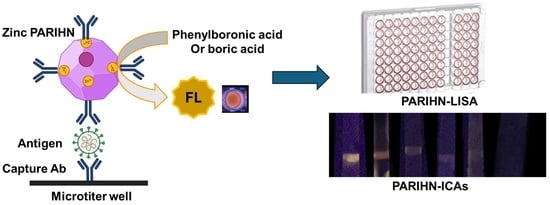
Polymerized Alizarin Red–Inorganic Hybrid Nanoarchitecture (PARIHN) as a Novel Fluorogenic Label for the Immunosorbent Assay of COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instruments and Materials
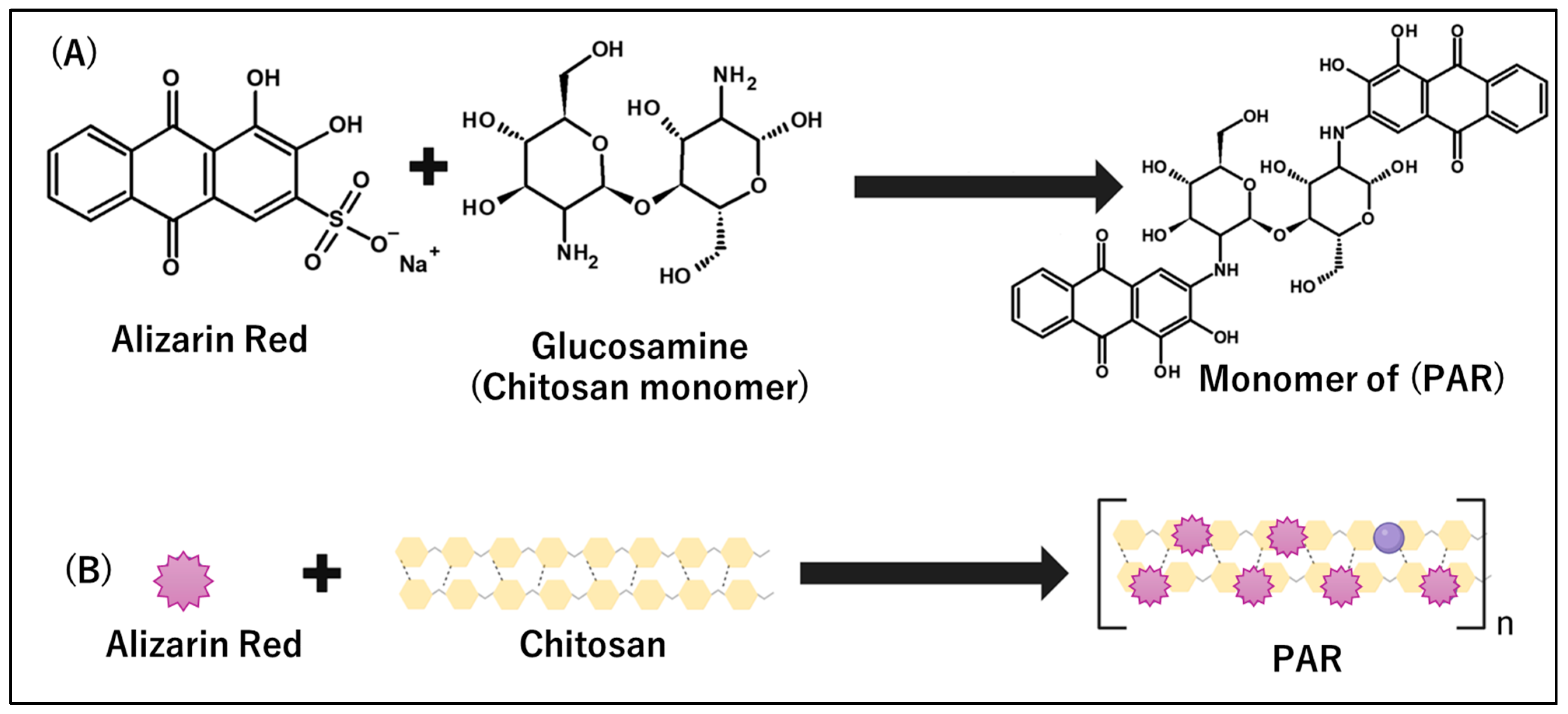
2.3. Synthesis of Polymerized Alizarin Red Using Deacetylated Chitosan as the Backbone
2.4. Determining the Number of Alizarin Number in Chitosan–Alizarin Polymer
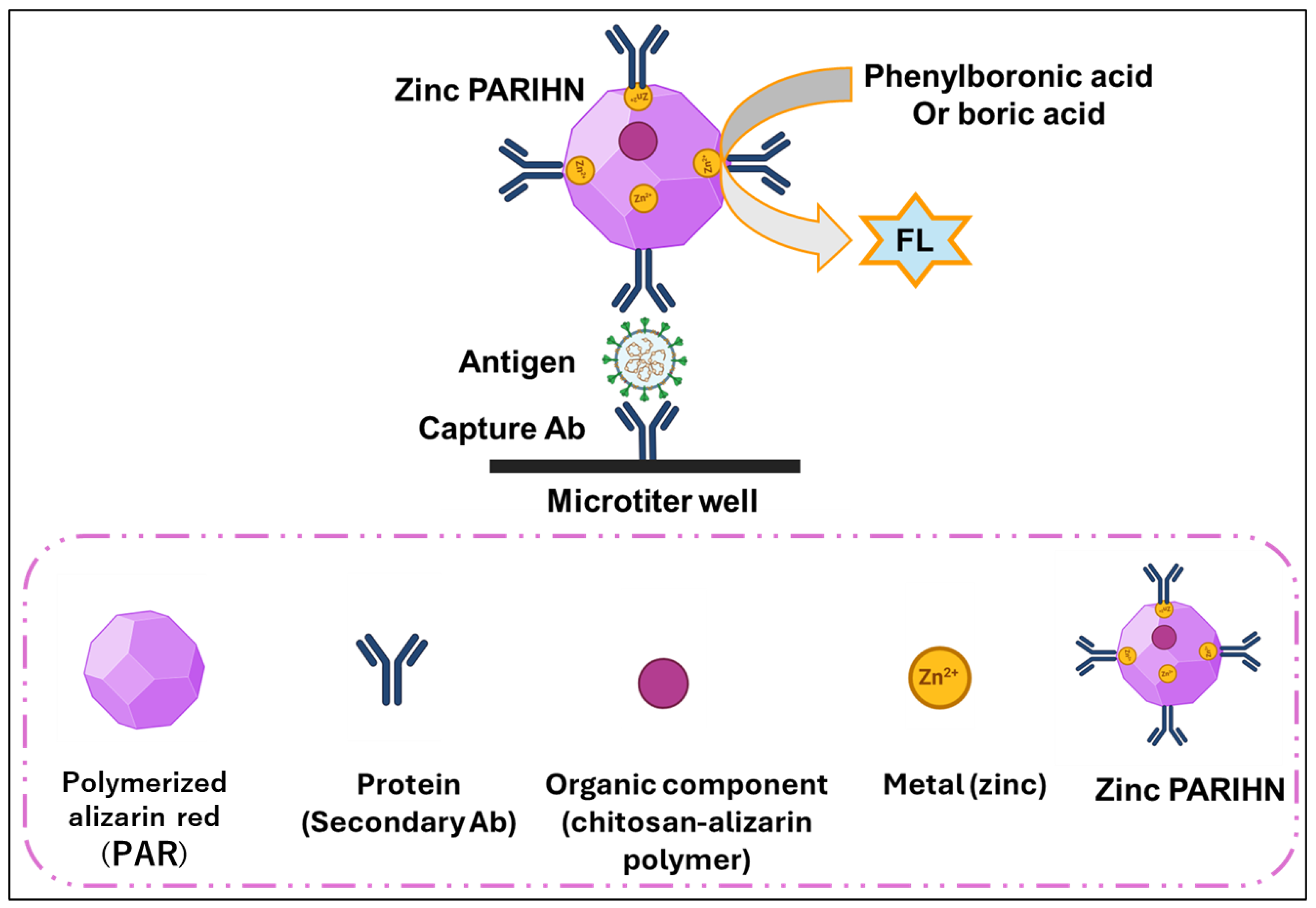
2.5. Synthesis of Zinc PARIHN with Commercially Available Secondary Ab of SARS-CoV-2 Nucleoprotein
2.6. Measuring the FL Intensity of PARIHN
2.7. ELISA vs. PARIHN-LISA for Determination of SARS-CoV-2 Nucleoprotein
2.8. Development of Immunochromatographic Assay Using Zinc PIHN for COVID-19 Through Modifying Commercially Available Rapid Testing Kit
3. Results and Discussion
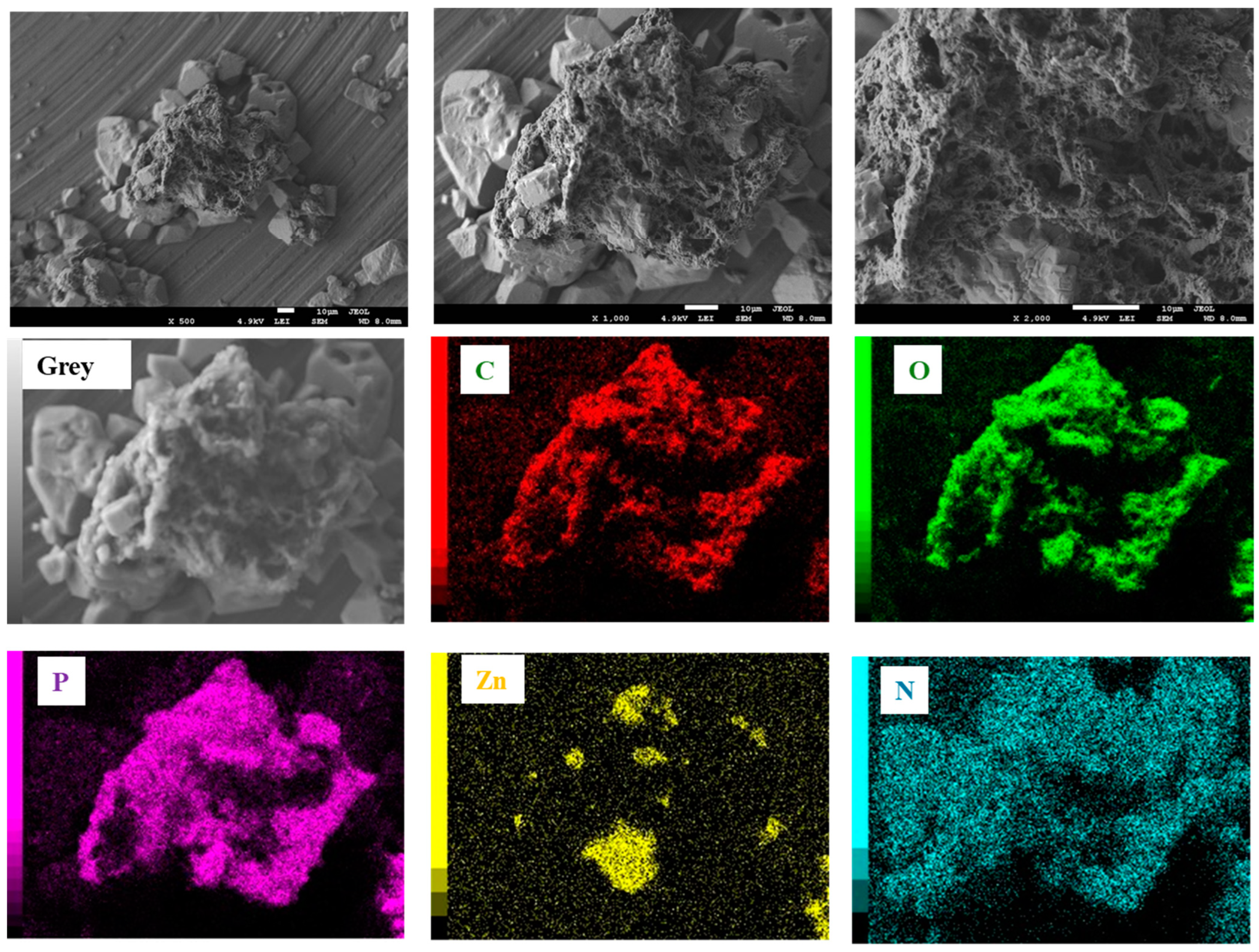
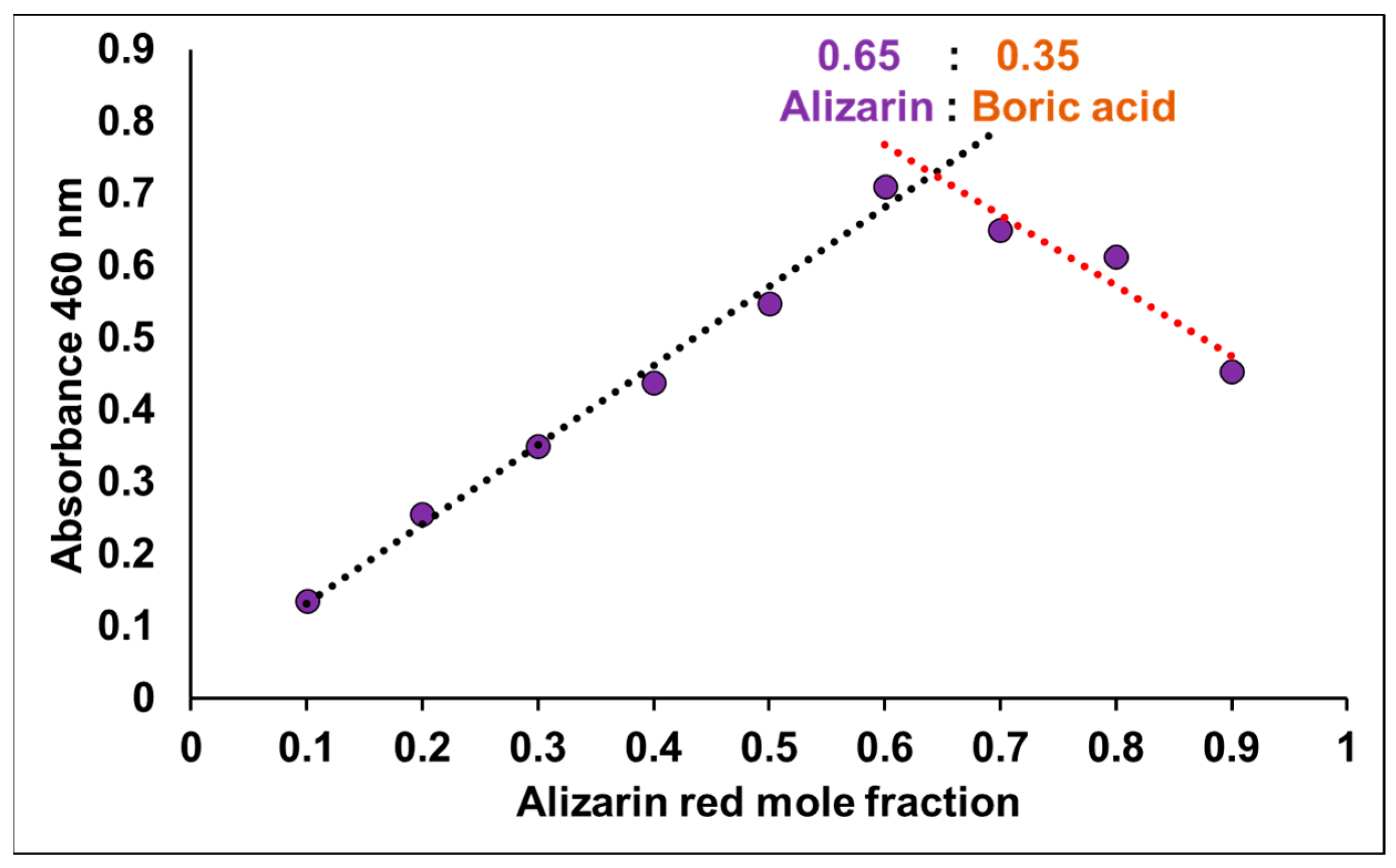
3.1. The Development, Characterization, and Optimization of PARIHN
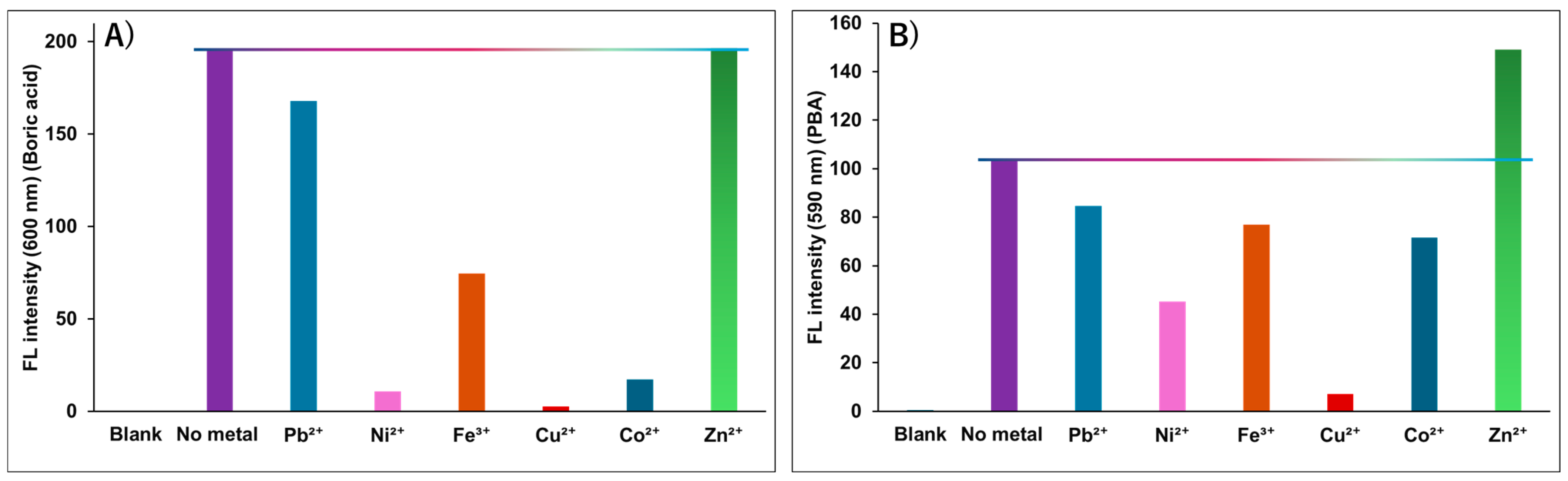
3.2. Study of the Reaction of PAR and PARIHN with Boric Acid Derivatives
3.3. Application of Zinc PIHN in PIHN-LISA for Detection of SARS-CoV-2 Nucleoprotein
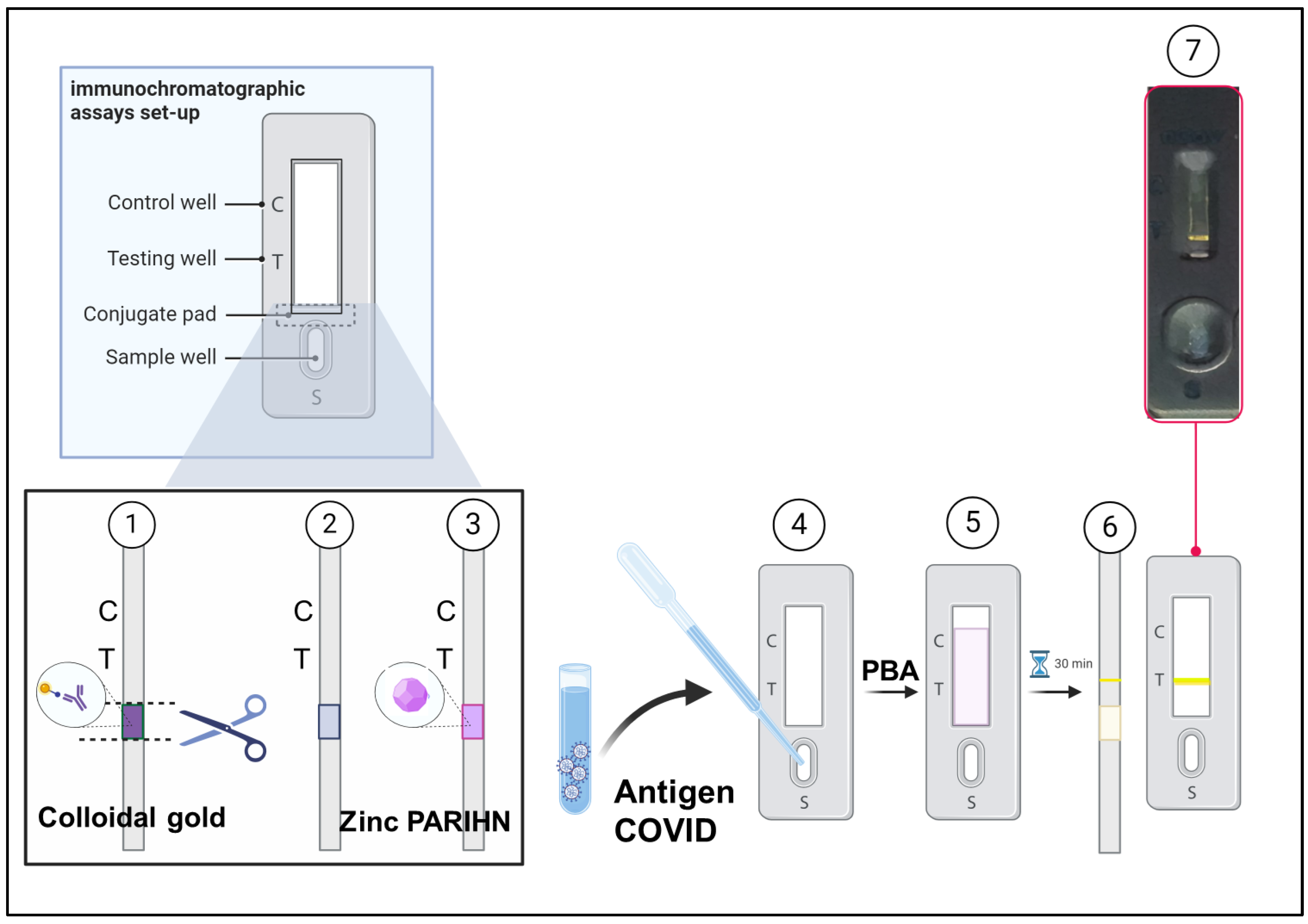
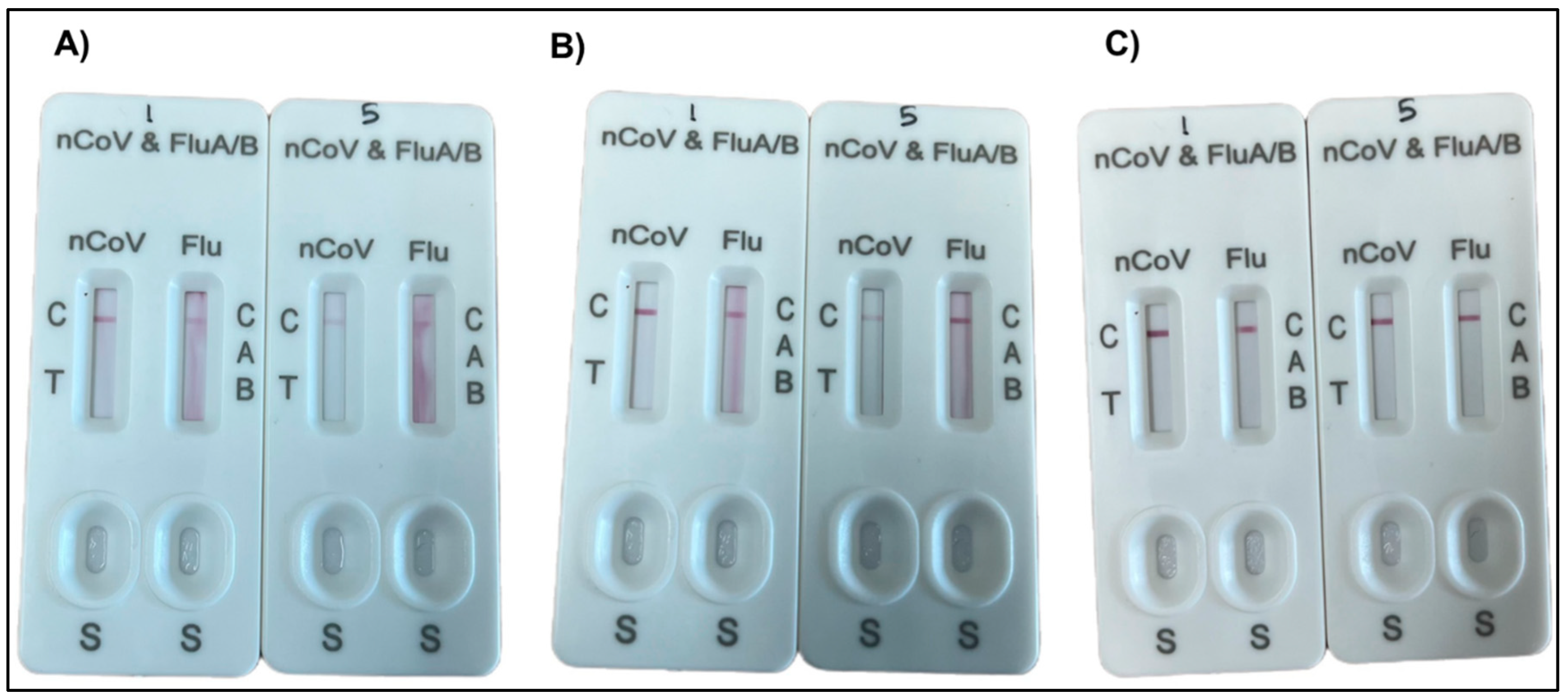
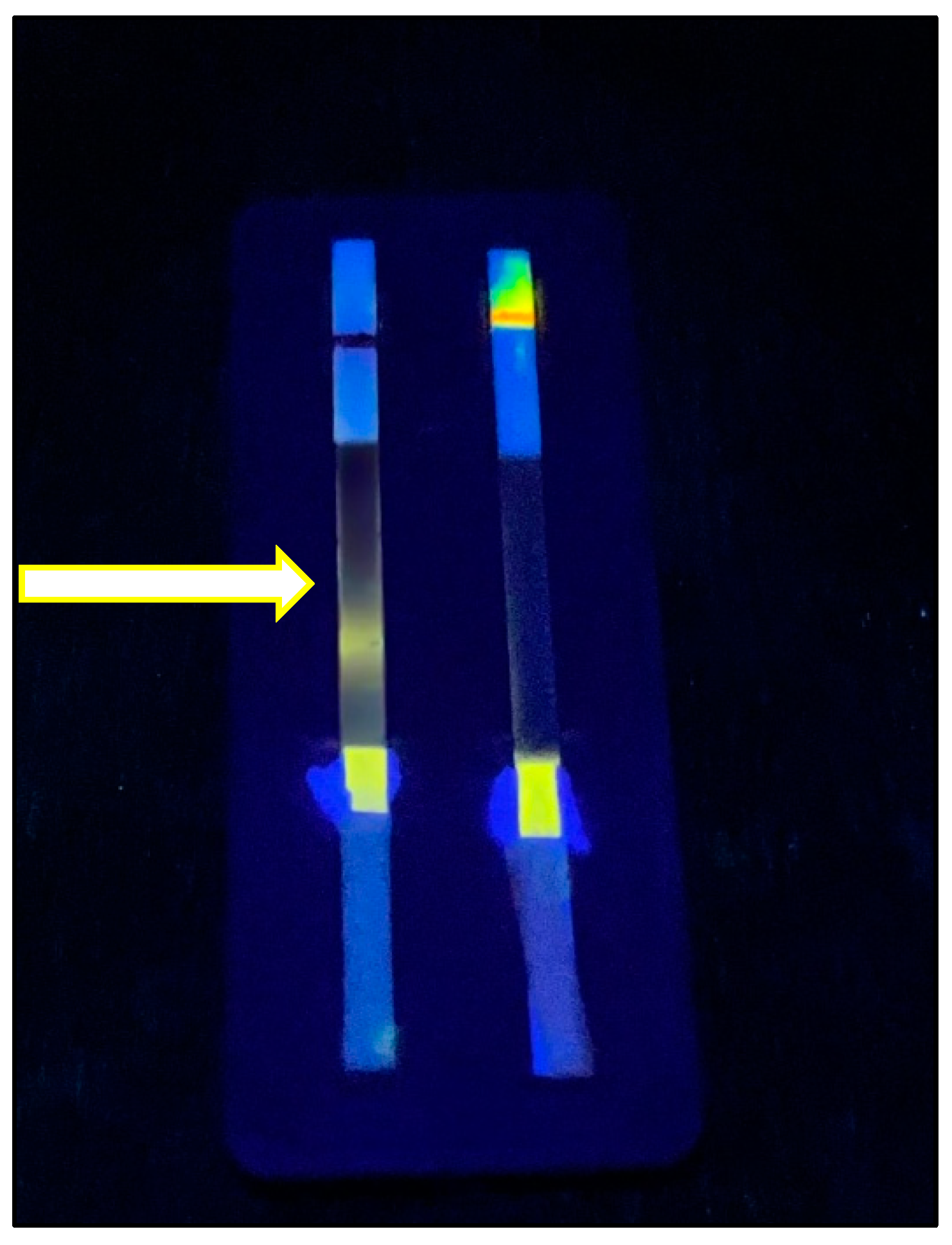
3.4. Performance of Zinc PIHN in Immunochromatographic Assay
3.5. Challenges and Future Directions of PARIHN as Labeling Tag for Immunoassays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PARIHN | Polymerized Alizarin red–inorganic hybrid nanoarchitecture |
| PAR | Polymerized Alizarin red |
| ICAs | immunochromatographic assay |
| QCCC | quinone–chitosan conjugation chemistry |
| PBA | phenylboronic acid |
| ROS | reactive oxygen species |
| FIAs | Fluorescence immunosorbent assays |
| PIHNs | protein–inorganic hybrid nanoarchitecture |
| DTT | dithiothreitol |
| ICAs | immunochromatographic assays |
| HRP | horseradish peroxidase |
| PBS | Phosphate-buffered saline |
| EDS | energy dispersive spectroscopy |
References
- Wu, Y.; Fu, Y.; Guo, J.; Guo, J. Single-Molecule Immunoassay Technology: Recent Advances. Talanta 2023, 265, 124903. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.-Y.; Lee, K.-H.; Park, Y.J.; Song, D.-Y.; Min, Y.-H.; Kim, D.-M. Revisiting ELISA with in Situ Amplification of Biomarkers to Boost Its Sensitivity. Sens. Actuators B Chem. 2025, 423, 136780. [Google Scholar] [CrossRef]
- Lyu, Z.; Ding, S.; Zhang, N.; Zhou, Y.; Cheng, N.; Wang, M.; Xu, M.; Feng, Z.; Niu, X.; Cheng, Y.; et al. Single-Atom Nanozymes Linked Immunosorbent Assay for Sensitive Detection of A β 1-40: A Biomarker of Alzheimer’s Disease. Research 2020, 2020, 4724505. [Google Scholar] [CrossRef]
- Liang, C.; Liu, B.; Li, J.; Lu, J.; Zhang, E.; Deng, Q.; Zhang, L.; Chen, R.; Fu, Y.; Li, C.; et al. A Nanoenzyme Linked Immunochromatographic Sensor for Rapid and Quantitative Detection of SARS-CoV-2 Nucleocapsid Protein in Human Blood. Sens. Actuators B Chem. 2021, 349, 130718. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, H.; Cheng, X.; Wang, Y.; Wei, H.; Rong, Z.; Wang, S. Simple and Ultrasensitive Nanozyme-Linked Immunosorbent Assay for SARS-CoV-2 Detection on a Syringe-Driven Filtration Device. ACS Appl. Mater. Interfaces 2024, 16, 44485–44492. [Google Scholar] [CrossRef]
- Shola David, M.; Kanayeva, D. Enzyme Linked Oligonucleotide Assay for the Sensitive Detection of SARS-CoV-2 Variants. Front. Cell. Infect. Microbiol. 2022, 12, 1017542. [Google Scholar] [CrossRef]
- Liu, D.; Ju, C.; Han, C.; Shi, R.; Chen, X.; Duan, D.; Yan, J.; Yan, X. Nanozyme Chemiluminescence Paper Test for Rapid and Sensitive Detection of SARS-CoV-2 Antigen. Biosens. Bioelectron. 2021, 173, 112817. [Google Scholar] [CrossRef] [PubMed]
- El-Maghrabey, M.; Magdy, G.; Hashem, H.M.; Amin, M.N.; Elgaml, A.; Radwan, A.S.; El-Sherbeny, M.A.; El-Shaheny, R. Comprehending COVID-19 Diagnostic Tests and Greenness Assessment of Its Reported Detection Methods. TrAC Trends Anal. Chem. 2023, 169, 117379. [Google Scholar] [CrossRef]
- El-Maghrabey, M.; Kishikawa, N.; Harada, S.; Ohyama, K.; Kuroda, N. Quinone-Based Antibody Labeling Reagent for Enzyme-Free Chemiluminescent Immunoassays. Application to Avidin and Biotinylated Anti-Rabbit IgG Labeling. Biosens. Bioelectron. 2020, 160, 112215. [Google Scholar] [CrossRef]
- El-Maghrabey, M.; Sato, Y.; Kaladari, F.; Kishikawa, N.; Kuroda, N. Development of Quinone Linked Immunosorbent Assay (QuLISA) Based on Using Folin’s Reagent as a Non-Enzymatic Tag: Application to Analysis of Food Allergens. Sens. Actuators B Chem. 2022, 368, 132167. [Google Scholar] [CrossRef]
- Kaladari, F.; El-Maghrabey, M.; Kishikawa, N.; Kuroda, N. Development of Signal Multiplication System for Quinone Linked Immunosorbent Assay (Multi-QuLISA) by Using Poly-l-Lysine Dendrigraft and 1,2-Naphthoquinone-4-Sulfonate as Enzyme-Free Tag. Talanta 2023, 253, 123911. [Google Scholar] [CrossRef]
- Xi, L.; Jiang, C.; Wang, F.; Zhang, X.; Huo, D.; Sun, M.; Dramou, P.; He, H. Recent Advances in Construction and Application of Metal-Nanozymes in Pharmaceutical Analysis. Crit. Rev. Anal. Chem. 2024, 54, 1661–1679. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yang, J.; Zhao, X.; Kong, R.; Wang, H.; You, J. Simple and Fast Determination of Catecholamines in Pharmaceutical Samples Using Ag + –3,3′,5,5′-Tetramethylbenzidine as a Colorimetric Probe. Anal. Methods 2015, 7, 6785–6790. [Google Scholar] [CrossRef]
- Ozinskas, A.J. Principles of Fluorescence Immunoassay. In Topics in Fluorescence Spectroscopy; Lakowicz, J.R., Ed.; Kluwer Academic Publishers: Boston, MA, USA, 1994; pp. 449–496. [Google Scholar]
- Dong, B.; Li, H.; Sun, J.; Mari, G.M.; Yu, X.; Ke, Y.; Li, J.; Wang, Z.; Yu, W.; Wen, K.; et al. Development of a Fluorescence Immunoassay for Highly Sensitive Detection of Amantadine Using the Nanoassembly of Carbon Dots and MnO2 Nanosheets as the Signal Probe. Sens. Actuators B Chem. 2019, 286, 214–221. [Google Scholar] [CrossRef]
- Su, D.; Li, H.; Zhou, R.; Zhao, L.; Li, A.; Liu, X.; Wang, C.; Jia, X.; Liu, F.; Sun, P.; et al. Embedding Proteins within Spatially Controlled Hierarchical Nanoarchitectures for Ultrasensitive Immunoassay. Anal. Chem. 2022, 94, 6271–6280. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Lei, J.; Zare, R.N. Protein–Inorganic Hybrid Nanoflowers. Nat. Nanotechnol. 2012, 7, 428–432. [Google Scholar] [CrossRef]
- Wei, T.; Du, D.; Zhu, M.-J.; Lin, Y.; Dai, Z. An Improved Ultrasensitive Enzyme-Linked Immunosorbent Assay Using Hydrangea-Like Antibody–Enzyme–Inorganic Three-in-One Nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 6329–6335. [Google Scholar] [CrossRef]
- Andrade, G.; Barbosa-Stancioli, E.F.; Mansur, A.A.P.; Vasconcelos, W.L.; Mansur, H.S. Design of Novel Hybrid Organic–Inorganic Nanostructured Biomaterials for Immunoassay Applications. Biomed. Mater. 2006, 1, 221–234. [Google Scholar] [CrossRef]
- Fukuda, M.; El-Maghrabey, M.H.; Kishikawa, N.; Ikemoto, K.; Kuroda, N. Ultrasensitive Determination of Pyrroloquinoline Quinone in Human Plasma by HPLC with Chemiluminescence Detection Using the Redox Cycle of Quinone. J. Pharm. Biomed. Anal. 2017, 145, 814–820. [Google Scholar] [CrossRef]
- Heyman, L.; Ferrarini, E.; Sanchez, L.; Barka, E.A.; Höfte, M. A Sensitive Chemiluminescence Method for Quantification of the Oxidative Burst in Grapevine Cells and Rice Roots. Plant Sci. 2021, 307, 110892. [Google Scholar] [CrossRef]
- Lu, C.; Song, G.; Lin, J.-M. Reactive Oxygen Species and Their Chemiluminescence-Detection Methods. TrAC Trends Anal. Chem. 2006, 25, 985–995. [Google Scholar] [CrossRef]
- Balzer, A.H.A.; Whitehurst, C.B. An Analysis of the Biotin–(Strept)Avidin System in Immunoassays: Interference and Mitigation Strategies. Curr. Issues Mol. Biol. 2023, 45, 8733–8754. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ezati, P.; Rhim, J.-W. Alizarin: Prospects and Sustainability for Food Safety and Quality Monitoring Applications. Colloids Surf. B Biointerfaces 2023, 223, 113169. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Jin, L. Assembly of Alizarin Red S/Boric Acid Ultrathin Films Based on Layered Double Hydroxide for Fluorescence Turn on Detection of Tiopronin. J. Mater. Chem. C 2016, 4, 3415–3421. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Yu, Y.; Cao, Y.; Guo, M.; Hu, X.; Wang, L.; Lin, B. Ratiometric Fluorescence Probe Based on Boric Acid-Modified Carbon Dots and Alizarin Red for Sensitive and Rapid Detection of Glyphosate. Microchim. Acta 2024, 191, 661. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Saji, V.S. Chitosan. In Polymeric Materials in Corrosion Inhibition; Elsevier: Amsteram, The Netherlands, 2022; pp. 131–153. [Google Scholar]
- Chandy, T.; Sharma, C.P. Chitosan-as a Biomaterial. Biomater. Artif. Cells Artif. Organs 1990, 18, 1–24. [Google Scholar] [CrossRef]
- Wang, G.-L.; Yuan, F.; Gu, T.; Dong, Y.; Wang, Q.; Zhao, W.-W. Enzyme-Initiated Quinone-Chitosan Conjugation Chemistry: Toward A General in Situ Strategy for High-Throughput Photoelectrochemical Enzymatic Bioanalysis. Anal. Chem. 2018, 90, 1492–1497. [Google Scholar] [CrossRef]
- Cao, Y.; Bu, T.; Wu, H.; Xi, J.; Wang, Y.; Xuan, C.; Jia, P.; Zheng, B.; Zhao, J.; Zhuang, Y.; et al. Ultrabright Fluorescent Nanorod-Based Immunochromatographic with Low Background for Advancing Detection Performance. Anal. Chem. 2023, 95, 16585–16592. [Google Scholar] [CrossRef]
- Hossain, M.; Iqbal, A. Production and Characterization of Chitosan from Shrimp Waste. J. Bangladesh Agric. Univ. 2014, 12, 153–160. [Google Scholar] [CrossRef]
- Abdou, E.S.; Nagy, K.S.A.; Elsabee, M.Z. Extraction and Characterization of Chitin and Chitosan from Local Sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef]
- Yin, W.; Zhu, L.; Xu, H.; Tang, Q.; Ma, Y.; Chou, S.-H.; He, J. Bio-Hybrid Nanoarchitectonics of Nanoflower-Based ELISA Method for the Detection of Staphylococcus Aureus. Sens. Actuators B Chem. 2022, 366, 132005. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, X.; Li, Y.; Yu, M.; Fu, S.; Qu, Z.; Li, Y.; Miao, J.; Xu, Y. Electrochemical Detection of SARS-CoV-2 Based on Copper Nanoflower-Triggered in Situ Growth of Electroactive Polymers. Analyst 2023, 148, 3659–3665. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Deng, Z.; Dou, L.; Guo, J.; Yang, C.; Dai, Z.; Wang, R.; Wang, Y.; Wang, J.; Zhang, D. Cascaded Antibody Directionality Relying on a Zinc-Based Nanocomposite for Performance Enhancement of Multiplex Lateral Flow Immunoassay. Chem. Eng. J. 2024, 481, 148493. [Google Scholar] [CrossRef]
- Tang, Q.; Zhang, L.; Tan, X.; Jiao, L.; Wei, Q.; Li, H. Bioinspired Synthesis of Organic–Inorganic Hybrid Nanoflowers for Robust Enzyme-Free Electrochemical Immunoassay. Biosens. Bioelectron. 2019, 133, 94–99. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Z.; Xin, Y.; Gu, Z.; Zhang, L.; Guo, X. Organic–Inorganic Hybrid Nanoflowers: A Comprehensive Review of Current Trends, Advances, and Future Perspectives. Coord. Chem. Rev. 2023, 489, 215191. [Google Scholar] [CrossRef]
- de Haan, M.P.; Balakrishnan, N.; Kuzmyn, A.R.; Li, G.; Willemen, H.M.; Seide, G.; Derksen, G.C.H.; Albada, B.; Zuilhof, H. Alizarin Grafting onto Ultrasmall ZnO Nanoparticles: Mode of Binding, Stability, and Colorant Studies. Langmuir 2021, 37, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Adewuyi, S.; Bisiriyu, I.; Akinremi, C. Zinc (II) Metal Ion Complexes of Chitosan: Toward Heterogeneous-Active Catalysts for the Polymerization of Vinyl Acetate. Ife J. Sci. 2015, 17, 749–754. [Google Scholar]
- Bandara, S.; Carnegie, C.; Johnson, C.; Akindoju, F.; Williams, E.; Swaby, J.M.; Oki, A.; Carson, L.E. Synthesis and Characterization of Zinc/Chitosan-Folic Acid Complex. Heliyon 2018, 4, e00737. [Google Scholar] [CrossRef]
- Legan, L.; Retko, K.; Ropret, P. Vibrational Spectroscopic Study on Degradation of Alizarin Carmine. Microchem. J. 2016, 127, 36–45. [Google Scholar] [CrossRef]
- Lampard, E.V.; Sedgwick, A.C.; Sombuttan, T.; Williams, G.T.; Wannalerse, B.; Jenkins, A.T.A.; Bull, S.D.; James, T.D. Dye Displacement Assay for Saccharides Using Benzoxaborole Hydrogels. ChemistryOpen 2018, 7, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Sugaya, T.; Iwatsuki, S.; Inamo, M.; Takagi, H.D.; Odani, A.; Ishihara, K. Detailed Reaction Mechanism of Phenylboronic Acid with Alizarin Red S in Aqueous Solution: Re-Investigation with Spectrophotometry and Fluorometry. ChemistrySelect 2017, 2, 2956–2964. [Google Scholar] [CrossRef]
- Tomsho, J.W.; Benkovic, S.J. Elucidation of the Mechanism of the Reaction between Phenylboronic Acid and a Model Diol, Alizarin Red S. J. Org. Chem. 2012, 77, 2098–2106. [Google Scholar] [CrossRef]
- Wang, F.; Huang, W.; Li, K.; Li, A.; Gao, W.; Tang, B. Study on the Fluorescence Enhancement in Lanthanum(III)–Carminic Acid–Cetyltrimethylammonium Bromide System and Its Analytical Application. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1946–1951. [Google Scholar] [CrossRef]
- Springsteen, G.; Wang, B. Alizarin Red S. as a General Optical Reporter for Studying the Binding of Boronic Acids with Carbohydrates. Chem. Commun. 2001, 17, 1608–1609. [Google Scholar] [CrossRef] [PubMed]
- Kubo, Y.; Kobayashi, A.; Ishida, T.; Misawa, Y.; James, T.D. Detection of Anions Using a Fluorescent Alizarin–Phenylboronic Acid Ensemble. Chem. Commun. 2005, 22, 2846. [Google Scholar] [CrossRef]
- Tobita, S.; Arakawa, M.; Tanaka, I. The Paramagnetic Metal Effect on the Ligand Localized S1 .Apprx. .Fwdarw. T1 Intersystem Crossing in the Rare-Earth-Metal Complexes with Methyl Salicylate. J. Phys. Chem. 1985, 89, 5649–5654. [Google Scholar] [CrossRef]
- Li, J.; Yin, C.; Huo, F. Development of Fluorescent Zinc Chemosensors Based on Various Fluorophores and Their Applications in Zinc Recognition. Dye. Pigment. 2016, 131, 100–133. [Google Scholar] [CrossRef]
- Volchkov, V.V.; Ivanov, V.L.; Uzhinov, B.M. Induced Intersystem Crossing at the Fluorescence Quenching of Laser Dye 7-Amino-1,3-Naphthalenedisulfonic Acid by Paramagnetic Metal Ions. J. Fluoresc. 2010, 20, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Brege, J.J.; Gallaway, C.; Barron, A.R. Fluorescence Quenching of Single-Walled Carbon Nanotubes with Transition-Metal Ions. J. Phys. Chem. C 2009, 113, 4270–4276. [Google Scholar] [CrossRef]
- Huang, H.; Bu, Y. Effect of Paramagnetic Open-Shell Gadolinium(III) Texaphyrin on Its Kinetics and Electronic Structures in Fluorescence and Phosphorescence Emission States. J. Phys. Chem. C 2019, 123, 28327–28335. [Google Scholar] [CrossRef]
- Golshan, M.; Gheitarani, B.; Safavi-Mirmahalleh, S.; Salami-Kalajahi, M. Rhodamine B-Modified Nanocrystalline Cellulose as Fluorescent Sensor for Fe 3+ Ion Detection. Macromol. Mater. Eng. 2025, 310, 2400285. [Google Scholar] [CrossRef]
- Gillis, B.S.; Arbieva, Z.; Gavin, I.M. Analysis of Lead Toxicity in Human Cells. BMC Genomics 2012, 13, 344. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Cheng, Y.; Zhou, H.; Sun, C.; Zhang, S. The SARS-CoV-2 Nucleocapsid Protein: Its Role in the Viral Life Cycle, Structure and Functions, and Use as a Potential Target in the Development of Vaccines and Diagnostics. Virol. J. 2023, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson Education Limited: Harlow, UK, 2010. [Google Scholar]
- Kobayashi, K.; Pillai, K.S.; Sakuratani, Y.; Abe, T.; Kamata, E.; Hayashi, M. Evaluation of Statistical Tools Used in Short-Term Repeated Dose Administration Toxicity Studies with Rodents. J. Toxicol. Sci. 2008, 33, 97–104. [Google Scholar] [CrossRef]




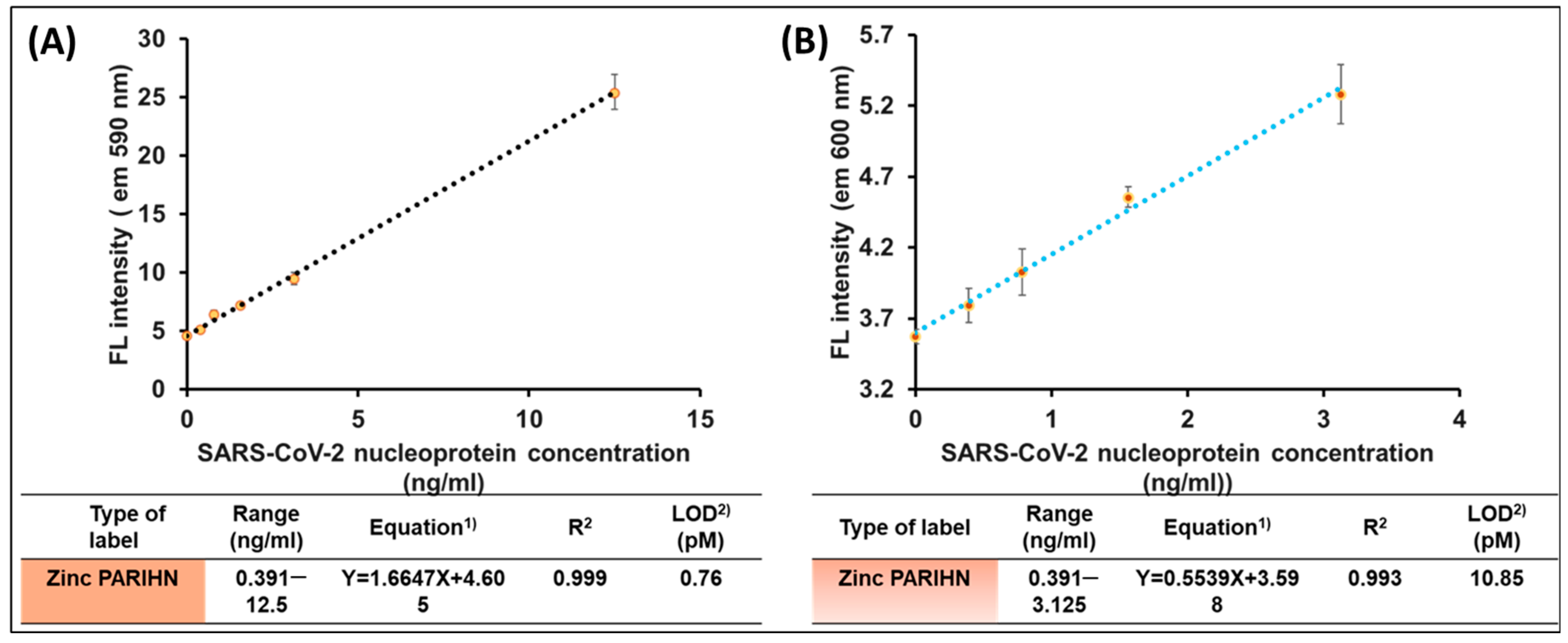


| Compound | Range | Correlation Coefficient | LOD (nM) | Detection Method | Used Reagent |
|---|---|---|---|---|---|
| PAR | 150.0–30,000 | 0.996 | 27.6 | Colorimetric | PBA |
| PAR | 3.0–300.0 | 0.998 | 0.10 | FL | PBA |
| PAR | 3.0–300.0 | 0.999 | 0.04 | FL | Boric acid |
| Zn2+ PARIHN | 0.05–10.0 | 0.977 | 0.016 | FL | PBA |
| Zn2+ PARIHN | 0.05–10.0 | 0.987 | 0.003 | FL | Boric acid |
| Type of Label Used | Type of Assay and Reagent | LOD (ng/mL) | LOD (pM) | Concentrations (ng/mL) | Recovery % ± SD |
|---|---|---|---|---|---|
| PARIHN | FL and PBA | 0.037 | 0.76 | 0.391 | 97.3 ± 4.8 |
| 0.783 | 108.0 ± 5.8 | ||||
| 1.563 | 99.5 ± 3.2 | ||||
| 3.125 | 96.8 ± 5.6 | ||||
| 12.5 | 100.2 ± 5.9 | ||||
| PARIHN | FL and boric acid | 0.521 | 10.85 | 0.391 | 100.6 ± 3.1 |
| 0.783 | 100.0 ± 4.1 | ||||
| 1.563 | 97.9 ± 1.6 | ||||
| 3.125 | 100.8 ± 3.9 | ||||
| Streptavidin–HRP complex | Colorimetric and TMB/H2O2 | 0.378 | 7.88 | 0.391 | 103.0 ± 7.0 |
| 0.783 | 98.5 ± 4.2 | ||||
| 1.563 | 95.9 ± 4.3 | ||||
| 3.125 | 101.5 ± 1.7 | ||||
| 12.5 | 99.30 ± 0.9 | ||||
| Brown–Forsythe test (p) | 0.4777 * | ||||
| Bartlett’s test (p) | 0.1578 * | ||||
| One-way ANOVA (p) | 0.9372 * | ||||
| Tested Concentration (ng/mL) | Recovery % | RSD |
|---|---|---|
| 0.05 | 104.63 | 1.82 |
| 0.1 | 97.13 | 12.92 |
| 0.2 | 99.59 | 2.76 |
| 0.8 | 98.58 | 0.84 |
| 1.0 | 101.01 | 2.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaladari, F.; El-Maghrabey, M.; Kishikawa, N.; El-Shaheny, R.; Kuroda, N. Polymerized Alizarin Red–Inorganic Hybrid Nanoarchitecture (PARIHN) as a Novel Fluorogenic Label for the Immunosorbent Assay of COVID-19. Biosensors 2025, 15, 256. https://doi.org/10.3390/bios15040256
Kaladari F, El-Maghrabey M, Kishikawa N, El-Shaheny R, Kuroda N. Polymerized Alizarin Red–Inorganic Hybrid Nanoarchitecture (PARIHN) as a Novel Fluorogenic Label for the Immunosorbent Assay of COVID-19. Biosensors. 2025; 15(4):256. https://doi.org/10.3390/bios15040256
Chicago/Turabian StyleKaladari, Fatema, Mahmoud El-Maghrabey, Naoya Kishikawa, Rania El-Shaheny, and Naotaka Kuroda. 2025. "Polymerized Alizarin Red–Inorganic Hybrid Nanoarchitecture (PARIHN) as a Novel Fluorogenic Label for the Immunosorbent Assay of COVID-19" Biosensors 15, no. 4: 256. https://doi.org/10.3390/bios15040256
APA StyleKaladari, F., El-Maghrabey, M., Kishikawa, N., El-Shaheny, R., & Kuroda, N. (2025). Polymerized Alizarin Red–Inorganic Hybrid Nanoarchitecture (PARIHN) as a Novel Fluorogenic Label for the Immunosorbent Assay of COVID-19. Biosensors, 15(4), 256. https://doi.org/10.3390/bios15040256