Tracking Traction Force Changes of Single Cells on the Liquid Crystal Surface
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Liquid Crystal Substrate
2.2. Preparation of Human Keratinocyte Cell Lines
2.3. Culturing Cells on the Liquid Crystal Substrates
2.4. Staining for Actin Fibers and Vinculin Accumulations
2.5. Quantification of Cell Traction Forces Using Cell Traction Force Mapping Software
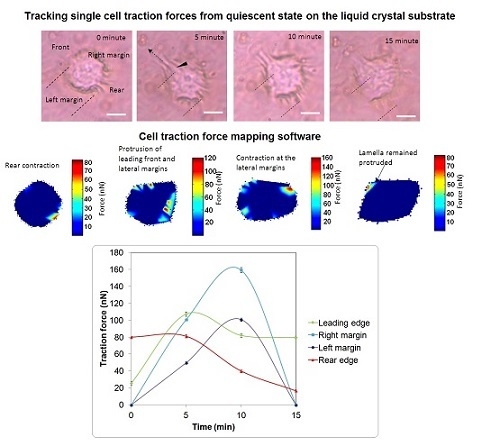
3. Results and Discussion





4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cross, K.J.; Mustoe, T.A. Growth factors in wound healing. Surg. Clin. North Am. 2003, 83, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Roure, O.D.; Saez, A.; Buguin, A.; Robert, H.A.; Chavrier, P.; Siberzan, P.; Benoit, L. Force mapping in epithelial cell migration. Proc. Natl. Acad. Sci. USA 2005, 102, 2390–2395. [Google Scholar] [CrossRef] [PubMed]
- Munevar, S.; Wang, Y.-L.; Dembo, M. Traction force microscopy of migrating normal and H-ras transformed 3T3 fibroblasts. Biophys. J. 2001, 80, 1744–1757. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.; Schwarz, U.; Riveline, D.; Goichberg, P.; Tzur, G.; Sabanay, I.; Mahalu, D.; Safran, S.; Bershadsky, A.; Addadi, L.; Geiger, B. Force and focal adhesion assembly: A close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001, 3, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Burton, K.; Taylor, D.L. Traction forces of cytokinesis measured with optically modified substrata. Nature 1997, 385, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Peschetola, V.; Laurent, V.M.; Duperray, A.; Michel, R.; Ambrosi, D.; Preziosi, L.; Verdier, C. Time-dependent traction force microscopy for cancer cells as a measure of invasiveness. Cytoskeleton 2013, 70, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.; Wild, P.; Stopak, D. Silicone rubber substrata: A new wrinkle in the study of cell locomotion. Science 1980, 208, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Burton, K.; Park, J.H.; Taylor, D.L. Keratocytes generate traction forces in two phases. Mol. Biol. Cell 1999, 10, 3745–3769. [Google Scholar] [CrossRef] [PubMed]
- Dembo, M.; Oliver, T.; Ishihara, A.; Jacobson, K. Imaging the traction stresses exerted by locomoting cells with the elastic subtratum method. Biophys. J. 1996, 70, 2008–2022. [Google Scholar] [CrossRef] [PubMed]
- Addae-Mensah, K.; Wikswo, J. Measurement techniques for cellular biomechanics in vitro. Exp. Biol. Med. 2008, 233, 792–809. [Google Scholar] [CrossRef]
- Misra, A.; Kumar, P. Periodic architecture for high performance shock absorbing composites. Nat. Sci. Rep. 2013, 3, 1–9. [Google Scholar]
- Kim, T.K.; Kim, J.K.; Jeong, O.C. Measurement of nonlinear mechanical properties of PDMS elastomer. J. Microeletron. Eng. 2011, 88, 1982–1985. [Google Scholar] [CrossRef]
- Tan, J.L.; Tien, J.; Pirone, D.M.; Gray, D.S.; Bhadriraju, K.; Chen, C.S. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proc. Natl. Acad. Sci. 2003, 100, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Style, R.W.; Bollyanskiy, R.; German, G.K.; Hyland, C.; MacMinn, C.W.; Mertz, A.F.; Wilen, L.A.; Xu, Y.; Dufresne, E.R. Traction force microscopy in physics and biology. Soft Matter 2014, 10, 4047–4055. [Google Scholar] [CrossRef] [PubMed]
- Beningo, K.A. Flexible substrata for the detection of cellular traction forces. Trends Cell Biol. 2002, 12, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Soon, C.F.; Youseffi, M.; Berends, R.F.; Blagden, N.; Denyer, M.C.T. Development of a novel liquid crystal based cell traction force transducer system. Biosens. Bioelectron. 2013, 39, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Soon, C.F.; Youseffi, M.; Blagden, N.; Berends, R.; Lobo, S.B.; Javid, F.A.; Denyer, M. Characterization and Biocompatibility Study of Nematic and Cholesteryl Liquid Crystals. In Proceedings of the World Congress on Engineering, London, UK, 1–3 July 2009; Volume 2, pp. 1872–1875.
- Soon, C.F.; Youseffi, M.; Gough, T.; Blagden, N.; Denyer, M.C.T. Rheological characterization of the time-dependent cholesteric based liquid crystals and in-situ verification. Mater. Sci. Eng. C 2011, 31, 1389–1397. [Google Scholar] [CrossRef]
- Soon, C.F.; Wan Omar, W.I.; Berends, R.F.; Nayan, N.; Basri, H.; Tee, K.S.; Youseffi, M.; Blagden, N.; Denyer, M.C.T. Biophysical characteristics of cells cultured on cholesteryl ester liquid crystals. Micron 2014, 56, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Oliver, T.; Dembo, M.; Jacobson, K. Traction forces in locomoting cells. Cell Motil. Cytoskelet. 1995, 31, 225–240. [Google Scholar] [CrossRef]
- Clubb, B.H.; Shivers, R.R. Extracellular matrix regulates microfilament and vinculin organization in c6-glioma cells. Acta Neuropathol. 1996, 91, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Owaribe, K.; Kodama, R.; Eguchi, G. Demonstration of contractility of circumferential actin bundles and its morphogenetic significance in pigmented epithelium in vitro and in vivo. Cell. Biol. 1981, 90, 507–514. [Google Scholar] [CrossRef]
- Mohl, C.; Kirchgebner, N.; Schafer, C.; Kupper, K.; Born, S.; Diez, G.; Goldmann, W.H.; Merkel, R.; Hoffman, B. Becoming stable and strong: The interplay between vinculin exchange dynamics and adhesion strength during adhesion site maturation. Cell Motil. Cytoskelet. 2009, 66, 350–364. [Google Scholar] [CrossRef]
- Gloushankova, N.A.; Alieva, N.A.; Krendel, M.F.; Bonder, E.M.; Feder, H.H.; Vasiliev, J.M.; Gelfand, I.M. Cell-cell contact changes the dynamics of lamellar activity in nontransformed epitheliocytes but not in their ra-transformed descendants. Proc. Natl. Acad. Sci. USA 1997, 94, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Kirfel, G.; Borm, B.; Rigort, A.; Herzog, V. The secretory amyloid precursor protein is a motogen for human epidermal keratinocytes. Eur. J. Cell Biol. 2002, 81, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Yam, P.Y.; Wilson, C.A.; Ji, L.; Hebert, B.; Barnhart, E.L.; Dye, N.A.; Wiseman, P.W.; Danuser, G.; Theriot, J.A. Actin-myosin network reorganization breaks symmetry at the cell rear to spontaneously initiate polarized cell motility. Cell. Biol. 2007, 178, 1207–1221. [Google Scholar] [CrossRef]
- Omelchenko, T.; Fetisova, E.; Ivanova, O.; Bonder, E.M.; Feder, H.; Vasiliev, J.M.; Gelfand, I.M. Contact interactions between epitheliocytes and fibroblasts: Formation of heterotypic cadherin-containing adhesion sites is accompanied by local cytoskeletal reorganization. Proc. Natl. Acad. Sci. 2001, 98, 8632–8637. [Google Scholar] [CrossRef] [PubMed]
- Kirfel, G.; Rigort, A.; Borm, B.; Schulte, C.; Herzog, V. Structural and compositional analysis of the keratinocyte migration track. Cell Motil. Cytoskelet. 2003, 55, 1–13. [Google Scholar] [CrossRef]
- Horwitz, A.R.; Parsons, J.T. Cell migration-moving on. Science 1999, 286, 1102–1103. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, E.A. Extracellular matrix and keratinocyte migration. Clin. Exp. Dermatol. 2001, 26, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Kirfel, G.; Herzog, H. Migration of epidermal keratinocytes: Mechanisms, regulation, and biological significance. Protoplasma 2004, 223, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, allosteris signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Pellegrin, S.; Mellor, H. Actin stress fibres. J. Cells Sci. 2007, 120, 3491–3499. [Google Scholar] [CrossRef]
- Hotulainen, P.; Lappalainen, P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Biol. 2006, 173, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.; Bacakova, L.; Newman, C.; Hategan, A.; Griffin, M.; Discher, D. Substrate compliance versus ligand density in cell on gel responses. Biophys. J. 2004, 86, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Takeo, M. Skin biomechanics from microscopic viewpoint: Mechanical properties and their measurement of horny layer, living epidermis, and dermis. Fagr. J. 2007, 35, 36–40. [Google Scholar]
- Hendriks, F.M.; Brokken, D.; Oomens, C.W.J.; Bader, D.L.; Baaijens, F.P.T. The relative contributions of different skin layers to the machanical behavior of human skin in vivo using suction experiment. Med. Eng. Phys. 2006, 28, 259–266. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soon, C.F.; Tee, K.S.; Youseffi, M.; Denyer, M.C.T. Tracking Traction Force Changes of Single Cells on the Liquid Crystal Surface. Biosensors 2015, 5, 13-24. https://doi.org/10.3390/bios5010013
Soon CF, Tee KS, Youseffi M, Denyer MCT. Tracking Traction Force Changes of Single Cells on the Liquid Crystal Surface. Biosensors. 2015; 5(1):13-24. https://doi.org/10.3390/bios5010013
Chicago/Turabian StyleSoon, Chin Fhong, Kian Sek Tee, Mansour Youseffi, and Morgan C. T. Denyer. 2015. "Tracking Traction Force Changes of Single Cells on the Liquid Crystal Surface" Biosensors 5, no. 1: 13-24. https://doi.org/10.3390/bios5010013
APA StyleSoon, C. F., Tee, K. S., Youseffi, M., & Denyer, M. C. T. (2015). Tracking Traction Force Changes of Single Cells on the Liquid Crystal Surface. Biosensors, 5(1), 13-24. https://doi.org/10.3390/bios5010013