A Sensitive and Fast microRNA Detection Platform Based on CRlSPR-Cas12a Coupled with Hybridization Chain Reaction and Photonic Crystal Microarray
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instrumentation
2.3. Preparation of Hairpins and HCRs
2.4. CRISPR/Cas12a Cleavage
2.5. Detection on Printed Photonic Crystal Microarrays
2.6. Gel Electrophoresis Analysis
3. Results and Discussion
3.1. Principles of miRNA Detection
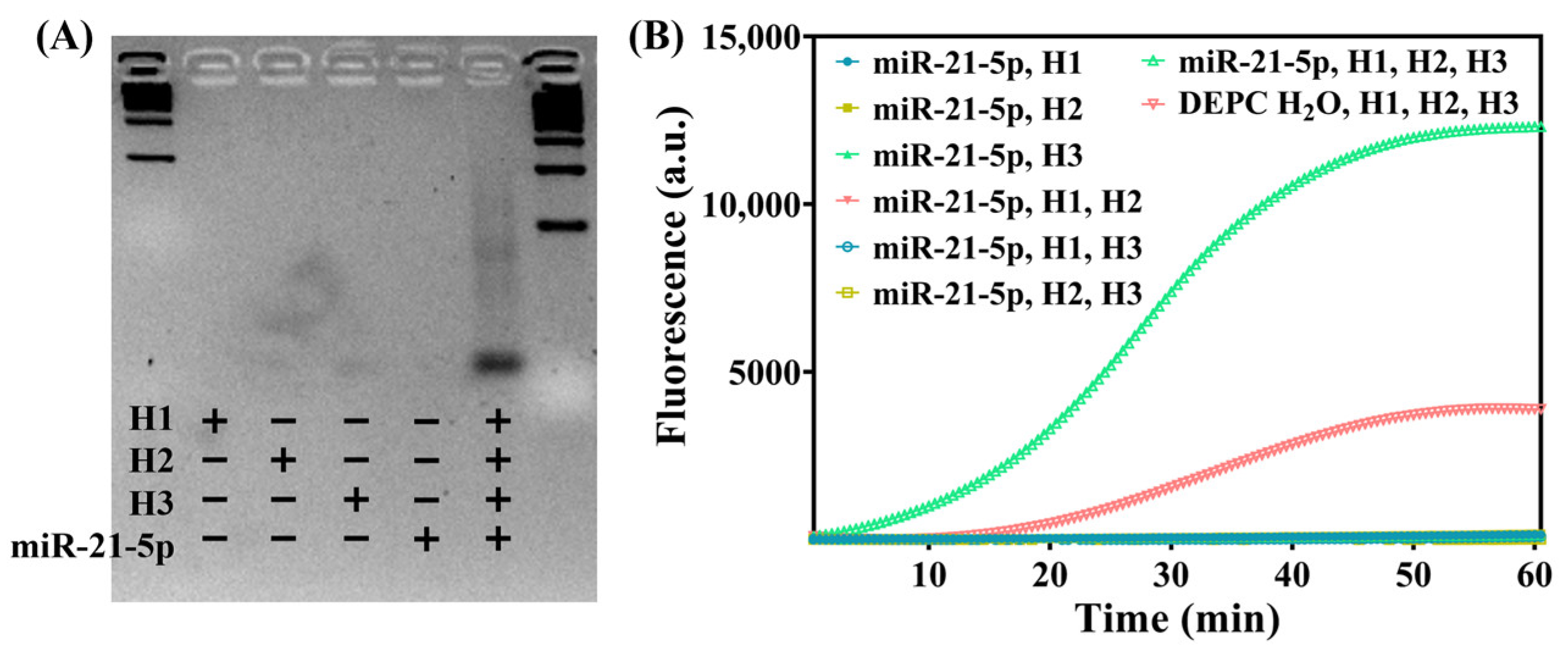
3.2. Feasibility of the Detection Strategy
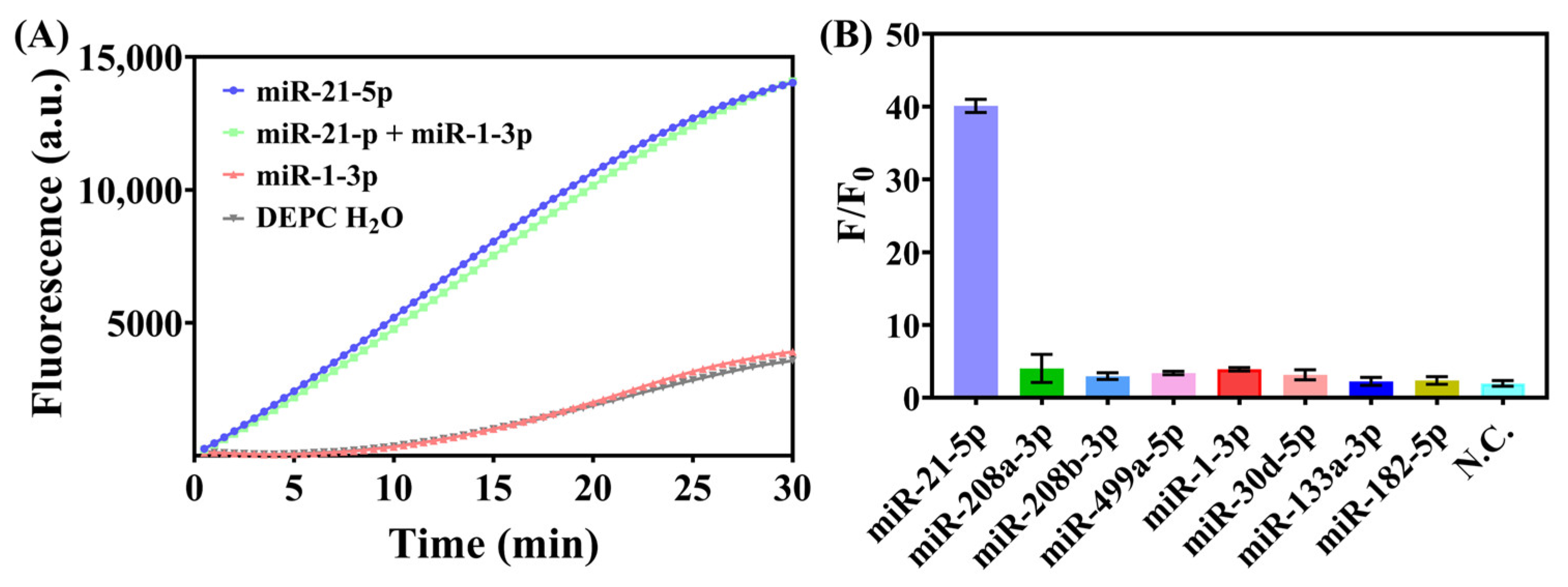
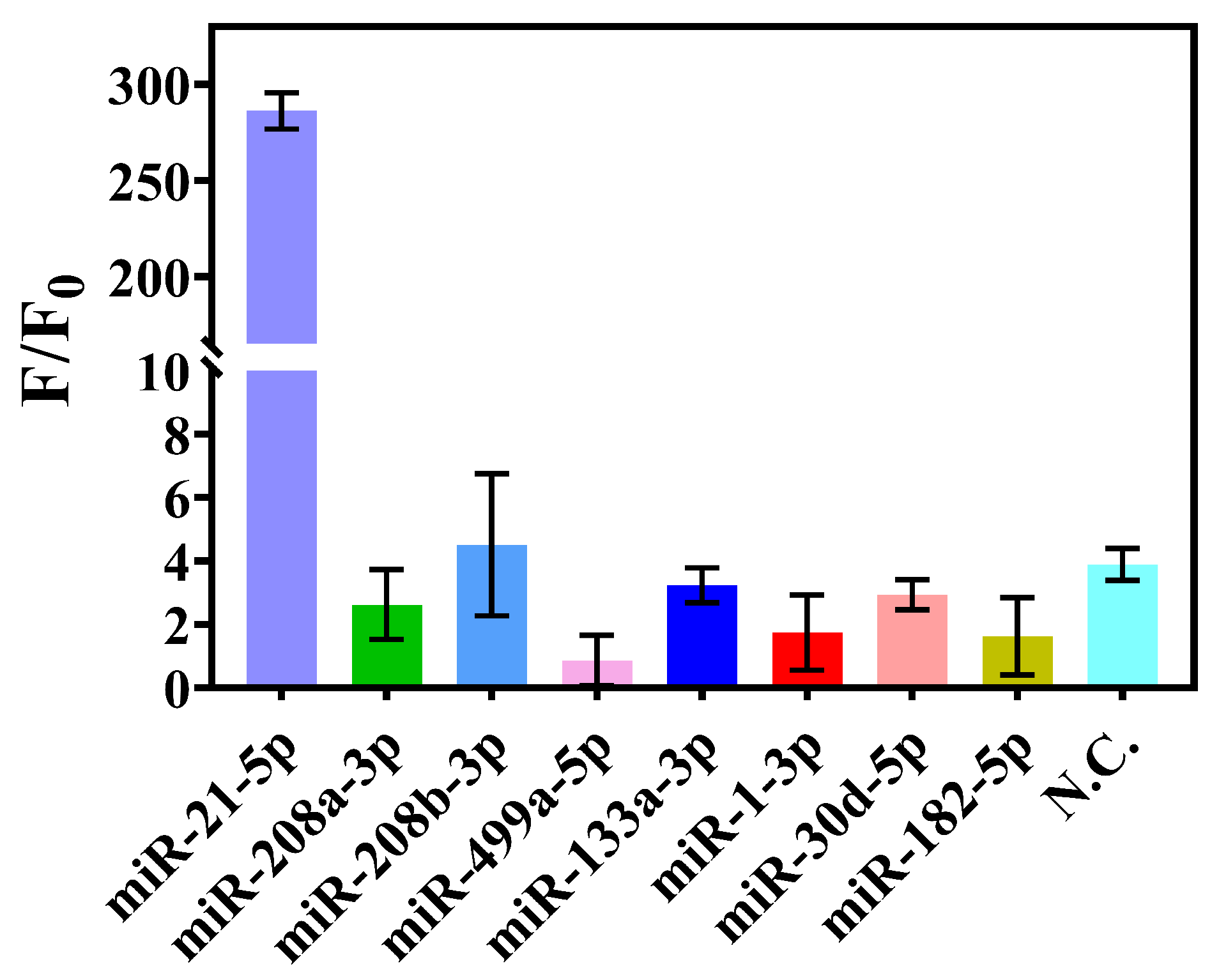
3.3. Investigation of Sensitivity and Specificity
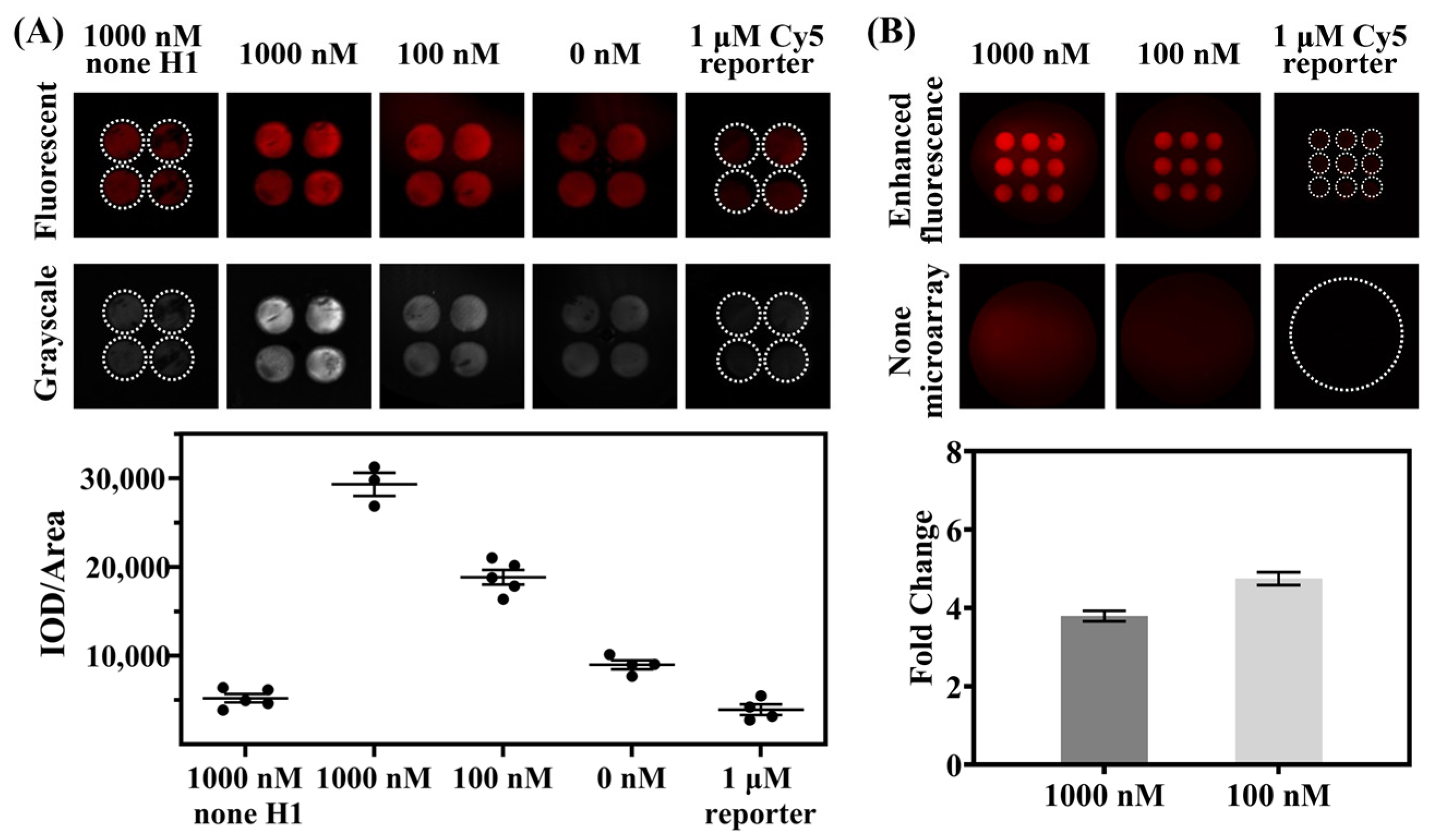
3.4. Detection on Photonic Crystal Microarrays for Enhanced Fluorescence
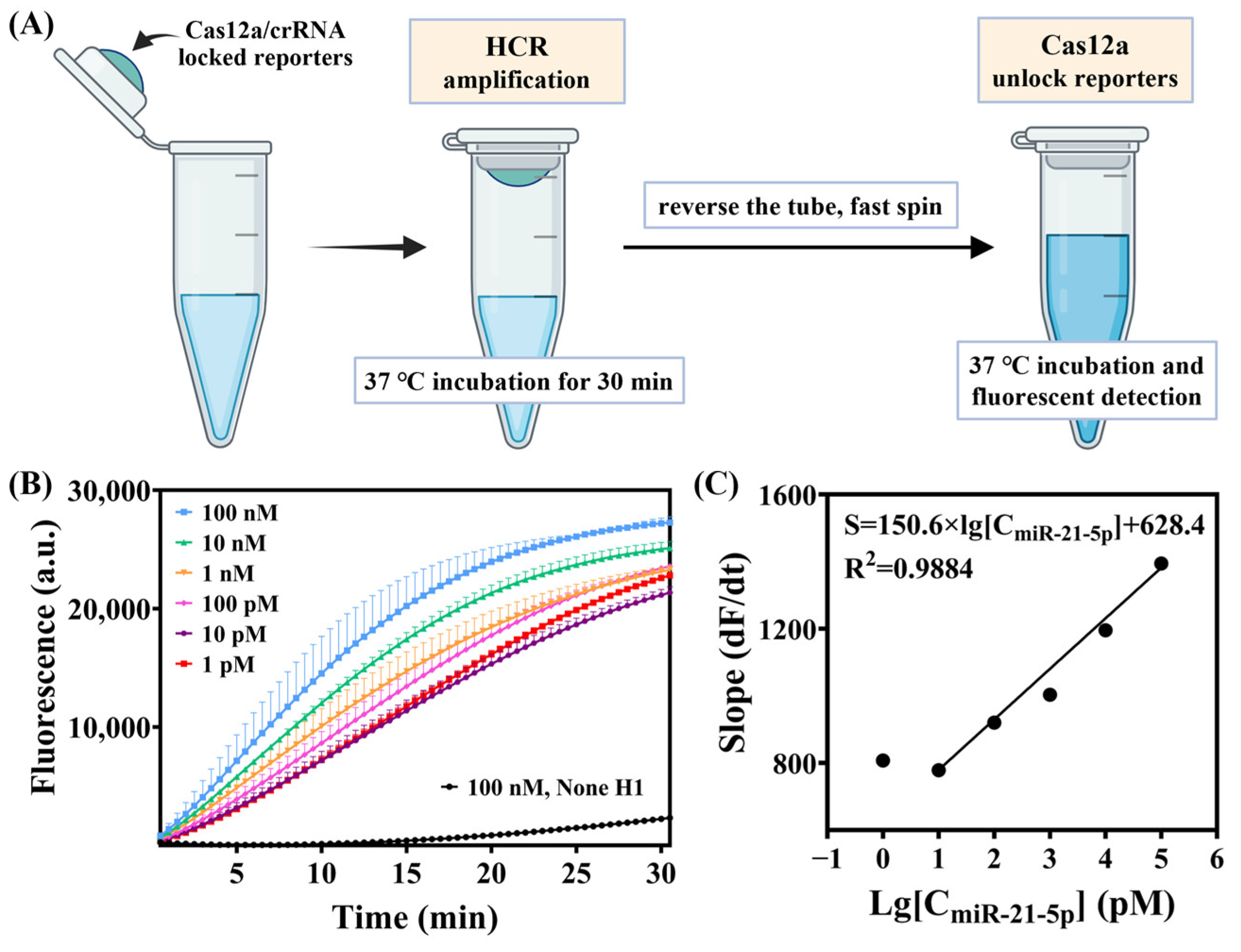
3.5. Optimization of Experimental Operations in a Pseudo One-Pot Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Caporali, A.; Anwar, M.; Devaux, Y.; Katare, R.; Martelli, F.; Srivastava, P.K.; Pedrazzini, T.; Emanueli, C. Non-coding RNAs as therapeutic targets and biomarkers in ischaemic heart disease. Nat. Rev. Cardiol. 2024, 21, 556–573. [Google Scholar] [CrossRef] [PubMed]
- Santovito, D.; Weber, C. Non-canonical features of microRNAs: Paradigms emerging from cardiovascular disease. Nat Rev Cardiol. 2022, 19, 620–638. [Google Scholar] [CrossRef] [PubMed]
- Barwari, T.; Joshi, A.; Mayr, M. MicroRNAs in Cardiovascular Disease. J. Am. Coll. Cardiol. 2016, 68, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- E, S.; Costa, M.C.; Kurc, S.; Drożdż, A.; Cortez-Dias, N.; Enguita, F.J. The circulating non-coding RNA landscape for biomarker research: Lessons and prospects from cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1085–1099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, H.; Wen, Y.; Tian, Z.; Hart, N.; Han, S.; Hughes, S.J.; Zeng, Y. A one-pot isothermal Cas12-based assay for the sensitive detection of microRNAs. Nat. Biomed. Eng. 2023, 7, 1583–1601. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2021, 360, 436–439, Erratum in Science 2021, 371, eabh0317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Myhrvold, C.; Freije, C.A.; Gootenberg, J.S.; Abudayyeh, O.O.; Metsky, H.C.; Durbin, A.F.; Kellner, M.J.; Tan, A.L.; Paul, L.M.; Parham, L.A.; et al. Field-deployable viral diagnostics using CRISPR-Cas13. Science 2018, 360, 444–448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gootenberg, J.S.; Abudayyeh, O.O.; Kellner, M.J.; Joung, J.; Collins, J.J.; Zhang, F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 2018, 360, 439–444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joung, J.; Ladha, A.; Saito, M.; Kim, N.G.; Woolley, A.E.; Segel, M.; Barretto, R.P.J.; Ranu, A.; Macrae, R.K.; Faure, G.; et al. Detection of SARS-CoV-2 with SHERLOCK One-Pot Testing. N. Engl. J. Med. 2020, 383, 1492–1494. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, R.A.; Puig, H.; Nguyen, P.Q.; Angenent-Mari, N.M.; Donghia, N.M.; McGee, J.P.; Dvorin, J.D.; Klapperich, C.M.; Pollock, N.R.; Collins, J.J. Ultrasensitive CRISPR-based diagnostic for field-applicable detection of Plasmodium species in symptomatic and asymptomatic malaria. Proc. Natl. Acad. Sci. USA 2020, 117, 25722–25731. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Puig, H.; Lee, R.A.; Najjar, D.; Tan, X.; Soeknsen, L.R.; Angenent-Mari, N.M.; Donghia, N.M.; Weckman, N.E.; Ory, A.; Ng, C.F.; et al. Minimally instrumented SHERLOCK (miSHERLOCK) for CRISPR-based point-of-care diagnosis of SARS-CoV-2 and emerging variants. Sci. Adv. 2021, 7, eabh2944. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rananaware, S.R.; Vesco, E.K.; Shoemaker, G.M.; Anekar, S.S.; Sandoval, L.S.W.; Meister, K.S.; Macaluso, N.C.; Nguyen, L.T.; Jain, P.K. Programmable RNA detection with CRISPR-Cas12a. Nat. Commun. 2023, 14, 5409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuan, A.; Sha, R.; Xie, W.; Qu, G.; Zhang, H.; Wang, H.; Le, X.C.; Jiang, G.; Peng, H. RNA-Activated CRISPR/Cas12a Nanorobots Operating in Living Cells. J. Am. Chem. Soc. 2024, 146, 26657–26666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, X.; Zou, X.; Ma, F.; Zhang, C.Y. CRISPR/Cas-Based MicroRNA Biosensors. Chemistry 2023, 29, e202203412. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Lei, X.; Qu, C. MicroRNA Sensors Based on CRISPR/Cas12a Technologies: Evolution From Indirect to Direct Detection. Crit. Rev. Anal. Chem. 2024, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Dirks, R.M.; Pierce, N.A. Triggered amplification by hybridization chain reaction. Proc. Natl. Acad. Sci. USA 2004, 101, 15275–15278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jia, H.Y.; Zhao, H.L.; Wang, T.; Chen, P.R.; Yin, B.C.; Ye, B.C. A programmable and sensitive CRISPR/Cas12a-based MicroRNA detection platform combined with hybridization chain reaction. Biosens. Bioelectron. 2022, 211, 114382. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Su, M.; Xue, B.; Cheng, L.; Lian, Z.; Yun, Y.; Yang, X.; Wang, X.; Xie, H.; Wang, H.; et al. Fast and Sensitive Detection of Protein Markers Using an All-Printing Photonic Crystal Microarray via Fingertip Blood. ACS Sens. 2023, 8, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hu, M.; Liu, A.A.; Lin, Y.; Liu, L.; Yu, B.; Zhou, X.; Pang, D.W. Detection of SARS-CoV-2 by CRISPR/Cas12a-Enhanced Colorimetry. ACS Sens. 2021, 6, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mei, Y.; Zhao, X.; Jiang, X. Reagents-Loaded, Automated Assay that Integrates Recombinase-Aided Amplification and Cas12a Nucleic Acid Detection for a Point-of-Care Test. Anal. Chem. 2020, 92, 14846–14852. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Tan, Z.; Chen, S.; Lei, C.; Nie, Z. Integrating CRISPR-Cas12a with a DNA circuit as a generic sensing platform for amplified detection of microRNA. Chem. Sci. 2020, 11, 7362–7368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Zhang, D.; Gan, X.; Liu, P.; Zheng, Q.; Yang, T.; Tian, G.; Ding, S.; Yan, Y. A Cascade Signal Amplification Based on Dynamic DNA Nanodevices and CRISPR/Cas12a Trans-cleavage for Highly Sensitive MicroRNA Sensing. ACS Synth. Biol. 2021, 10, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Thum, T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat. Rev. Cardiol. 2019, 16, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Poller, W.; Dimmeler, S.; Heymans, S.; Zeller, T.; Haas, J.; Karakas, M.; Leistner, D.M.; Jakob, P.; Nakagawa, S.; Blankenberg, S.; et al. Non-coding RNAs in cardiovascular diseases: Diagnostic and therapeutic perspectives. Eur. Heart J. 2018, 39, 2704–2716. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Alessandra, Y.; Devanna, P.; Limana, F.; Straino, S.; Di Carlo, A.; Brambilla, P.G.; Rubino, M.; Carena, M.C.; Spazzafumo, L.; De Simone, M.; et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur. Heart J. 2010, 31, 2765–2773. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, G.K.; Zhu, J.Q.; Zhang, J.T.; Li, Q.; Li, Y.; He, J.; Qin, Y.W.; Jing, Q. Circulating microRNA: A novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur. Heart J. 2010, 31, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction). J. Mol. Cell. Cardiol. 2016, 94, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Wu, Y.; Qin, F.; Su, M.; Cheng, N.; Zhang, J.; Li, C.; Lian, Z.; Yang, X.; Cheng, L.; et al. All-printed point-of-care immunosensing biochip for one drop blood diagnostics. Lab Chip 2022, 22, 3008–3014. [Google Scholar] [CrossRef] [PubMed]
- Lian, Z.; Wu, T.; Wang, H.; Chi, J.; Cheng, L.; Xie, D.; Pan, X.; Hu, Y.; Tan, Z.; Chen, S.; et al. At-Home COVID-19 Rapid Antigen Test Down to 0.03 pg mL-1 of Nucleocapsid Protein. Small 2023, 19, e2301162. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Wei, D.; Dang, L.; Xu, X.; Huang, S.; Li, L.; Wu, S.; Wu, J.; Liu, X.; et al. CoHIT: A one-pot ultrasensitive ERA-CRISPR system for detecting multiple same-site indels. Nat. Commun. 2024, 15, 5014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; van der Oost, J.; Regev, A.; et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, B.; Qiao, B.; Jia, L.; Chi, J.; Su, M.; Song, Y.; Du, J. A Sensitive and Fast microRNA Detection Platform Based on CRlSPR-Cas12a Coupled with Hybridization Chain Reaction and Photonic Crystal Microarray. Biosensors 2025, 15, 233. https://doi.org/10.3390/bios15040233
Xue B, Qiao B, Jia L, Chi J, Su M, Song Y, Du J. A Sensitive and Fast microRNA Detection Platform Based on CRlSPR-Cas12a Coupled with Hybridization Chain Reaction and Photonic Crystal Microarray. Biosensors. 2025; 15(4):233. https://doi.org/10.3390/bios15040233
Chicago/Turabian StyleXue, Bingjie, Bokang Qiao, Lixin Jia, Jimei Chi, Meng Su, Yanlin Song, and Jie Du. 2025. "A Sensitive and Fast microRNA Detection Platform Based on CRlSPR-Cas12a Coupled with Hybridization Chain Reaction and Photonic Crystal Microarray" Biosensors 15, no. 4: 233. https://doi.org/10.3390/bios15040233
APA StyleXue, B., Qiao, B., Jia, L., Chi, J., Su, M., Song, Y., & Du, J. (2025). A Sensitive and Fast microRNA Detection Platform Based on CRlSPR-Cas12a Coupled with Hybridization Chain Reaction and Photonic Crystal Microarray. Biosensors, 15(4), 233. https://doi.org/10.3390/bios15040233





