Variation in Gas and Volatile Compound Emissions from Human Urine as It Ages, Measured by an Electronic Nose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
| Demographic Data | Diabetes Medication | Frequency | |
|---|---|---|---|
| Male (%) | 44 (61) | OHA | 39 |
| Female (%) | 28 (39) | Insulin | 7 |
| Mean age | 56 | OHA + Insulin | 18 |
| Median age | 59 | Nil | 8 |
| Mean BMI | 39 | HbA1c | (mmol/mol) |
| Median BMI | 39 | Mean HbA1c | 67 |
| Median Hb A1c | 57 | ||
2.2. Urine Collection, Storage and Transfer
2.3. FAIMS Analysis

2.4. Analysis Methodology


3. Results and Discussion
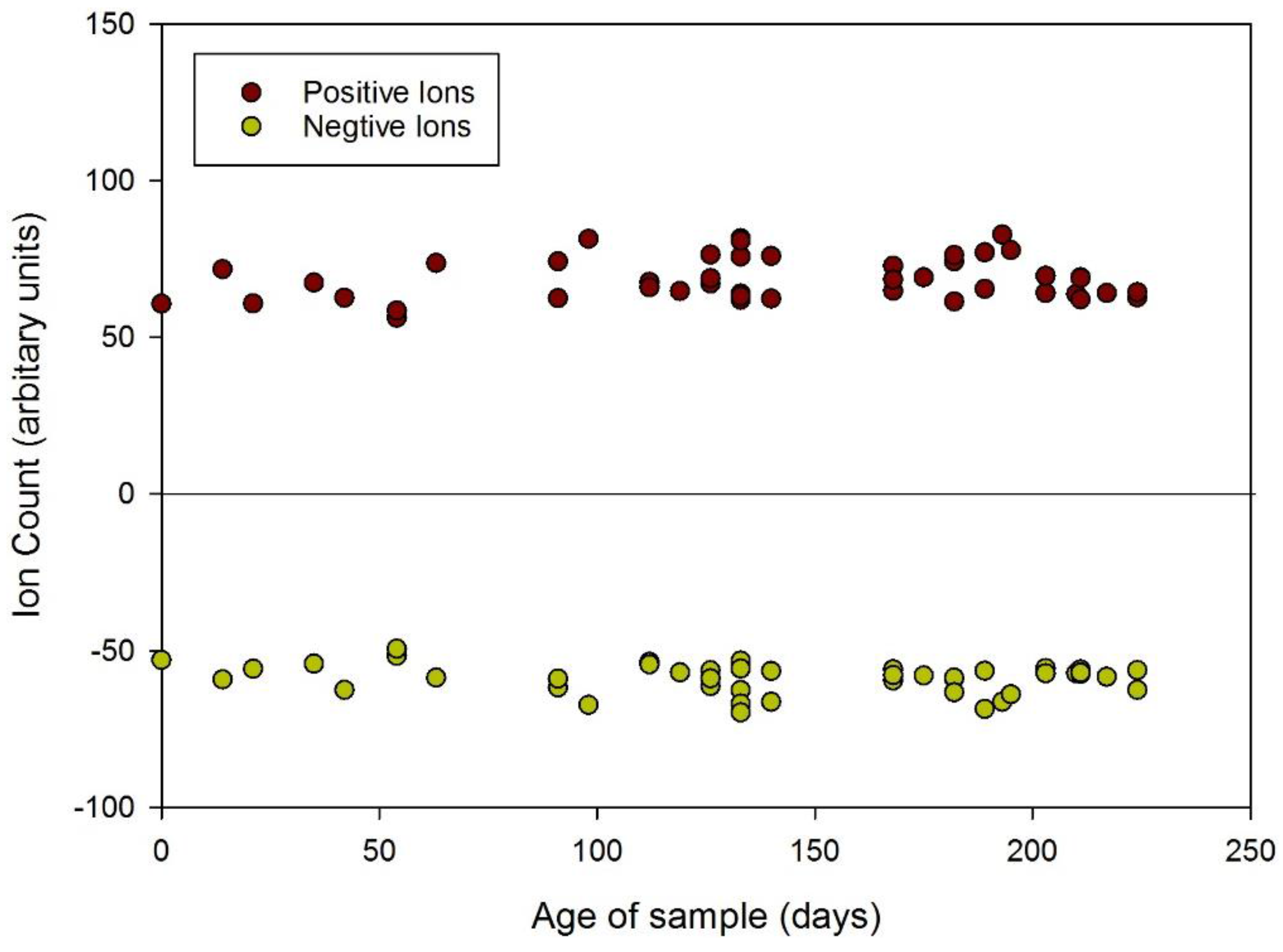
3.1. Results

| Positive Ions (Arbitrary Units) | ||||||
| Year | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 |
| Age (days) | 0–147 | 148–503 | 504–859 | 860–1215 | 1216–1572 | 1572–1617 |
| Average | 68.2 | 67.0 | 57.0 | 53.4 | 62.3 | 63.4 |
| s.d. | 7.4 | 8.6 | 6.5 | 3.8 | 17.7 | 12.6 |
| % | 100.0 | 98.2 | 83.6 | 78.3 | 91.3 | 93.0 |
| Negative Ions (Arbitrary Units) | ||||||
| Year | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 |
| Age (days) | 0–147 | 148–503 | 504–859 | 860–1215 | 1216–1572 | 1572–1617 |
| Average | −58.4 | −58.2 | −48.5 | −44.9 | −48.6 | −44.7 |
| s.d. | 5.3 | 7.8 | 3.5 | 4.7 | 7.0 | 5.2 |
| % | 100.0 | 99.7 | 83.0 | 76.9 | 83.2 | 76.5 |
| Threshold Values (Positive Ions Only) | ||||||
| Year | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 |
| Age (days) | 0–147 | 148–503 | 504–859 | 860–1215 | 1216–1572 | 1572–1617 |
| Average | 54.3 | 55.5 | 53.2 | 54.3 | 52.8 | 52.3 |
| s.d. | 2.3 | 2.2 | 2.2 | 1.8 | 2.1 | 1.5 |
| % | 100.0 | 102.2 | 98.0 | 100 | 97.2 | 96.3 |

| Year | May 2014–March 2014 | February 2014–December 2013 | November 2013–Septeber 2013 | August 2013–June 2013 | May 2013–March 2013 |
|---|---|---|---|---|---|
| Age (days) | 0–88 | 89–178 | 179–269 | 270–356 | 357–445 |
| Average | 64.0 | 70.0 | 69.0 | No data | 64.4 |
| s.d. | 6.3 | 6.8 | 6.7 | No data | 12.1 |
3.2. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Persaud, K.; Dodd, G. Analysis of discrimination mechanisms in the mammalian olfactory system using a model nose. Nature 1982, 299, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Kateb, B.; Ryan, M.A.; Homer, M.L.; Lara, L.M.; Yin, Y.; Higa, K.; Chen, M.Y. Sniffing out cancer using the JPL electronic nose: A pilot study of a novel approach to detection and differentiation of brain cancer. NeuroImage 2009, 47, T5–T9. [Google Scholar] [CrossRef] [PubMed]
- Bruins, M.; Rahim, Z.; Bos, A.; van de Sande, W.W.; Endtz, H.P.; van Belkum, A. Diagnosis of active tuberculosis by e-nose analysis of exhaled air. Tuberculosis 2013, 93, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.G.; Persaud, K.C.; Pisanelli, A.M. Wound-state monitoring for burn patients using E-Nose/SPME system. ETRI J. 2010, 32, 440–446. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; Covington, J.A.; Harmston, C.; Nwokolo, C.U. Review article: Next generation diagnostic modalities in gastroenterology-gas phase volatile compound biomarker detection. Aliment. Pharmacol. Ther. 2014, 39, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.; Baietto, M. Advances in electronic-nose technologies developed for biomedical applications. Sensors 2011, 11, 1105–1176. [Google Scholar] [CrossRef] [PubMed]
- Covington, J.A.; der Schee, M.V.; Edge, A.S.L.; Boyle, B.; Savage, R.S.; Arasaradnam, R.P. The application of FAIMS gas analysis in medical diagnostics. Analyst 2015, 140, 6775–6781. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P.; Mores, N.; Trové, A.; Mondino, C.; Barnes, P.J. The electronic nose in respiratory medicine. Respiration 2013, 85, 72–84. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.; di Natale, C.; Falconi, C.; Martinelli, E.; Paolesse, R.; Pennazza, G.; Santonico, M.; Sterk, P.J. Detection and identification of cancers by the electronic nose. Expert Opin. Med. Diagn. 2013, 6, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Covington, J.A.; Westenbrink, E.W.; Ouaret, N.; Harbord, R.; Bailey, C.; O’Connell, N.; Cullis, J.; Williams, N.; Nwokolo, C.U.; Bardhan, K.D.; et al. Application of a novel tool for diagnosing bile acid diarrhoea. Sensors 2013, 13, 11899–11912. [Google Scholar] [CrossRef] [PubMed]
- Arasaradnam, R.P.; Ouaret, N.; Thomas, M.G.; Quraishi, N.; Heatherington, E.; Nwokolo, C.U.; Bardhan, K.D.; Covington, J.A. A novel tool for noninvasive diagnosis and tracking of patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Sykes, B.D. Urine stability for metabolomic studies: effects of preparation and storage. Metabolomics 2007, 3, 19–27. [Google Scholar]
- Gika, H.G.; Theodoridis, G.A.; Wilson, I.D. Liquid chromatography and ultra-performance liquid chromatography-mass spectrometry fingerprinting of human urine: Sample stability under different handling and storage conditions for metabonomics studies. J. Chromatogr. A 2008, 1189, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Broadhurst, D.; Ellis, D.I.; Brown, M.; Halsall, A.; O’Hagan, S.; Spasic, I.; Tseng, A.; Kell, D.B. A GC-TOF-MS study of the stability of serum and urine metabolomes during the UK Biobank sample collection and preparation protocols. Int. J. Epidemiol. 2008, 37, i23–i30. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Burden, H.; Persad, R.; Whittington, K.; de Lacy Costello, B.; Ratcliffe, N.M.; Probert, C.S. A comparative study of the analysis of human urine headspace using gas chromatography-mass spectrometry. J. Breath Res. 2008, 2. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Kumar, S.; Abbassi-Ghadi, N.; Španěl, P.; Smith, D.; Hanna, G.B. Selected ion flow tube mass spectrometry analysis of volatile metabolites in urine headspace for the profiling of gastro-esophageal cancer. Anal. Chem. 2013, 85, 3409–3416. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.; Grigsby, C.C.; Smith, B.R.; Rizki, M.M.; Preti, G. Changes in volatile compounds of human urine as it ages: Their interaction with water. J. Chromatogr. B 2013, 941, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.; Grigsby, C.C.; Preti, G.; Rizki, M.M.; Yamazaki, K.; Beauchamp, G.K. Changes in volatile compounds of mouse urine as it ages: Their interactions with water and urinary proteins. Physiol. Behav. 2013, 120, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Buryakov, I.A.; Krylov, E.V.; Makas, A.L.; Nazarov, E.G.; Pervukhin, V.V.; Rasulev, U.K. Ion division by their mobility in high-tension alternating electric field. Sov. Tech. Phys. Lett. 1991, 17, 446–447. [Google Scholar]
- Guevremont, R. High-field asymmetric waveform ion mobility spectrometry: A new tool for mass spectrometry. J. Chromatogr. A 2004, 1058, 3–19. [Google Scholar] [CrossRef]
- Guo, D.; Wang, Y.; Li, L.; Wang, X.; Luo, J. Precise determination of nonlinear function of ion mobility for explosives and drugs at high electric fields for microchip FAIMS. J. Mass Spectrom. 2015, 50, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xie, Y.F.; Zhao, W.J.; Li, P.; Qian, H.; Wang, X.Z. Rapid microchip-based FAIMS determination of trimethylamine, an indicator of pork deterioration. Anal. Methods 2014, 6, 2965–2972. [Google Scholar] [CrossRef]
- Xia, Y.Q.; Steven, W.T.; Mohammed, J. LC-FAIMS-MS/MS for quantification of apeptide in plasma and evaluation of FAIMS global selectivity from plasma components. Anal. Chem. 2008, 80, 7137–7143. [Google Scholar] [CrossRef] [PubMed]
- Lambertus, G.R.; Fix, C.S.; Reidy, S.M.; Miller, R.A.; Wheeler, D.; Nazarov, E.; Sacks, R. Silicon microfabricated column with microfabricated differential mobility spectrometer for GC analysis of volatile organic compounds. Anal. Chem. 2005, 77, 7563–7757. [Google Scholar] [CrossRef] [PubMed]
- Wilks, A.; Hart, M.; Koehl, A.; Somerville, J.; Boyle, B.; Ruiz-Alonso, D. Characterization of a miniature, ultra-high-field, ion mobility spectrometer. Int. J. Ion Mobil. Spectrom. 2012, 15, 199–222. [Google Scholar] [CrossRef]
- Gabryelski, W.; Wu, F.; Froese, K.L. Comparison of high-field asymmetric waveform ion mobility spectrometry with GC methods in analysis of haloacetic acids in drinking water. Anal. Chem. 2003, 75, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Krylov, E.V. Comparison of the planar and coaxial field asymmetrical waveform ion mobility spectrometer (FAIMS). Int. J. Mass Spectrom. 2003, 225, 39–51. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esfahani, S.; Sagar, N.M.; Kyrou, I.; Mozdiak, E.; O’Connell, N.; Nwokolo, C.; Bardhan, K.D.; Arasaradnam, R.P.; Covington, J.A. Variation in Gas and Volatile Compound Emissions from Human Urine as It Ages, Measured by an Electronic Nose. Biosensors 2016, 6, 4. https://doi.org/10.3390/bios6010004
Esfahani S, Sagar NM, Kyrou I, Mozdiak E, O’Connell N, Nwokolo C, Bardhan KD, Arasaradnam RP, Covington JA. Variation in Gas and Volatile Compound Emissions from Human Urine as It Ages, Measured by an Electronic Nose. Biosensors. 2016; 6(1):4. https://doi.org/10.3390/bios6010004
Chicago/Turabian StyleEsfahani, Siavash, Nidhi M. Sagar, Ioannis Kyrou, Ella Mozdiak, Nicola O’Connell, Chuka Nwokolo, Karna D. Bardhan, Ramesh P. Arasaradnam, and James A. Covington. 2016. "Variation in Gas and Volatile Compound Emissions from Human Urine as It Ages, Measured by an Electronic Nose" Biosensors 6, no. 1: 4. https://doi.org/10.3390/bios6010004
APA StyleEsfahani, S., Sagar, N. M., Kyrou, I., Mozdiak, E., O’Connell, N., Nwokolo, C., Bardhan, K. D., Arasaradnam, R. P., & Covington, J. A. (2016). Variation in Gas and Volatile Compound Emissions from Human Urine as It Ages, Measured by an Electronic Nose. Biosensors, 6(1), 4. https://doi.org/10.3390/bios6010004







