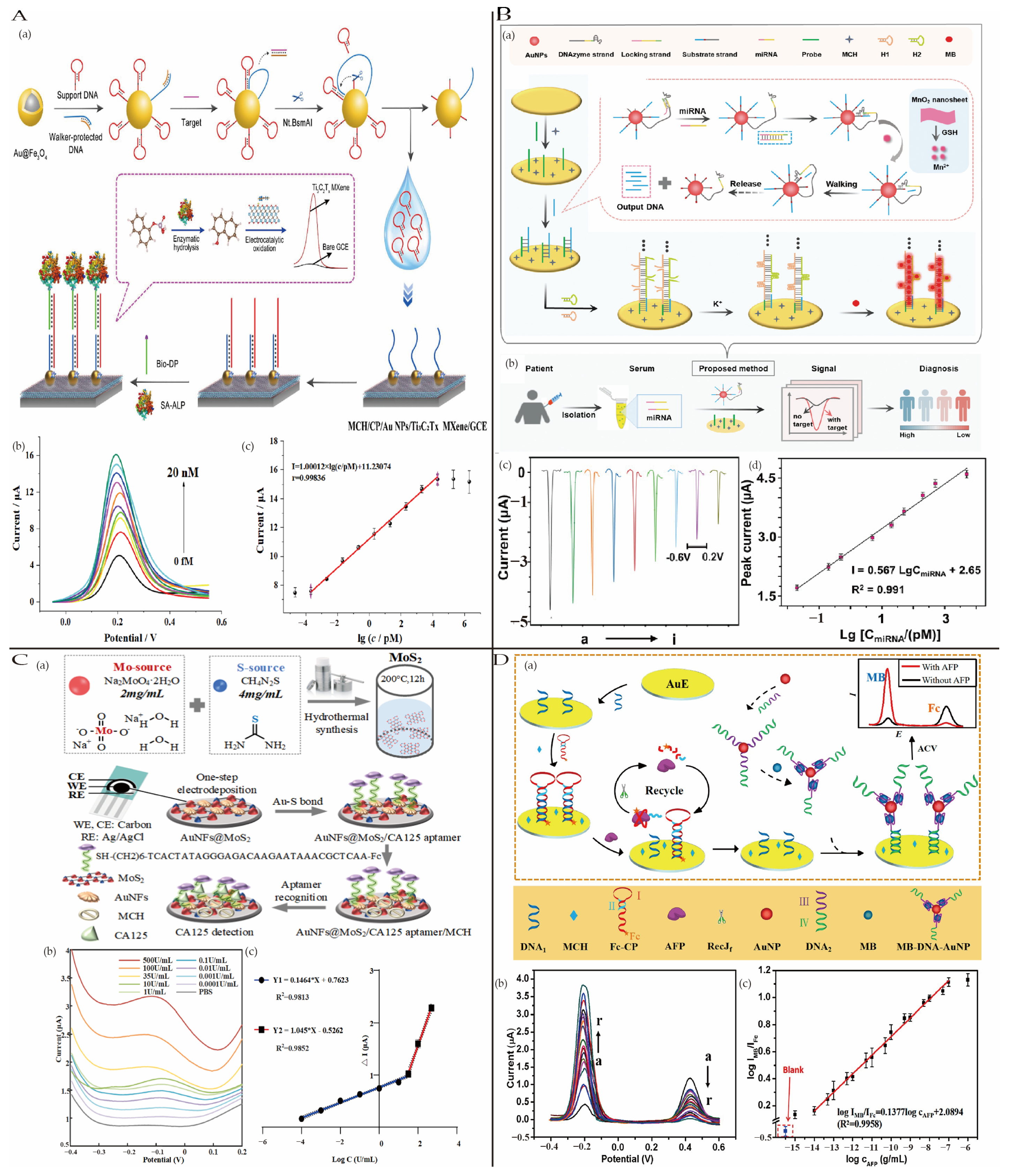
4.1.4. Other Cancers
4.2. Infectious Diseases
4.2.1. Coronavirus Disease 2019
4.2.2. Acquired Immune Deficiency Syndrome
4.2.3. Monkeypox
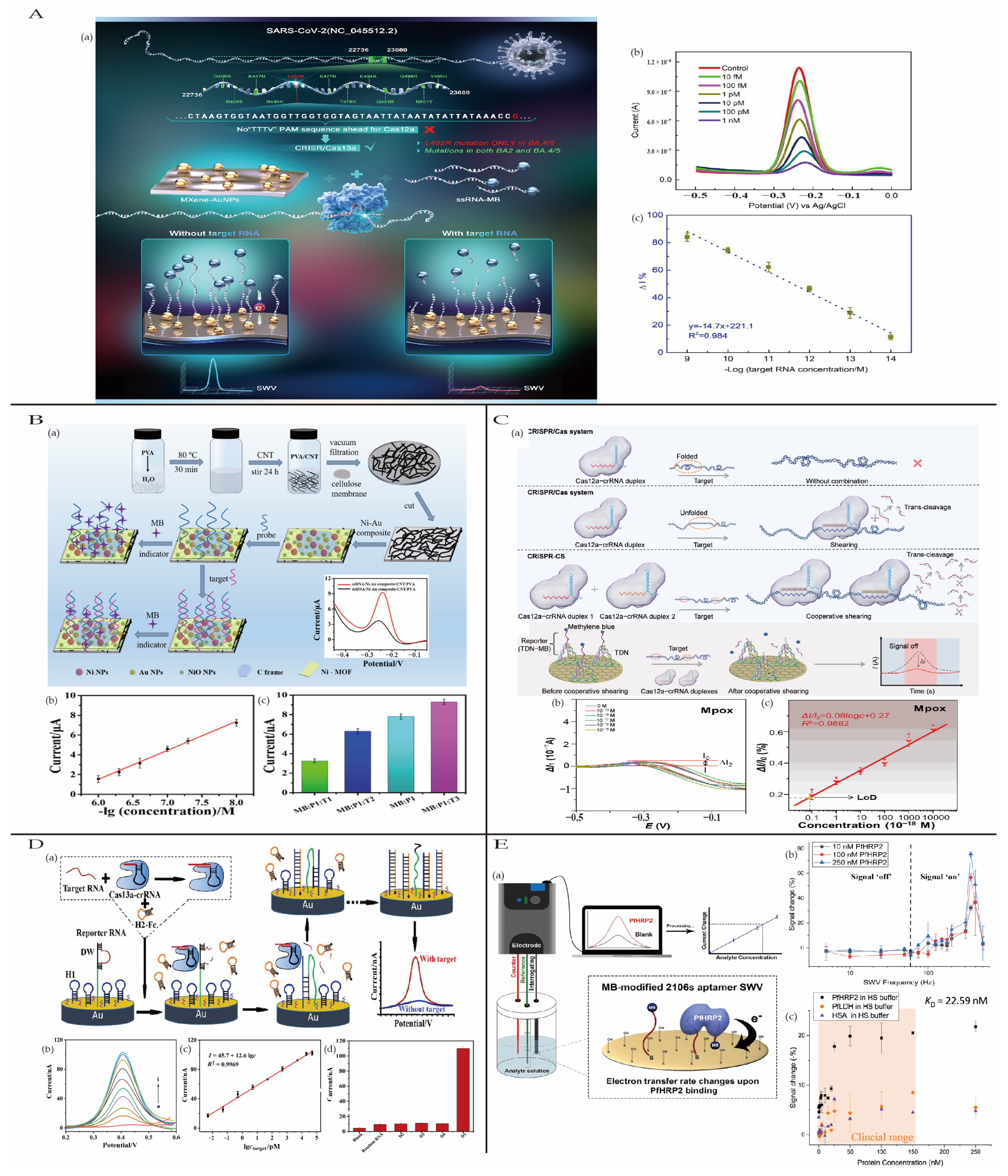
4.2.4. Dengue Fever
4.2.5. Malaria
4.3. Inflammatory Diseases
4.3.1. Sepsis
4.3.2. Viral Hepatitis
4.3.3. Other Inflammatory Diseases
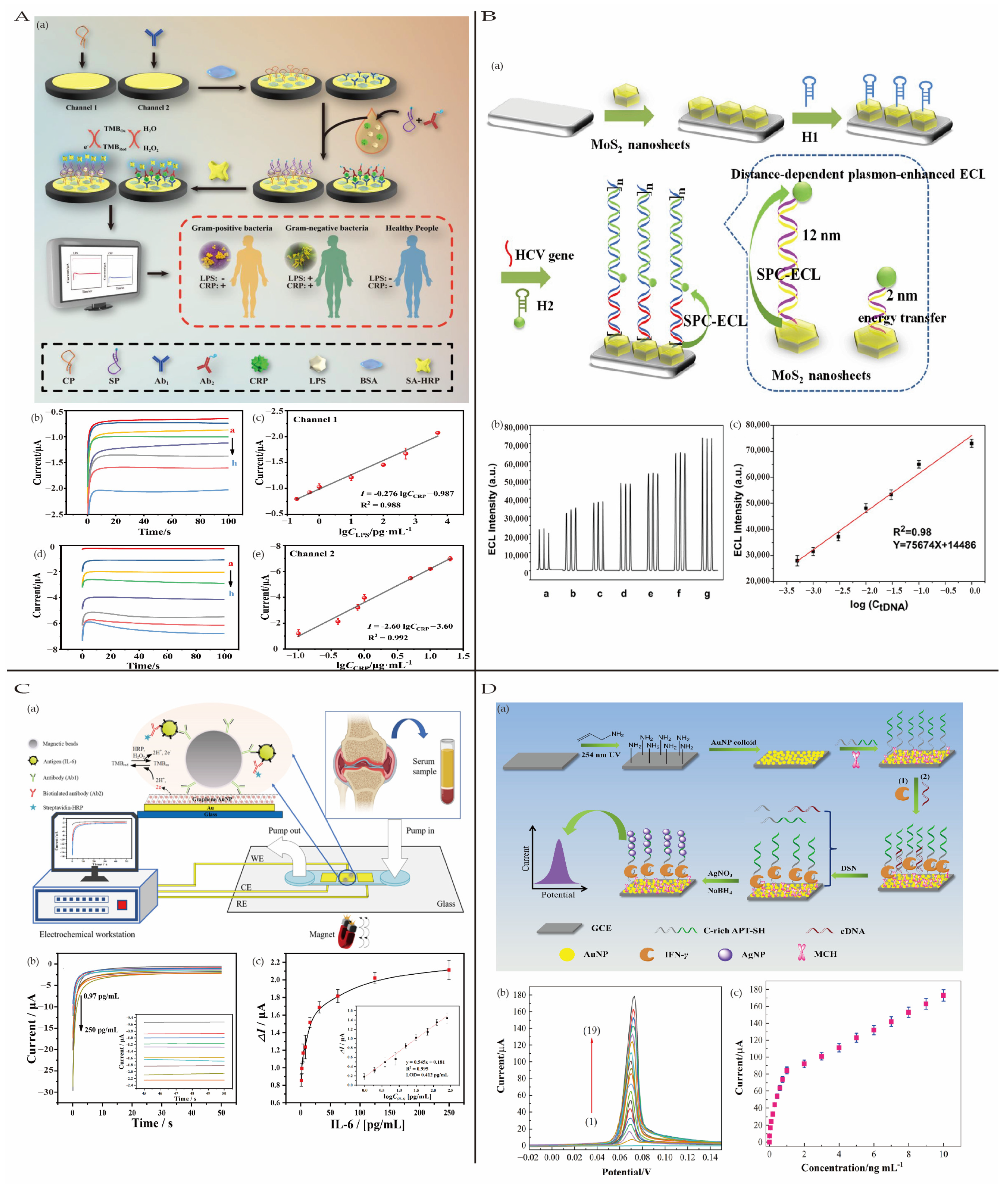
4.3.4. Inflammatory Biomarkers
4.4. Neurodegenerative Diseases
4.4.1. Alzheimer’s Disease
4.4.2. Parkinson’s Disease
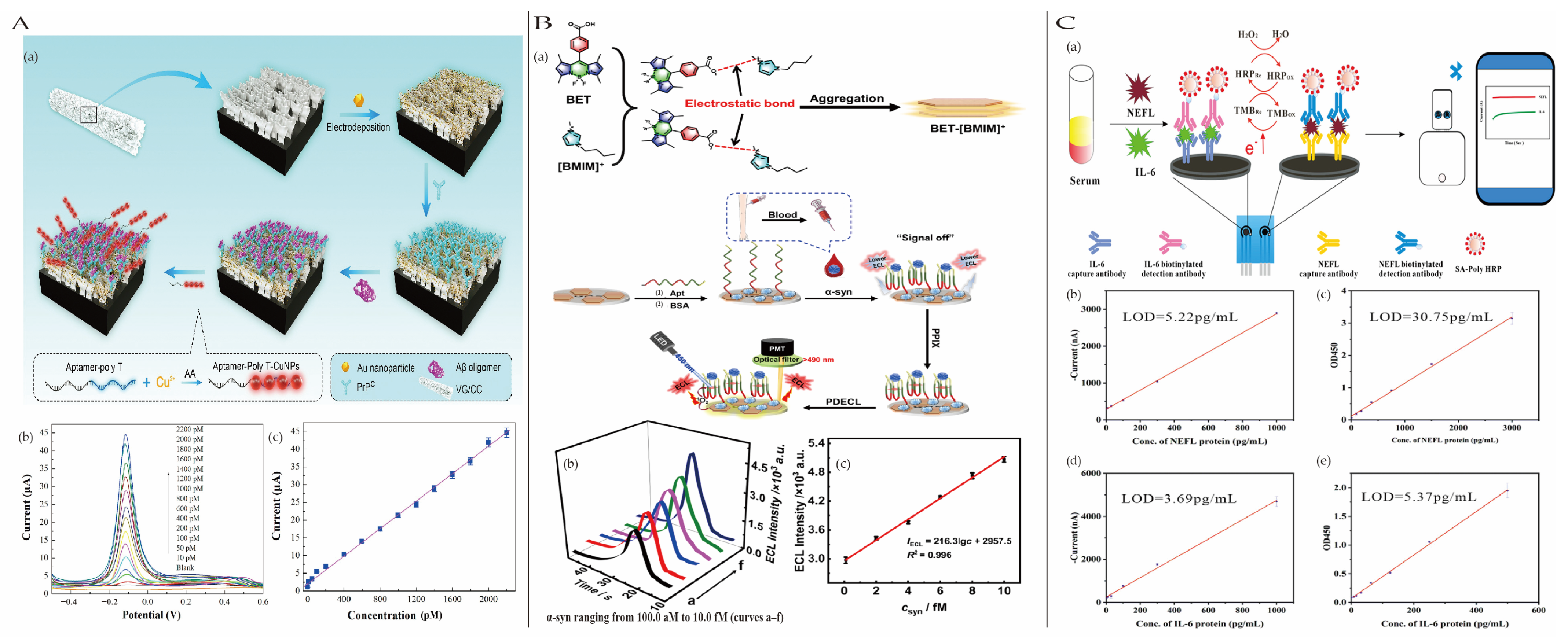
4.4.3. Other Neurodegenerative Disease Biomarkers
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-Care Diagnostics for Infectious Diseases: From Methods to Devices. Nano Today 2021, 37, 101092. [Google Scholar] [CrossRef] [PubMed]
- Paladiya, C.; Kiani, A. Nano Structured Sensing Surface: Significance in Sensor Fabrication. Sens. Actuators B Chem. 2018, 268, 494–511. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Cancelliere, R.; Cosio, T.; Campione, E.; Corvino, M.; D’Amico, M.P.; Micheli, L.; Signori, E.; Contini, G. Label-Free Electrochemical Immunosensor as a Reliable Point-of-Care Device for the Detection of Interleukin-6 in Serum Samples from Patients with Psoriasis. Front. Chem. 2023, 11, 1251360. [Google Scholar] [CrossRef]
- Cancelliere, R.; Paialunga, E.; Grattagliano, A.; Micheli, L. Label-Free Electrochemical Immunosensors: A Practical Guide. TrAC Trends Anal. Chem. 2024, 180, 117949. [Google Scholar] [CrossRef]
- Kumar, A.; Mahato, K.; Purohit, B.; Chandra, P. Commercial Aspects and Market Pull of Biosensors in Diagnostic Industries. In Miniaturized Biosensing Devices: Fabrication and Applications; Chandra, P., Mahato, K., Eds.; Springer Nature: Singapore, 2022; pp. 351–368. [Google Scholar]
- Pollard, T.D.; Ong, J.J.; Goyanes, A.; Orlu, M.; Gaisford, S.; Elbadawi, M.; Basit, A.W. Electrochemical Biosensors: A Nexus for Precision Medicine. Drug Discov. Today 2021, 26, 69–79. [Google Scholar] [CrossRef]
- Ilyin, S.E.; Belkowski, S.M.; Plata-Salamán, C.R. Biomarker Discovery and Validation: Technologies and Integrative Approaches. Trends Biotechnol. 2004, 22, 411–416. [Google Scholar] [CrossRef]
- Bensalah, K.; Montorsi, F.; Shariat, S.F. Challenges of Cancer Biomarker Profiling. Eur. Urol. 2007, 52, 1601–1609. [Google Scholar] [CrossRef]
- Ahmad, A.; Imran, M.; Ahsan, H. Biomarkers as Biomedical Bioindicators: Approaches and Techniques for the Detection, Analysis, and Validation of Novel Biomarkers of Diseases. Pharmaceutics 2023, 15, 1630. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X.; Aihara, K.; Chen, L. Early Diagnosis of Complex Diseases by Molecular Biomarkers, Network Biomarkers, and Dynamical Network Biomarkers. Med. Res. Rev. 2014, 34, 455–478. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Devarajan, P. Characteristics of an Ideal Biomarker of Kidney Diseases. In Biomarkers of Kidney Disease; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–24. [Google Scholar]
- Ziemssen, T.; Akgün, K.; Brück, W. Molecular Biomarkers in Multiple Sclerosis. J. Neuroinflamm. 2019, 16, 272. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Freitas, A.C.; Pereira, R.; Duarte, A.C.; Rocha Santos, T.A.P. Recent Developments in Recognition Elements for Chemical Sensors and Biosensors. TrAC Trends Anal. Chem. 2015, 68, 2–17. [Google Scholar] [CrossRef]
- Majdinasab, M.; Mitsubayashi, K.; Marty, J.L. Optical and Electrochemical Sensors and Biosensors for the Detection of Quinolones. Trends Biotechnol. 2019, 37, 898–915. [Google Scholar] [CrossRef]
- Bordbar, M.M.; Sheini, A.; Hashemi, P.; Hajian, A.; Bagheri, H. Disposable Paper-Based Biosensors for the Point-of-Care Detection of Hazardous Contaminations—A Review. Biosensors 2021, 11, 316. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical Biosensors. Chem. Soc. Rev. 2010, 39, 1747. [Google Scholar] [CrossRef] [PubMed]
- Perumal, V.; Hashim, U. Advances in Biosensors: Principle, Architecture and Applications. J. Appl. Biomed. 2014, 12, 1–15. [Google Scholar] [CrossRef]
- Malekzad, H.; Zangabad, P.S.; Mirshekari, H.; Karimi, M.; Hamblin, M.R. Noble Metal Nanoparticles in Biosensors: Recent Studies and Applications. Nanotechnol. Rev. 2017, 6, 301–329. [Google Scholar] [CrossRef]
- Lei, J.; Ju, H. Signal Amplification Using Functional Nanomaterials for Biosensing. Chem. Soc. Rev. 2012, 41, 2122–2134. [Google Scholar] [CrossRef]
- Dou, Y.; Li, Z.; Su, J.; Song, S. A Portable Biosensor Based on Au Nanoflower Interface Combined with Electrochemical Immunochromatography for POC Detection of Prostate-Specific Antigen. Biosensors 2022, 12, 259. [Google Scholar] [CrossRef]
- Pourakbari, R.; Shadjou, N.; Yousefi, H.; Isildak, I.; Yousefi, M.; Rashidi, M.-R.; Khalilzadeh, B. Recent Progress in Nanomaterial-Based Electrochemical Biosensors for Pathogenic Bacteria. Microchim. Acta 2019, 186, 820. [Google Scholar] [CrossRef]
- Mu, Z.; Tian, J.; Wang, J.; Zhou, J.; Bai, L. A New Electrochemical Aptasensor for Ultrasensitive Detection of Endotoxin Using Fe-MOF and AgNPs Decorated P-N-CNTs as Signal Enhanced Indicator. Appl. Surf. Sci. 2022, 573, 151601. [Google Scholar] [CrossRef]
- Hong, G.; Diao, S.; Antaris, A.L.; Dai, H. Carbon Nanomaterials for Biological Imaging and Nanomedicinal Therapy. Chem. Rev. 2015, 115, 10816–10906. [Google Scholar] [CrossRef] [PubMed]
- Speranza, G. Carbon Nanomaterials: Synthesis, Functionalization and Sensing Applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Sun, S.; Jiang, X.; Ai, Y.; Xu, W.; Xie, L.; Sun, H.; Liang, Q. Oligo-Layer Graphene Stabilized Fully Exposed Fe-Sites for Ultra-Sensitivity Electrochemical Detection of Dopamine. Biosens. Bioelectron. 2022, 211, 114367. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Jang, J. Conducting-Polymer Nanomaterials for High-Performance Sensor Applications: Issues and Challenges. Adv. Funct. Mater. 2009, 19, 1567–1576. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, Y.; Chen, Q.; Zhao, H.; Gao, B.; Zhang, T. A Flexible Electrochemical Biosensor Based on Functionalized Poly(3,4-Ethylenedioxythiophene) Film to Detect Lactate in Sweat of the Human Body. J. Colloid. Interface Sci. 2022, 617, 454–462. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, J.; Dong, J.; Wen, L.; Hu, Z.; He, C.; Xu, F.; Huo, D.; Hou, C. Simultaneous Detection of Exosomal microRNAs by Nucleic Acid Functionalized Disposable Paper-Based Sensors. Chem. Eng. J. 2022, 438, 135594. [Google Scholar] [CrossRef]
- Yang, T.; Xu, C.; Liu, C.; Ye, Y.; Sun, Z.; Wang, B.; Luo, Z. Conductive Polymer Hydrogels Crosslinked by Electrostatic Interaction with PEDOT:PSS Dopant for Bioelectronics Application. Chem. Eng. J. 2022, 429, 132430. [Google Scholar] [CrossRef]
- Yuan, R.; Li, H.-K.; He, H. Recent Advances in Metal/Covalent Organic Framework-Based Electrochemical Aptasensors for Biosensing Applications. Dalton Trans. 2021, 50, 14091–14104. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Khan, A.; Ghosh, T.; Kim, J.T.; Rhim, J.-W. Advances and Prospects for Biochar Utilization in Food Processing and Packaging Applications. Sustain. Mater. Technol. 2024, 39, e00831. [Google Scholar] [CrossRef]
- Cancelliere, R.; Cianciaruso, M.; Carbone, K.; Micheli, L. Biochar: A Sustainable Alternative in the Development of Electrochemical Printed Platforms. Chemosensors 2022, 10, 344. [Google Scholar] [CrossRef]
- Cao, L.; Ding, Q.; Liu, M.; Lin, H.; Yang, D.-P. Biochar-Supported Cu2+/Cu+ Composite as an Electrochemical Ultrasensitive Interface for Ractopamine Detection. ACS Appl. Bio Mater. 2021, 4, 1424–1431. [Google Scholar] [CrossRef]
- Marcisz, K.; Kaniewska, K.; Karbarz, M. Smart Functionalized Thin Gel Layers for Electrochemical Sensors, Biosensors and Devices. Curr. Opin. Electrochem. 2020, 23, 57–64. [Google Scholar] [CrossRef]
- Nishat, Z.S.; Hossain, T.; Islam, N.; Phan, H.; Wahab, A.; Moni, M.A.; Salomon, C.; Amin, M.A.; Ibn Sina, A.A.; Hossain, S.A.; et al. Hydrogel Nanoarchitectonics: An Evolving Paradigm for Ultrasensitive Biosensing. Small 2022, 18, 2107571. [Google Scholar] [CrossRef]
- Zhang, S.; Wright, G.; Yang, Y. Materials and Techniques for Electrochemical Biosensor Design and Construction. Biosens. Bioelectron. 2000, 15, 273–282. [Google Scholar] [CrossRef]
- Tan, H.W.; Choong, Y.Y.C.; Kuo, C.N.; Low, H.Y.; Chua, C.K. 3D Printed Electronics: Processes, Materials and Future Trends. Prog. Mater. Sci. 2022, 127, 100945. [Google Scholar] [CrossRef]
- Liu, X.; Gao, X.; Yang, L.; Zhao, Y.; Li, F. Metal–Organic Framework-Functionalized Paper-Based Electrochemical Biosensor for Ultrasensitive Exosome Assay. Anal. Chem. 2021, 93, 11792–11799. [Google Scholar] [CrossRef] [PubMed]
- Noč, L.; Jerman, I. Review of the Spectrally Selective (CSP) Absorber Coatings, Suitable for Use in SHIP. Sol. Energy Mater. Sol. Cells 2022, 238, 111625. [Google Scholar] [CrossRef]
- Hu, D.; Cui, H.; Wang, X.; Luo, F.; Qiu, B.; Cai, W.; Huang, H.; Wang, J.; Lin, Z. Highly Sensitive and Selective Photoelectrochemical Aptasensors for Cancer Biomarkers Based on MoS2/Au/GaN Photoelectrodes. Anal. Chem. 2021, 93, 7341–7347. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, F.; Zhang, B.; Li, S.; Shang, H. Precisely Controlled CdS/NiO Nanomaterials by Atomic Layer Deposition for Excellent Photoelectrochemical Biosensor. J. Alloys Compd. 2022, 928, 167052. [Google Scholar] [CrossRef]
- Xu, M.; Chen, K.; Zhu, L.; Zhang, S.; Wang, M.; He, L.; Zhang, Z.; Du, M. MOF@COF Heterostructure Hybrid for Dual-Mode Photoelectrochemical–Electrochemical HIV-1 DNA Sensing. Langmuir 2021, 37, 13479–13492. [Google Scholar] [CrossRef]
- Liu, Y.; Goebl, J.; Yin, Y. Templated Synthesis of Nanostructured Materials. Chem. Soc. Rev. 2013, 42, 2610–2653. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zuo, Y.Y. Molecular and Colloidal Self-Assembly at the Oil–Water Interface. Curr. Opin. Colloid. Interface Sci. 2022, 62, 101639. [Google Scholar] [CrossRef]
- Xia, T.; Liu, G.; Wang, J.; Hou, S.; Hou, S. MXene-Based Enzymatic Sensor for Highly Sensitive and Selective Detection of Cholesterol. Biosens. Bioelectron. 2021, 183, 113243. [Google Scholar] [CrossRef]
- He, L.; Huang, R.; Xiao, P.; Liu, Y.; Jin, L.; Liu, H.; Li, S.; Deng, Y.; Chen, Z.; Li, Z.; et al. Current Signal Amplification Strategies in Aptamer-Based Electrochemical Biosensor: A Review. Chin. Chem. Lett. 2021, 32, 1593–1602. [Google Scholar] [CrossRef]
- Kong, L.; Li, H.; Zhang, X.; Zhuo, Y.; Chai, Y.; Yuan, R. A Novel Ratiometric Electrochemical Biosensor Using Only One Signal Tag for Highly Reliable and Ultrasensitive Detection of miRNA-21. Anal. Chem. 2022, 94, 5167–5172. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Dong, S. Nucleic Acid Biosensors: Recent Advances and Perspectives. Anal. Chem. 2017, 89, 189–215. [Google Scholar] [CrossRef]
- Su, J.; Liu, W.; Chen, S.; Deng, W.; Dou, Y.; Zhao, Z.; Li, J.; Li, Z.; Yin, H.; Ding, X.; et al. A Carbon-Based DNA Framework Nano–Bio Interface for Biosensing with High Sensitivity and a High Signal-to-Noise Ratio. ACS Sens. 2020, 5, 3979–3987. [Google Scholar] [CrossRef]
- Song, L.; Zhuge, Y.; Zuo, X.; Li, M.; Wang, F. DNA Walkers for Biosensing Development. Adv. Sci. 2022, 9, 2200327. [Google Scholar] [CrossRef]
- Miao, P.; Chai, H.; Tang, Y. DNA Hairpins and Dumbbell-Wheel Transitions Amplified Walking Nanomachine for Ultrasensitive Nucleic Acid Detection. ACS Nano 2022, 16, 4726–4733. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Z.; Gan, L.; Fan, D.; Sun, X.; Qian, Z.; Liu, X.; Huang, Y. Signal Amplification-Based Biosensors and Application in RNA Tumor Markers. Sensors 2023, 23, 4237. [Google Scholar] [CrossRef]
- Wang, X.; Lu, D.; Liu, Y.; Wang, W.; Ren, R.; Li, M.; Liu, D.; Liu, Y.; Liu, Y.; Pang, G. Electrochemical Signal Amplification Strategies and Their Use in Olfactory and Taste Evaluation. Biosensors 2022, 12, 566. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yan, M.; Xue, Y.; Huang, J.; Yang, X. Electrochemical Immunosensor for Cardiac Troponin I Detection Based on Covalent Organic Framework and Enzyme-Catalyzed Signal Amplification. Anal. Chem. 2021, 93, 13572–13579. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, M.; Shadjou, N.; de la Guardia, M. Iron and Iron-Oxide Magnetic Nanoparticles as Signal-Amplification Elements in Electrochemical Biosensing. TrAC Trends Anal. Chem. 2015, 72, 1–9. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, E. Electrochemical Biosensors Based on Magnetic Micro/Nano Particles. Electrochim. Acta 2012, 84, 62–73. [Google Scholar] [CrossRef]
- Xue, L.; Guo, R.; Huang, F.; Qi, W.; Liu, Y.; Cai, G.; Lin, J. An Impedance Biosensor Based on Magnetic Nanobead Net and MnO2 Nanoflowers for Rapid and Sensitive Detection of Foodborne Bacteria. Biosens. Bioelectron. 2021, 173, 112800. [Google Scholar] [CrossRef]
- Cui, M.; Che, Z.; Gong, Y.; Li, T.; Hu, W.; Wang, S. A Graphdiyne-Based Protein Molecularly Imprinted Biosensor for Highly Sensitive Human C-Reactive Protein Detection in Human Serum. Chem. Eng. J. 2022, 431, 133455. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, J.; Li, B.; Liu, J.; Xu, J.-J.; Chen, H.-Y. Dual-Mode SERS and Electrochemical Detection of miRNA Based on Popcorn-like Gold Nanofilms and Toehold-Mediated Strand Displacement Amplification Reaction. Anal. Chem. 2021, 93, 6120–6127. [Google Scholar] [CrossRef]
- Ma, Y.; Leng, Y.; Huo, D.; Zhao, D.; Zheng, J.; Zhao, P.; Yang, H.; Li, F.; Hou, C. A Portable Sensor for Glucose Detection in Huangshui Based on Blossom-Shaped Bimetallic Organic Framework Loaded with Silver Nanoparticles Combined with Machine Learning. Food Chem. 2023, 429, 136850. [Google Scholar] [CrossRef]
- Tarannum, N.; Khatoon, S.; Dzantiev, B.B. Perspective and Application of Molecular Imprinting Approach for Antibiotic Detection in Food and Environmental Samples: A Critical Review. Food Control 2020, 118, 107381. [Google Scholar] [CrossRef]
- Ganju, S.; Gogate, P.R. A Review on Approaches for Efficient Recovery of Whey Proteins from Dairy Industry Effluents. J. Food Eng. 2017, 215, 84–96. [Google Scholar] [CrossRef]
- Han, Q.; Wang, H.; Wang, J. Multi-Mode/Signal Biosensors: Electrochemical Integrated Sensing Techniques. Adv. Funct. Mater. 2024, 34, 2403122. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent Advances in Electrochemical Biosensors: Applications, Challenges, and Future Scope. Biosensors 2021, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Yue, Y.; Zhang, Y.; Zhang, Z.; Zhou, H.S. Advancing Biosensors with Machine Learning. ACS Sens. 2020, 5, 3346–3364. [Google Scholar] [CrossRef]
- Molinara, M.; Cancelliere, R.; Di Tinno, A.; Ferrigno, L.; Shuba, M.; Kuzhir, P.; Maffucci, A.; Micheli, L. A Deep Learning Approach to Organic Pollutants Classification Using Voltammetry. Sensors 2022, 22, 8032. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Nagai, H.; Kim, Y.H. Cancer Prevention from the Perspective of Global Cancer Burden Patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef]
- Chikkaveeraiah, B.V.; Bhirde, A.A.; Morgan, N.Y.; Eden, H.S.; Chen, X. Electrochemical Immunosensors for Detection of Cancer Protein Biomarkers. ACS Nano 2012, 6, 6546–6561. [Google Scholar] [CrossRef]
- Whiteley, A.E.; Price, T.T.; Cantelli, G.; Sipkins, D.A. Leukaemia: A Model Metastatic Disease. Nat. Rev. Cancer 2021, 21, 461–475. [Google Scholar] [CrossRef]
- Grimwade, D.; Ivey, A.; Huntly, B.J.P. Molecular Landscape of Acute Myeloid Leukemia in Younger Adults and Its Clinical Relevance. Blood 2016, 127, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Y.; Huang, L.-X.; Xu, Z.-W.; Wang, P.; Lei, Y.; Liu, A.-L. Efficient Determination of PML/RARα Fusion Gene by the Electrochemical DNA Biosensor Based on Carbon Dots/Graphene Oxide Nanocomposites. Int. J. Nanomed. 2021, 16, 3497–3508. [Google Scholar] [CrossRef]
- Sawyers, C.L. Chronic Myeloid Leukemia. N. Engl. J. Med. 1999, 340, 1330–1340. [Google Scholar] [CrossRef]
- Yu, R.; Xue, J.; Wang, Y.; Qiu, J.; Huang, X.; Chen, A.; Xue, J. Novel Ti3C2Tx MXene Nanozyme with Manageable Catalytic Activity and Application to Electrochemical Biosensor. J. Nanobiotechnol. 2022, 20, 119. [Google Scholar] [CrossRef]
- Yang, L.-Y.; Huang, J.-L.; Lin, Y.; Cai, Q.-Q.; Zheng, Y.-J.; Wu, Y.; Chen, J.-Y.; Lin, X.-H. A Split-Type Electrochemical Biosensor Using Enzyme-Linked DNA Magnetic Beads Realizes the Detection of BCR/ABLp210 Fusion Gene in Clinical Samples: Duplex Ligation Chain Reaction Coupled with OR Logic Gate Design. Chem. Eng. J. 2024, 479, 147683. [Google Scholar] [CrossRef]
- Poon, C.; Haderi, A.; Roediger, A.; Yuan, M. Should We Screen for Lung Cancer? A 10-Country Analysis Identifying Key Decision-Making Factors. Health Policy 2022, 126, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, H.; Jiang, Y.; Fu, W.; Liu, X.; Zhong, R.; Cheng, B.; Zhu, F.; Xiang, Y.; He, J.; et al. Advances in Lung Cancer Screening and Early Detection. Cancer Biol. Med. 2022, 19, 591–608. [Google Scholar] [CrossRef]
- Villalobos, P.; Wistuba, I.I. Lung Cancer Biomarkers. Hematol./Oncol. Clin. N. Am. 2017, 31, 13–29. [Google Scholar] [CrossRef]
- Meng, F.; Yu, W.; Chen, C.; Guo, S.; Tian, X.; Miao, Y.; Ma, L.; Zhang, X.; Yu, Y.; Huang, L.; et al. A Versatile Electrochemical Biosensor for the Detection of Circulating MicroRNA toward Non-Small Cell Lung Cancer Diagnosis. Small 2022, 18, 2200784. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhuang, X.; Tian, C.; Fu, X.; Luan, F. Ru(Bpy)32+ Encapsulated Cyclodextrin Based Metal Organic Framework with Improved Biocompatibility for Sensitive Electrochemiluminescence Detection of CYFRA21-1 in Cell. Biosens. Bioelectron. 2021, 190, 113371. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, L.; Guntupalli, S.R. Treatment of Epithelial Ovarian Cancer. BMJ 2020, 371, m3773. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, G.; Li, M.; Guo, P.; Xiao, Y.; Ji, H.; Hao, Y. Global Patterns and Trends in Ovarian Cancer Incidence: Age, Period and Birth Cohort Analysis. BMC Cancer 2019, 19, 984. [Google Scholar] [CrossRef]
- Kobayashi, E.; Ueda, Y.; Matsuzaki, S.; Yokoyama, T.; Kimura, T.; Yoshino, K.; Fujita, M.; Kimura, T.; Enomoto, T. Biomarkers for Screening, Diagnosis, and Monitoring of Ovarian Cancer. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1902–1912. [Google Scholar] [CrossRef]
- Xu, X.; Tang, L.; Yu, Y.; Zhang, J.; Zhou, X.; Zhou, T.; Xuan, C.; Tian, Q.; Pan, D. Cooperative Amplification of Prussian Blue as a Signal Indicator and Functionalized Metal-Organic Framework-Based Electrochemical Biosensor for an Ultrasensitive HE4 Assay. Biosens. Bioelectron. 2024, 262, 116541. [Google Scholar] [CrossRef]
- Hu, C.; Qin, Z.; Fu, J.; Gao, Q.; Chen, C.; Tan, C.S.; Li, S. Aptamer-Based Carbohydrate Antigen 125 Sensor with Molybdenum Disulfide Functional Hybrid Materials. Anal. Biochem. 2023, 675, 115213. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, C.; Zhang, C.; Wen, K.; Zhu, Y. Bi-Directionally Amplified Ratiometric Electrochemical Aptasensor for the Ultrasensitive Detection of Alpha-Fetoprotein. Sens. Actuators B Chem. 2020, 323, 128666. [Google Scholar] [CrossRef]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, G.; Chen, J.; Wang, L.; Hu, Q.; Wu, J.; Zhang, W.; Song, M.; Qiao, J.; Xu, C. Electrochemical Biosensors for Measurement of Colorectal Cancer Biomarkers. Anal. Bioanal. Chem. 2021, 413, 2407–2428. [Google Scholar] [CrossRef]
- Wang, M.; Pan, Y.; Wu, S.; Sun, Z.; Wang, L.; Yang, J.; Yin, Y.; Li, G. Detection of Colorectal Cancer-Derived Exosomes Based on Covalent Organic Frameworks. Biosens. Bioelectron. 2020, 169, 112638. [Google Scholar] [CrossRef]
- Li, M.; Li, Z.; Wang, P.; Ma, Q. A Novel Bimetallic MXene Derivative QD-Based ECL Sensor for miRNA-27a-3p Detection. Biosens. Bioelectron. 2023, 228, 115225. [Google Scholar] [CrossRef] [PubMed]
- Sauzay, C.; Petit, A.; Bourgeois, A.-M.; Barbare, J.-C.; Chauffert, B.; Galmiche, A.; Houessinon, A. Alpha-Foetoprotein (AFP): A Multi-Purpose Marker in Hepatocellular Carcinoma. Clin. Chim. Acta 2016, 463, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Xie, C.; Li, Q.; Yang, M.; Li, S.; Li, H.; Xia, F. Electrochemical Biosensors for the Analysis of Breast Cancer Biomarkers: From Design to Application. Anal. Chem. 2022, 94, 269–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, J.; Lu, L.; Yang, X.; Xia, J.; Zhang, F.; Wang, Z. Competitive Electrochemical Aptasensor Based on a cDNA-Ferrocene/MXene Probe for Detection of Breast Cancer Marker Mucin1. Anal. Chim. Acta 2020, 1094, 18–25. [Google Scholar] [CrossRef]
- Yang, C.; Guo, Q.; Lu, Y.; Zhang, B.; Nie, G. Ultrasensitive “Signal-on” Electrochemiluminescence Immunosensor for Prostate-Specific Antigen Detection Based on Novel Nanoprobe and Poly(Indole-6-Carboxylic Acid)/Flower-like Au Nanocomposite. Sens. Actuators B Chem. 2020, 303, 127246. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, Y.; Huang, Q.; Huang, J.; Tao, Y.; Chen, J.; Li, H.-Y.; Liu, H. Electrochemical Protein Biosensors for Disease Marker Detection: Progress and Opportunities. Microsyst. Nanoeng. 2024, 10, 65. [Google Scholar] [CrossRef]
- Morens, D.M.; Folkers, G.K.; Fauci, A.S. The Challenge of Emerging and Re-Emerging Infectious Diseases. Nature 2004, 430, 242–249. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Hu, Z.; Yan, X.; Gao, Q.; Li, X.; Zheng, J.; Li, B.; Wu, Y.; Liao, Y. Advancing Aggregation-Induced Emission-Derived Biomaterials in Viral, Tuberculosis, and Fungal Infectious Diseases. Aggregate 2024, e715. [Google Scholar] [CrossRef]
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 Infection: Emergence, Transmission, and Characteristics of Human Coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, F.; Xie, W.; Zhou, T.-C.; OuYang, J.; Jin, L.; Li, H.; Zhao, C.-Y.; Zhang, L.; Wei, J.; et al. Ultrasensitive Supersandwich-Type Electrochemical Sensor for SARS-CoV-2 from the Infected COVID-19 Patients Using a Smartphone. Sens. Actuators B Chem. 2021, 327, 128899. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yisimayi, A.; Jian, F.; Song, W.; Xiao, T.; Wang, L.; Du, S.; Wang, J.; Li, Q.; Chen, X.; et al. BA.2.12.1, BA.4 and BA.5 Escape Antibodies Elicited by Omicron Infection. Nature 2022, 608, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Tegally, H.; Moir, M.; Everatt, J.; Giovanetti, M.; Scheepers, C.; Wilkinson, E.; Subramoney, K.; Makatini, Z.; Moyo, S.; Amoako, D.G.; et al. Emergence of SARS-CoV-2 Omicron Lineages BA.4 and BA.5 in South Africa. Nat. Med. 2022, 28, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, C.; Yuan, Y.; Xie, Z.; Li, T.; Huang, H.; Li, S.; Deng, J.; Lin, H.; Shi, Z.; et al. CRISPR-Cas13a-Powered Electrochemical Biosensor for the Detection of the L452R Mutation in Clinical Samples of SARS-CoV-2 Variants. J. Nanobiotechnol. 2023, 21, 141. [Google Scholar] [CrossRef]
- Fatin, M.F.; Ruslinda, A.R.; Md Arshad, M.K.; Tee, K.K.; Ayub, R.M.; Hashim, U.; Kamarulzaman, A.; Gopinath, S.C.B. HIV-1 Tat Biosensor: Current Development and Trends for Early Detection Strategies. Biosens. Bioelectron. 2016, 78, 358–366. [Google Scholar] [CrossRef]
- Cai, Q.; Wu, D.; Li, H.; Jie, G.; Zhou, H. Versatile Photoelectrochemical and Electrochemiluminescence Biosensor Based on 3D CdSe QDs-DNA Nanonetwork-SnO2 Nanoflower Coupled with DNA Walker Amplification for HIV Detection. Biosens. Bioelectron. 2021, 191, 113455. [Google Scholar] [CrossRef]
- Lu, Q.; Su, T.; Shang, Z.; Jin, D.; Shu, Y.; Xu, Q.; Hu, X. Flexible Paper-Based Ni-MOF Composite/AuNPs/CNTs Film Electrode for HIV DNA Detection. Biosens. Bioelectron. 2021, 184, 113229. [Google Scholar] [CrossRef]
- Lum, F.-M.; Torres-Ruesta, A.; Tay, M.Z.; Lin, R.T.P.; Lye, D.C.; Rénia, L.; Ng, L.F.P. Monkeypox: Disease Epidemiology, Host Immunity and Clinical Interventions. Nat. Rev. Immunol. 2022, 22, 597–613. [Google Scholar] [CrossRef]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef]
- Chandran, M.; Chellasamy, G.; Veerapandian, M.; Dhanasekaran, B.; Kumar Arumugasamy, S.; Govindaraju, S.; Yun, K. Fabrication of Label-Free Immunoprobe for Monkeypox A29 Detection Using One-Step Electrodeposited Molybdenum Oxide-Graphene Quantum Rods. J. Colloid. Interface Sci. 2024, 660, 412–422. [Google Scholar] [CrossRef]
- Zhao, J.; Kong, D.; Zhang, G.; Zhang, S.; Wu, Y.; Dai, C.; Chen, Y.; Yang, Y.; Liu, Y.; Wei, D. An Efficient CRISPR/Cas Cooperative Shearing Platform for Clinical Diagnostics Applications. Angew. Chem. Int. Ed. 2024, 63, e202411705. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Q.; Wu, J.; Lin, Y.; Ju, H. A Sensitive Electrochemical Method for Rapid Detection of Dengue Virus by CRISPR/Cas13a-Assisted Catalytic Hairpin Assembly. Anal. Chim. Acta 2021, 1187, 339131. [Google Scholar] [CrossRef]
- Lo, Y.; Cheung, Y.-W.; Wang, L.; Lee, M.; Figueroa-Miranda, G.; Liang, S.; Mayer, D.; Tanner, J.A. An Electrochemical Aptamer-Based Biosensor Targeting Plasmodium Falciparum Histidine-Rich Protein II for Malaria Diagnosis. Biosens. Bioelectron. 2021, 192, 113472. [Google Scholar] [CrossRef]
- Huang, C.-C.; Hsu, C.-C.; Guo, H.-R.; Su, S.-B.; Lin, H.-J. Dengue Fever Mortality Score: A Novel Decision Rule to Predict Death from Dengue Fever. J. Infect. 2017, 75, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Duffy, P.E.; Gorres, J.P.; Healy, S.A.; Fried, M. Malaria Vaccines: A New Era of Prevention and Control. Nat. Rev. Microbiol. 2024, 22, 756–772. [Google Scholar] [CrossRef]
- Krampa, F.D.; Aniweh, Y.; Kanyong, P.; Awandare, G.A. Recent Advances in the Development of Biosensors for Malaria Diagnosis. Sensors 2020, 20, 799. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Muhammad Khan, A.; Khan, A.; Shal, B.; Aziz, A.; Ahmed, M.N.; Khan, S. The Newly Synthesized Compounds (NCHDH and NTHDH) Attenuates LPS-Induced Septicemia and Multi-Organ Failure via Nrf2/HO1 and HSP/TRVP1 Signaling in Mice. Chem.-Biol. Interact. 2020, 329, 109220. [Google Scholar] [CrossRef]
- Cavaillon, J.-M.; Chrétien, F. From Septicemia to Sepsis 3.0—From Ignaz Semmelweis to Louis Pasteur. Microbes Infect. 2019, 21, 213–221. [Google Scholar] [CrossRef]
- Sheini, A. A Point-of-Care Testing Sensor Based on Fluorescent Nanoclusters for Rapid Detection of Septicemia in Children. Sens. Actuators B Chem. 2021, 328, 129029. [Google Scholar] [CrossRef]
- Lu, T.-C.; Yang, Y.-J.; Zhong, Y.; Qiu, Q.-Z.; Chen, Z.-H.; Chen, Y.-Z.; Lei, Y.; Liu, A.-L. Simultaneous Detection of C-Reactive Protein and Lipopolysaccharide Based on a Dual-Channel Electrochemical Biosensor for Rapid Gram-Typing of Bacterial Sepsis. Biosens. Bioelectron. 2024, 243, 115772. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global Burden of Liver Disease: 2023 Update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- Iannacone, M.; Guidotti, L.G. Immunobiology and Pathogenesis of Hepatitis B Virus Infection. Nat. Rev. Immunol. 2022, 22, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-Z.; Liu, J.-L.; Chen, Y.-F.; Chai, Y.-Q.; Li, Z.-H.; Yuan, R. Boron and Nitrogen-Codoped Carbon Dots as Highly Efficient Electrochemiluminescence Emitters for Ultrasensitive Detection of Hepatitis B Virus DNA. Anal. Chem. 2022, 94, 7601–7608. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.-M.; Negro, F.; Aghemo, A.; Berenguer, M.; Dalgard, O.; Dusheiko, G.; Marra, F.; Puoti, M.; Wedemeyer, H. EASL Recommendations on Treatment of Hepatitis C: Final Update of the Series☆. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nie, Y.; Wang, M.; Zhang, Q.; Ma, Q. Distance-Dependent Plasmon-Enhanced Electrochemiluminescence Biosensor Based on MoS2 Nanosheets. Biosens. Bioelectron. 2020, 148, 111823. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 320, 1360. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, D.; Li, X.; Yuan, J. Microfluidic Electrochemical Magnetoimmunosensor for Ultrasensitive Detection of Interleukin-6 Based on Hybrid of AuNPs and Graphene. Talanta 2022, 240, 123173. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Dong, H.; Liu, Z.; Wang, L.; Li, Q.; Ren, J.; Zhang, Y.; Xu, M. Target-Induced Silver Nanocluster Generation for Highly Sensitive Electrochemical Aptasensor towards Cell-Secreted Interferon-γ. Biosens. Bioelectron. 2022, 203, 114042. [Google Scholar] [CrossRef]
- Blauwet, L.A.; Cooper, L.T. Myocarditis. Prog. Cardiovasc. Dis. 2010, 52, 274–288. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; He, Y.; Gao, J.; Li, W.; Cheng, L.; Sun, F.; Xia, P.; Wang, Q. A Generic and Non-Enzymatic Electrochemical Biosensor Integrated Molecular Beacon-like Catalyzed Hairpin Assembly Circuit with MOF@Au@G-Triplex/Hemin Nanozyme for Ultrasensitive Detection of miR-721. Biosens. Bioelectron. 2022, 203, 114051. [Google Scholar] [CrossRef]
- Wu, J.; Liang, L.; Zhang, M.; Zhu, R.; Wang, Z.; Yin, Y.; Yin, B.; Weng, T.; Fang, S.; Xie, W.; et al. Single-Molecule Identification of the Conformations of Human C-Reactive Protein and Its Aptamer Complex with Solid-State Nanopores. ACS Appl. Mater. Interfaces 2022, 14, 12077–12088. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Liu, Z.; Yan, Y.; Chen, J.; Yang, R.; Huang, Q.; Jin, M.; Shui, L. Triple Signal-Enhancing Electrochemical Aptasensor Based on Rhomboid Dodecahedra Carbonized-ZIF67 for Ultrasensitive CRP Detection. Biosens. Bioelectron. 2022, 207, 114129. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Lu, D.; Guo, D.; Xin, X. Dual-Optofluidic Waveguide in-Line Fiber Biosensor for Real-Time Label-Free Detection of Interferon-Gamma with Temperature Compensation. Opt. Express 2020, 28, 10491–10504. [Google Scholar] [CrossRef]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, 2004433. [Google Scholar] [CrossRef]
- Cancelliere, R.; Di Tinno, A.; Di Lellis, A.M.; Contini, G.; Micheli, L.; Signori, E. Cost-Effective and Disposable Label-Free Voltammetric Immunosensor for Sensitive Detection of Interleukin-6. Biosens. Bioelectron. 2022, 213, 114467. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, S.; Wang, Y.; Feng, W.; Li, B.; Jiao, J.; Han, B.; Chen, Q. A Novel Oriented Immunosensor Based on AuNPs-Thionine-CMWCNTs and Staphylococcal Protein A for Interleukin-6 Analysis in Complicated Biological Samples. Anal. Chim. Acta 2020, 1140, 145–152. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Q.; Chen, J.; Yao, J.; Jin, M.; Wang, X.; Akinoglu, E.M.; Zhang, M.; Li, N.; Shui, L. Nanoparticle-Assisted Sacrificial Synthesis of Hierarchical Porous Carbon Composite for Rapid Sample Enrichment and Ultrasensitive Label-Free Immunosensing of Interleukin-6 Biomarker. J. Electroanal. Chem. 2021, 883, 115068. [Google Scholar] [CrossRef]
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of Neurodegenerative Diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Scheltens, P.; Blennow, K.; Breteler, M.M.B.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; der Flier, W.M.V. Alzheimer’s Disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- 2020 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2020, 16, 391–460. [CrossRef]
- Zhou, Y.; Lv, Y.; Dong, H.; Liu, L.; Mao, G.; Zhang, Y.; Xu, M. Ultrasensitive Assay of Amyloid-Beta Oligomers Using Au-Vertical Graphene/Carbon Cloth Electrode Based on Poly(Thymine)-Templated Copper Nanoparticles as Probes. Sens. Actuators B Chem. 2021, 331, 129429. [Google Scholar] [CrossRef]
- Song, Y.; Xu, T.; Zhu, Q.; Zhang, X. Integrated Individually Electrochemical Array for Simultaneously Detecting Multiple Alzheimer’s Biomarkers. Biosens. Bioelectron. 2020, 162, 112253. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Darweesh, S.; Llibre-Guerra, J.; Marras, C.; Luciano, M.S.; Tanner, C. The Epidemiology of Parkinson’s Disease. Lancet 2024, 403, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the Diagnosis of Parkinson’s Disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Qiu, Z.; Liang, H.; Huang, F.; Wei, C.; Cui, J.; Song, Z.; Tang, Q.; Liao, X.; Liu, Z.; et al. Proximity Hybridization Induced Molecular Machine for Signal-on Electrochemical Detection of α-Synuclein Oligomers. Talanta 2024, 271, 125720. [Google Scholar] [CrossRef]
- Song, S.-S.; Liu, W.; Bao, J.-Y.; Zhu, H.-T.; Wang, A.-J.; Song, P.; Yuan, P.-X.; Feng, J.-J. Photodynamic-Assisted Electrochemiluminescence Enhancement toward Advanced BODIPY for Precision Diagnosis of Parkinson. Anal. Chem. 2024, 96, 8586–8593. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, L.; Dou, Y.; Liu, X.; Song, S.; Jiang, H.; Fan, C. Electrochemical Biosensor for Point-of-Care Testing of Low-Abundance Biomarkers of Neurological Diseases. Anal. Chem. 2024, 96, 10332–10340. [Google Scholar] [CrossRef]
- Chang, Y.; Chen, Y.; Shao, Y.; Li, B.; Wu, Y.; Zhang, W.; Zhou, Y.; Yu, Z.; Lu, L.; Wang, X.; et al. Solid-Phase Microextraction Integrated Nanobiosensors for the Serial Detection of Cytoplasmic Dopamine in a Single Living Cell. Biosens. Bioelectron. 2021, 175, 112915. [Google Scholar] [CrossRef]
- Zhao, R.; Li, D.; Yin, N.; Guo, Z.; Wang, D.; Yao, X. The High Sensitive and Selective Detection of Dopamine Based on Its Electropolymerization by Electrochemical Surface Plasmon Resonance. Sens. Actuators B Chem. 2022, 370, 132401. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, S.; Zang, X.; Ding, M.; Ding, C. Ratiometric Antifouling Electrochemical Biosensors Based on Designed Y-Shaped Peptide and MXene Loaded with Au@ZIF-67 and Methylene Blue. Microchim. Acta 2023, 191, 5. [Google Scholar] [CrossRef]
- Li, F.; Yang, W.; Zhao, B.; Yang, S.; Tang, Q.; Chen, X.; Dai, H.; Liu, P. Ultrasensitive DNA-Biomacromolecule Sensor for the Detection Application of Clinical Cancer Samples. Adv. Sci. 2022, 9, 2102804. [Google Scholar] [CrossRef]
- Huang, X.; Liang, B.; Huang, S.; Liu, Z.; Yao, C.; Zheng, S.; Zhang, T.; Liu, Z.; Wang, Y.; Wu, Y.; et al. Vertical Graphene-Based Multiparametric Sensing Array for Integration of Smart Catheter to Electrochemically Monitor Peritoneal Dialysis. Adv. Mater. 2025, 37, 2412302. [Google Scholar] [CrossRef] [PubMed]
| Cancer | Biomarker | Materials | Detection Methods | LOD | Range | Ref. |
|---|---|---|---|---|---|---|
| APL | PML/RARα fusion gene | CDs/GO/GCE | DPV | 83 pM | 2.5 × 10−10–2.25 × 10−9 M | [74] |
| CML | BCR/ABL fusion gene | Ti3C2Tx MXene | DPV | 0.05 fM | 0.2 fM–20 nM | [76] |
| CML | BCR/ABLp210 transcript | Enzyme-linked DNA-coating MBs | CA | 1 aM | 1.0 × 10−18–5.0 × 10−14 M 1.0 × 10−15–1.0 × 10−12 M | [77] |
| NSCLC | miRNA | G-quadruplex/dsDNA | LSV | 5.68 fM | 20 fM to 5 nM | [81] |
| NSCLC | CYFRA21-1 | CD-MOF@Ru(bpy)32+ | ECL | 0.006 ng/mL | 0.1–50 ng/mL | [82] |
| OC | HE4 | TiMOF-KB@AuNPs | DPV | 0.02 ng/mL | 0.1–80 ng/mL | [87] |
| OC | CA125 | AuNFs@MoS2/CA125 aptamer/MCH | DPV | 0.0001 U/mL | 0.0001–35 U/mL 35–500 U/mL | [88] |
| CRC | Exosomes | HRP-pSC4-AuNPs@COFs | CA | 160 particles/μL | 5 × 102 to 107 particles/μL | [92] |
| GC | miRNA-27a-3p | Mo2TiC2 QDs | ECL | 1 fM | 1 fM–10 nM | [93] |
| Liver cancer | AFP | MB-DNA-AuNP | ACV | 269.4 ag/mL | 10 fg/mL–100 ng/mL | [89] |
| Breast cancer | MUC1 | cDNA-ferrocene/MXene | SWV | 0.33 pM | 1.0 pM–10 μM | [96] |
| Prostate cancer | PSA | AuNP/GQDs-PEI-GO | ECL | 0.44 pg/mL | 0.001 ng/mL–100 ng/mL | [97] |
| Infectious Diseases | Biomarker | Materials | Detection Methods | LOD | Range | Ref. |
|---|---|---|---|---|---|---|
| COVID-19 | SARS-CoV-2 RNA | SCX8-RGO | DPV | 200 copies/mL | 10−17–10−12 M | [103] |
| Omicron | crRNA | AuE-MXene-AuNPs | SWV | 1 fM | 1 nM–10 fM | [106] |
| AIDS | HIV DNA | 3D CdSe QDs-DNA/SnO2 nanolowers | ECL/PEC | 1.38 fM | 0.5 μM–5 fM | [108] |
| AIDS | HIV DNA | Ni-MOF/AuNPs/CNTs | DPV | 0.13 nM | 10 nM–1 μM | [109] |
| Monkeypox | dsDNA | CRISPR-CS | DPV | 9.5 × 10−20 M | 10−21 M–10−12 M | [113] |
| Dengue | DENV-1 RNA | CRISPR/Cas13a | ACV | 0.78 fM | 5 fM–50 nM | [114] |
| Malaria | PfHRP2 | Aptamers | SWV | 3.73 nM | - | [115] |
| Inflammatory Diseases | Biomarker | Materials | Detection Methods | LOD | Range | Ref. |
|---|---|---|---|---|---|---|
| Sepsis | CRP LPS | LPS aptamers CRP antibodies | CA | LPS (0.343 pg/mL) CRP (0.05 μg/mL) | LPS (0.5–1000 pg/mL) CRP (0.1–20 μg/mL) | [122] |
| Hepatitis B | HBV-DNA | BN-CDs | ECL | 18.08 aM | 100 aM–1 nM | [125] |
| Hepatitis C | HCV gene | S-BN QDs | ECL | 0.17 pmoL/L | 5 pmoL/L–1 nmoL/L | [127] |
| RA | IL-6 | AuNPs/graphene | CA | 0.42 pg/mL | 0.97–250 pg/mL | [129] |
| Acute myocarditis | miR-721 | MOF@Au@G-triplex/hemin nanozyme | CA | 0.25 fM | 0.5 fM–1 nM | [132] |
| - | CRP | AuNPs@C-ZIF67 | DPV | 0.44 pg/mL | 10 pg/mL–10 μg/mL | [134] |
| - | IFN-γ | AgNCs | LSV | 1.7 pg/mL | 5–1000 pg/mL 1–10 ng/mL | [130] |
| - | IL-6 | Ab-SPA-Cys-AuNPs-THI-CMWCNTs | SWV | 2.87 pg/mL | 0.01–800 ng/mL | [138] |
| - | IL-6 | NMC@AuNPs | DPV | 0.14 pg/mL | 0.5–1200 pg/mL | [139] |
| Neurodegenerative Diseases | Biomarker | Materials | Detection Methods | LOD | Range | Ref. |
|---|---|---|---|---|---|---|
| AD | Aβ oligomers | Au-VG/CC | DPV | 3.5 pM | 10–2200 pM | [143] |
| AD | Tau ApoE4 Amyloid-β miRNA-101 | Gold nanodendrites/PDMS mini-pillar | SWV | Tau (5.91 × 10−11 mg/mL) ApoE4 (7.14 × 10−11 mg/mL) Amyloid-β (8.6 × 10−12 mg/mL) miRNA-101 (91.4 pM) | 10−10–10−7 | [144] |
| PD | α-synuclein oligomer | Mg2+-DNAzyme exhibit | DPV | 0.57 fg/mL | 1 fg/mL–10 pg/mL | [147] |
| PD | α-syn | BET-[BMIm] | PDECL | 63 aM | 100.0 aM–10.0 fM | [148] |
| - | NEFL IL-6 | CAb/SA-poly-HRP 80 | CA | NEFL (5.22 pg/mL) IL-6 (3.69 pg/mL) | NEFL (5.22–1000 pg/mL) IL-6 (3.69–1000 pg/mL) | [149] |
| - | DA | MHA | Amperometry | 1.4 pM | 0.01–1000 nM | [151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Xin, X.; Su, J.; Song, S. Research Progress of Electrochemical Biosensors for Diseases Detection in China: A Review. Biosensors 2025, 15, 231. https://doi.org/10.3390/bios15040231
Cui H, Xin X, Su J, Song S. Research Progress of Electrochemical Biosensors for Diseases Detection in China: A Review. Biosensors. 2025; 15(4):231. https://doi.org/10.3390/bios15040231
Chicago/Turabian StyleCui, Haoran, Xianglin Xin, Jing Su, and Shiping Song. 2025. "Research Progress of Electrochemical Biosensors for Diseases Detection in China: A Review" Biosensors 15, no. 4: 231. https://doi.org/10.3390/bios15040231
APA StyleCui, H., Xin, X., Su, J., & Song, S. (2025). Research Progress of Electrochemical Biosensors for Diseases Detection in China: A Review. Biosensors, 15(4), 231. https://doi.org/10.3390/bios15040231







