Point-of-Care-Testing in Acute Stroke Management: An Unmet Need Ripe for Technological Harvest
Abstract
:1. Introduction
1.1. Stroke—A Leading Cause of Death
1.2. Gaps in the Current System
1.3. Rapid Diagnosis Improves Stroke Care
2. Stroke Prognostic Care Shows Painful Needs
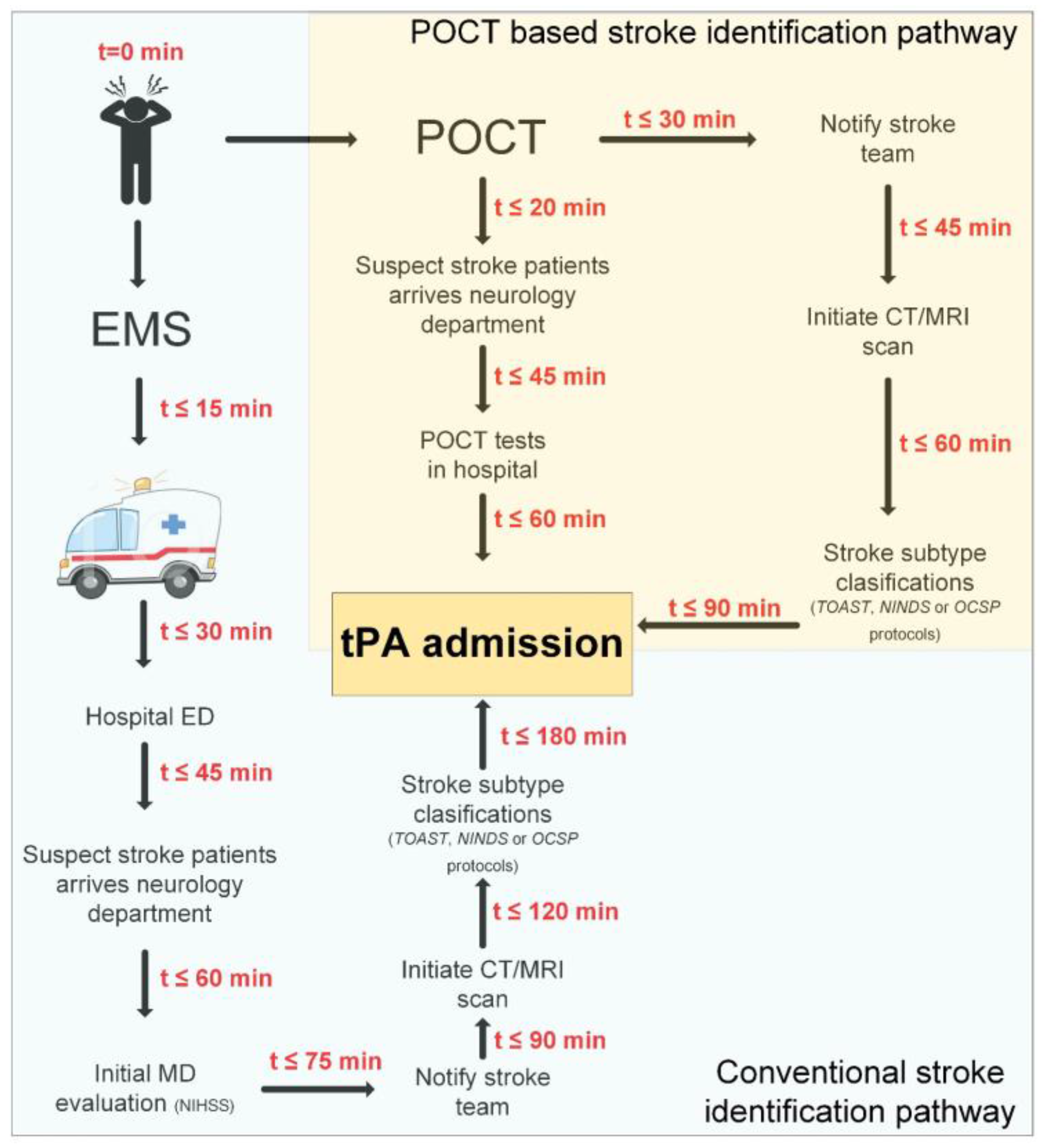
2.1. Pre-Hospital: From Symptoms Onset to ED Admission
2.2. In-Hospital: Stroke Classification
2.2.1. Ischemic vs. Hemorrhagic Stroke
2.2.2. Ischemic Stroke Subtypes
2.3. Post-Hospital: Recovery and Prevention of Stroke Reoccurrence
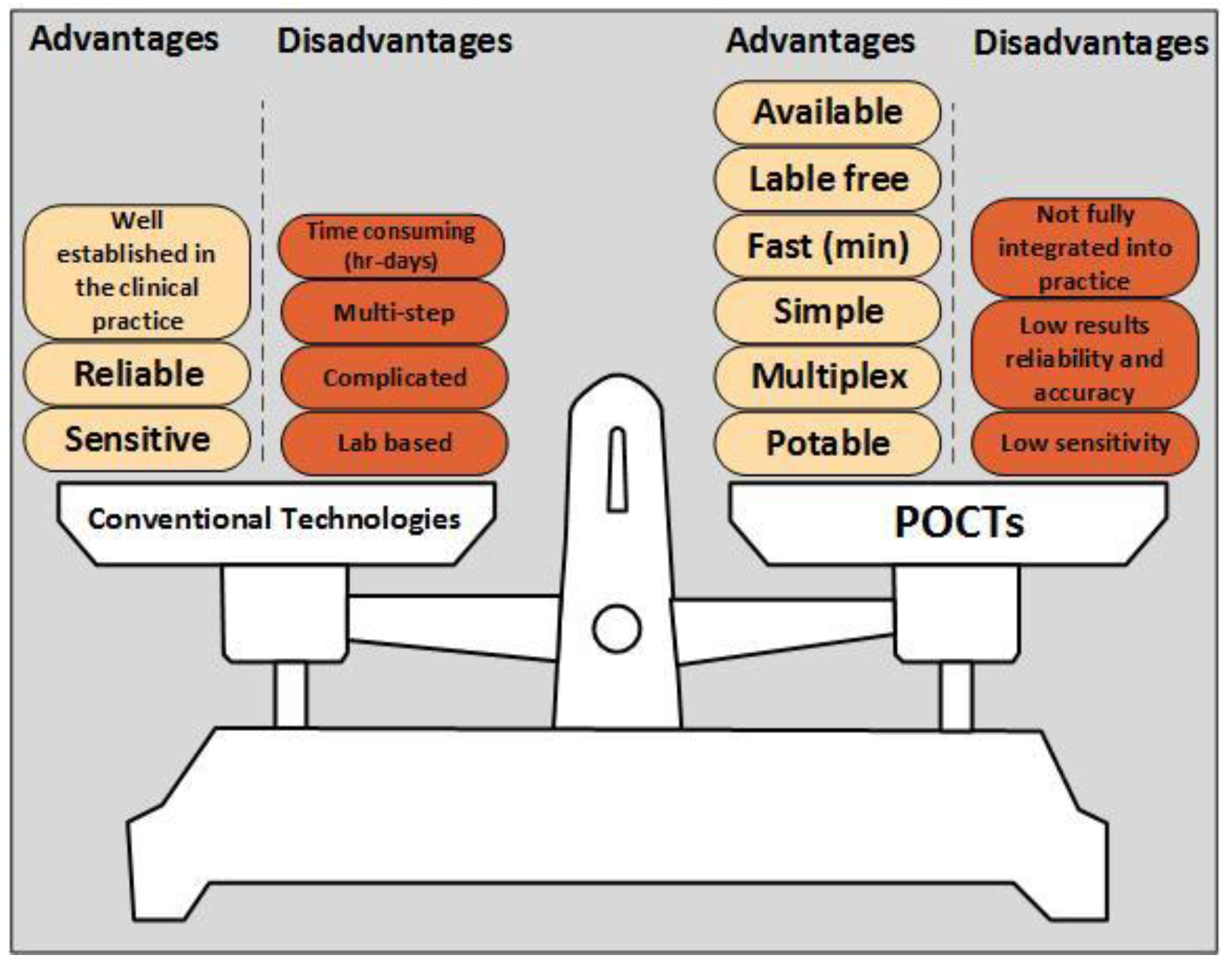
3. POCTs Expedite Stroke Prognostics
3.1. POCTs in Pre-Hospital Setting: Telemedicine and Mobile Stroke Unit
3.2. POCTs in In-Hospital Setting: Coagumeters, Blood-Count, Blood-Chemistry and Biomarkers
3.2.1. Coagumeters
3.2.2. Blood-Count POCT
3.2.3. Blood-Chemistry POCTs
3.2.4. POCTs for Biomarkers Measurement
3.3. POCTs in Post-Hospital Setting: Aspirin Resistance and Biomarkers
3.3.1. POCT for Biomarkers Measurement
3.3.2. Aspirin Resistance POCTs
4. Key Elements in Novel Stroke-POCTs
4.1. What is an Ideal Brain Biomarker
4.2. Specific Stroke Related Biomarkers
4.3. Multiplex and Quantitative Detection
4.4. POCT-based Sensors
4.5. Novel POCT-based Sensors Platforms
4.6. Use of Data-Mining for Efficient POCT Clinical Integration
5. Future Trends
Acknowledgments
Conflicts of Interest
References
- Strong, K.; Mathers, C.; Bonita, R. Preventing stroke: Saving lives around the world. Lancet Neurolol. 2007, 6, 182–187. [Google Scholar] [CrossRef]
- Bhavna, J. Stroke Diagnostics and Therapeutics: Global Markets; BCC research: Wellesley, MA, USA, 2015. [Google Scholar]
- Goldstein, L.B.; Adams, R.; Alberts, M.J.; Appel, L.J.; Brass, L.M.; Bushnell, C.D.; Culebras, A.; Degraba, T.J.; Gorelick, P.B.; Guyton, J.R.; et al. Primary prevention of ischemic stroke: A guideline from the american heart association/american stroke association stroke council: Cosponsored by the atherosclerotic peripheral vascular disease interdisciplinary working group; cardiovascular nursing council; clinical cardiology council; nutrition, physical activity, and metabolism council; and the quality of care and outcomes research interdisciplinary working group: The American academy of neurology affirms the value of this guideline. Stroke 2006, 37, 1583–1633. [Google Scholar] [PubMed]
- Allender, S.; Scarborough, P.; Peto, V.; Rayner, M.; Leal, J.; Luengo-Fernandez, R.; Gray, A. European Cardiovascular Disease Statistics; European Heart Network: Brussels, UK, 2008. [Google Scholar]
- Charles, P.W.; Jan, V.G.; Martin, S.D.; Joanna, M.W.; John, M.B.; Graeme, J.H.; Peter, A.G.S.; Gabriel, R.; Peter, L.; Cathie, S.; et al. Stroke: Practical Management, 3rd ed.; Wiley-Blackwell: Tokyo, Japan, 2008. [Google Scholar]
- Great Britain: National Audit Office. Reducing brain damage: Faster access to better stroke care; National Audit Office: London, UK, 2005. [Google Scholar]
- Kidwell, C.S.; Chalela, J.A.; Saver, J.L.; Starkman, S.; Hill, M.D.; Demchuk, A.M.; Butman, J.A.; Patronas, N.; Alger, J.R.; Latour, L.L.; et al. Comparison of mri and ct for detection of acute intracerebral hemorrhage. Jama 2004, 292, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Weinstein, J.R.; Murphy, S.P. Stroke: Basic and clinical. Adv. Neurobiol. 2017, 15, 281–293. [Google Scholar] [PubMed]
- Patel, R.A.G.; White, C.J. Stroke treatment and prevention. Prog. Cardiovasc. Dis. 2017, 59, 525–526. [Google Scholar] [CrossRef] [PubMed]
- Tsivgoulis, G.; Kargiotis, O.; Alexandrov, A.V. Intravenous thrombolysis for acute ischemic stroke: A bridge between two centuries. Expert Rev. Neurother. 2017, 17, 819–837. [Google Scholar] [CrossRef] [PubMed]
- Glickman, S.W.; Phillips, S.; Anstrom, K.J.; Laskowitz, D.T.; Cairns, C.B. Discriminative capacity of biomarkers for acute stroke in the emergency department. J. Emerg. Med. 2011, 41, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Caplan, L.R. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995, 333, 1581–1587. [Google Scholar]
- Adams, H.P., Jr.; del Zoppo, G.; Alberts, M.J.; Bhatt, D.L.; Brass, L.; Furlan, A.; Grubb, R.L.; Higashida, R.T.; Jauch, E.C.; Kidwell, C.; et al. Guidelines for the early management of adults with ischemic stroke: A guideline from the american heart association/american stroke association stroke council, clinical cardiology council, cardiovascular radiology and intervention council, and the atherosclerotic peripheral vascular disease and quality of care outcomes in research interdisciplinary working groups: The american academy of neurology affirms the value of this guideline as an educational tool for neurologists. Circulation 2007, 115, e478–e534. [Google Scholar] [PubMed]
- Ng, G.J.; Quek, A.M.; Cheung, C.; Arumugam, T.V.; Seet, R.C. Stroke biomarkers in clinical practice: A critical appraisal. Neurochem. Int. 2017, 107, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.; Pizzi, M.A.; Brent Peel, J.; Alejos, D.; Mbabuike, N.; Brown, B.L.; Hodge, D.; David Freeman, W. Update on neurocritical care of stroke. Curr. Cardiol. Reports 2017, 19, 67. [Google Scholar] [CrossRef] [PubMed]
- Khaku, A.D. Stroke; Statpearls: Treasure Island, FL, USA, 2017. [Google Scholar]
- Audebert, H.J.; Saver, J.L.; Starkman, S.; Lees, K.R.; Endres, M. Prehospital stroke care: New prospects for treatment and clinical research. Neurology 2013, 81, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Lees, K.R.; Bluhmki, E.; von Kummer, R.; Brott, T.G.; Toni, D.; Grotta, J.C.; Albers, G.W.; Kaste, M.; Marler, J.R.; Hamilton, S.A.; et al. Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ecass, atlantis, ninds, and epithet trials. Lancet 2010, 375, 1695–1703. [Google Scholar] [CrossRef]
- Hacke, W.; Kaste, M.; Bluhmki, E.; Brozman, M.; Davalos, A.; Guidetti, D.; Larrue, V.; Lees, K.R.; Medeghri, Z.; Machnig, T.; et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Sandercock, P.; Wardlaw, J.M.; Lindley, R.I.; Dennis, M.; Cohen, G.; Murray, G.; Innes, K.; Venables, G.; Czlonkowska, A.; Kobayashi, A.; et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [ist-3]): A randomised controlled trial. Lancet 2012, 379, 2352–2363. [Google Scholar] [PubMed]
- Leys, D.; Ringelstein, E.B.; Kaste, M.; Hacke, W. Facilities available in european hospitals treating stroke patients. Stroke; J. Cereb. Circ. 2007, 38, 2985–2991. [Google Scholar] [CrossRef] [PubMed]
- Nolte, C.H.; Audebert, H.J. [management of acute ischemic stroke]. Dtsch. Med. Wochenschr. 2015, 140, 1583–1586. [Google Scholar] [PubMed]
- Evenson, K.R.; Rosamond, W.D.; Morris, D.L. Prehospital and in-hospital delays in acute stroke care. Neuroepidemiology 2001, 20, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.J.; Yan, B. Minimising time to treatment: Targeted strategies to minimise time to thrombolysis for acute ischaemic stroke. Intern. Med. J. 2013, 43, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, A.; Garcia-Berrocoso, T.; Rodriguez, N.; Llombart, V.; Ribo, M.; Molina, C.; Montaner, J. Ischemic stroke outcome: A review of the influence of post-stroke complications within the different scenarios of stroke care. Eur. J. Intern. Med. 2016, 29, 9–21. [Google Scholar] [CrossRef] [PubMed]
- McMullan, J.T.; Knight, W.A.; Clark, J.F.; Beyette, F.R.; Pancioli, A. Time-critical neurological emergencies: The unfulfilled role for point-of-care testing. Intern. J. Emerg. Med. 2010, 3, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Rooney, K.D.; Schilling, U.M. Point-of-care testing in the overcrowded emergency department—Can it make a difference? Crit. Care 2014, 18, 692. [Google Scholar] [CrossRef] [PubMed]
- Cummins, B.M.; Ligler, F.S.; Walker, G.M. Point-of-care diagnostics for niche applications. Biotech. Adv. 2016, 34, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Vasan, A.S.; Mahadeo, D.M.; Doraiswami, R.; Huang, Y.; Pecht, M. Point-of-care biosensor system. Front. Biosci. 2013, 5, 39–71. [Google Scholar] [CrossRef]
- Chalela, J.A.; Kidwell, C.S.; Nentwich, L.M.; Luby, M.; Butman, J.A.; Demchuk, A.M.; Hill, M.D.; Patronas, N.; Latour, L.; Warach, S. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: A prospective comparison. Lancet 2007, 369, 293–298. [Google Scholar] [CrossRef]
- Dzialowski, I.; Hill, M.D.; Coutts, S.B.; Demchuk, A.M.; Kent, D.M.; Wunderlich, O.; von Kummer, R. Extent of early ischemic changes on computed tomography (CT) before thrombolysis. Prognostic Value of the Alberta Stroke Program Early CT Score in ECASS II. Stroke 2006, 37, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Dunbabin, D.W.; Sandercock, P.A. Investigation of acute stroke: What is the most effective strategy? Postgrad. Med. J. 1991, 67, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.C. Functional imaging in stroke recovery. Stroke 2004, 35, 2695–2698. [Google Scholar] [CrossRef] [PubMed]
- Schellinger, P.D.; Chalela, J.A.; Kang, D.W.; Latour, L.L.; Warach, S. Diagnostic and prognostic value of early mr imaging vessel signs in hyperacute stroke patients imaged <3 hours and treated with recombinant tissue plasminogen activator. Am. J. Neuroradiol. 2005, 26, 618–624. [Google Scholar] [PubMed]
- Schellinger, P.D.; Jansen, O.; Fiebach, J.B.; Hacke, W.; Sartor, K. A standardized mri stroke protocol. Comparison with CT in Hyperacute Intracerebral Hemorrhage. Stroke 1999, 30, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Caplan, L.R. Stroke: A Clinical Approach, 4th ed.; Saunders Elsevier: Philadelphia, PA, USA, 2009. [Google Scholar]
- Jiang, Q.; Zhang, Z.G.; Chopp, M. Mri of stroke recovery. Stroke 2010, 41, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.J.; Kidwell, C.S.; Alger, J.; Starkman, S.; Saver, J.L. Impact on stroke subtype diagnosis of early diffusion-weighted magnetic resonance imaging and magnetic resonance angiography. Stroke 2000, 31, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Chollet, F.; Albucher, J.F. Strategies to augment recovery after stroke. Curr. Treat. Options Neurol. 2012, 14, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Heller, S.L.; Heier, L.A.; Watts, R.; Schwartz, T.H.; Zelenko, N.; Doyle, W.; Devinsky, O. Evidence of cerebral reorganization following perinatal stroke demonstrated with fmri and dti tractography. Clin. Imag. 2005, 29, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; You, S.H.; Kwon, Y.H.; Hallett, M.; Kim, J.H.; Jang, S.H. Longitudinal fmri study for locomotor recovery in patients with stroke. Neurology 2006, 67, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Jang, S.H.; Chang, Y.; Byun, W.M.; Son, S.; Ahn, S.H. Bilateral primary sensori-motor cortex activation of post-stroke mirror movements: An fMRI study. Neuroreport 2003, 14, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Eaton, K.P.; Szaflarski, J.P.; Altaye, M.; Ball, A.L.; Kissela, B.M.; Banks, C.; Holland, S.K. Reliability of fMRI for studies of language in post-stroke aphasia subjects. Neuroimage 2008, 41, 311–322. [Google Scholar] [CrossRef] [PubMed]
- O'Sullivan, M.; Morris, R.G.; Huckstep, B.; Jones, D.K.; Williams, S.C.; Markus, H.S. Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J. Neurol. Neurosurg. Psychiatry 2004, 75, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, Z.G.; Chopp, M. Mri evaluation of white matter recovery after brain injury. Stroke 2010, 41, S112–S113. [Google Scholar] [CrossRef] [PubMed]
- Kunimatsu, A.; Aoki, S.; Masutani, Y.; Abe, O.; Mori, H.; Ohtomo, K. Three-dimensional white matter tractography by diffusion tensor imaging in ischaemic stroke involving the corticospinal tract. Neuroradiology 2003, 45, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.S.; Barnett, H.J.; Beebe, H.G.; Bernstein, E.F.; Brener, B.J.; Brott, T.; Caplan, L.R.; Day, A.; Goldstone, J.; Hobson, R.W.; et al. Guidelines for carotid endarterectomy. A multidisciplinary consensus statement from the ad hoc committee, american heart association. Stroke 1995, 26, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Barnett, H.J.M. Stroke Pathophysiology, Diagnosis, and Management, 2nd ed.; Churchill Livingstone: New York, NY, USA, 1992. [Google Scholar]
- American Heart & Stroke Association. Available online: https://www.heart.org/HEARTORG/General/Heart-and-Stroke-AssociationStatistics_UCM_319064_SubHomePage.jsp (accessed on 1 April 2017).
- Govindarajan, P.; Ghilarducci, D.; McCulloch, C.; Pierog, J.; Bloom, E.; Johnston, C. Comparative evaluation of stroke triage algorithms for emergency medical dispatchers (meds): Prospective cohort study protocol. BMC Neurol. 2011, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Wojner-Alexandrov, A.W.; Alexandrov, A.V.; Rodriguez, D.; Persse, D.; Grotta, J.C. Houston paramedic and emergency stroke treatment and outcomes study (hopsto). Stroke 2005, 36, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Gardiner, F.; Neeman, T.; Jones, B.; Gawarikar, Y. The cost-effectiveness of a stroke unit in providing enhanced patient outcomes in an australian teaching hospital. Available online: http://www.sciencedirect.com/science/article/pii/S1052305717302550 (accessed on 3 August 2017).
- Miller, E.C.; Blum, C.; Rostanski, S.K. Developing a stroke center. Stroke 2017, 48, e155–e156. [Google Scholar] [CrossRef] [PubMed]
- Watkins, C.L.; Leathley, M.J.; Jones, S.P.; Ford, G.A.; Quinn, T.; Sutton, C.J. Training emergency services' dispatchers to recognise stroke: An interrupted time-series analysis. BMC Health. Serv. Res. 2013, 13, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.Z.; Reeves, M.J.; Jacobs, B.S.; Birbeck, G.L.; Kothari, R.U.; Hickenbottom, S.L.; Mullard, A.J.; Wehner, S.; Maddox, K.; Majid, A. Iv tissue plasminogen activator use in acute stroke: Experience from a statewide registry. Neurology 2006, 66, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, L.; Rusterholtz, T.; Fery-Lemonnier, E.; Woimant, F.; Leroyer, J.; Hommel, M. Improving stroke care: a French health-care organiser’s perspective. Intern. J. Stroke. 2011, 6, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Katz, B.S.; Adeoye, O.; Sucharew, H.; Broderick, J.P.; McMullan, J.; Khatri, P.; Widener, M.; Alwell, K.S.; Moomaw, C.J.; Kissela, B.M.; et al. Estimated impact of emergency medical service triage of stroke patients on comprehensive stroke centers: An urban population-based study. Stroke 2017, 48, 2164–2170. [Google Scholar] [CrossRef] [PubMed]
- Waqar Faiz, K.; Sundseth, A.; Thommessen, B.; Ronning, O.M. Prehospital path in acute stroke. Tidsskr. Nor. 2017, 137, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Brott, T.; Adams, H.P., Jr.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V.; et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Assis, Z.A.; Menon, B.K.; Goyal, M. Imaging department organization in a stroke center and workflow processes in acute stroke. Available online: http://www.sciencedirect.com/science/article/pii/S0720048X17302607 (accessed on 3 August 2017).
- Ferro, J.M.; Massaro, A.R.; Mas, J.L. Aetiological diagnosis of ischaemic stroke in young adults. Lancet. Neurol. 2010, 9, 1085–1096. [Google Scholar] [CrossRef]
- Thomas, S.M.; Thomas, S.R. Stroke and Transient Ischemic Attack. Available online: https://scholar.google.com/scholar?q=Thomas%2C+S.%3B+Maldonado%2C+M.D.%3B+Thomas%2C+S.%3B+Riles%2C+M.D.%3B+F.A.C.S.+Stroke+and+transient+ischemic+attack-1+1+stroke+and+transient+ischemic+attack.+Vasc.+Syst.+2007&btnG=&hl=en&as_sdt=0%2C5 (accessed on 3 August 2017).
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation 2013, 127, e6–e245. [Google Scholar] [CrossRef] [PubMed]
- Radu, R.A.; Terecoasa, E.O.; Bajenaru, O.A.; Tiu, C. Etiologic classification of ischemic stroke: Where do we stand? Clin. Neurol. Neurosurg. 2017, 159, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Amarenco, P.; Bogousslavsky, J.; Caplan, L.R.; Donnan, G.A.; Hennerici, M.G. Classification of stroke subtypes. Cerebrovasc. Dis. 2009, 27, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Landau, W.M.; Nassief, A. Editorial comment--time to burn the toast. Stroke 2005, 36, 902–904. [Google Scholar] [PubMed]
- Amarenco, P. Patent foramen ovale and the risk of stroke: Smoking gun guilty by association? Heart 2005, 91, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Gokcal, E.; Niftaliyev, E.; Asil, T. Etiological classification of ischemic stroke in young patients: A comparative study of TOAST, CCS, and ASCO. Acta. Neurologica. Belgica. 2017, 1–6. [Google Scholar]
- Sacco, R.L.; Ellenberg, J.H.; Mohr, J.P.; Tatemichi, T.K.; Hier, D.B.; Price, T.R.; Wolf, P.A. Infarcts of undetermined cause: The nincds stroke data bank. Ann. Neurol. 1989, 25, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Bamford, J.; Sandercock, P.; Dennis, M.; Burn, J.; Warlow, C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991, 337, 1521–1526. [Google Scholar] [CrossRef]
- Lindley, R.I.; Warlow, C.P.; Wardlaw, J.M.; Dennis, M.S.; Slattery, J.; Sandercock, P.A. Interobserver reliability of a clinical classification of acute cerebral infarction. Stroke 1993, 24, 1801–1804. [Google Scholar] [CrossRef] [PubMed]
- Teo, K.; Slark, J. A systematic review of studies investigating the care of stroke survivors in long-term care facilities. Disabil. Rehabil. 2016, 38, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Price, C.P. Point of care testing. BMJ 2001, 322, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- St John, A.; Price, C.P. Existing and emerging technologies for point-of-care testing. Clin. Biochem. Rev. 2014, 35, 155–167. [Google Scholar] [PubMed]
- Yoo, E.H.; Lee, S.Y. Glucose biosensors: An overview of use in clinical practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Maghsoudlou, D. Enzyme-linked immunosorbent assay (elisa). Basics Br. J. Hospital Med. 2016, 77, C98–C101. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.E.; Ebinger, M.; Rozanski, M.; Waldschmidt, C.; Wendt, M.; Winter, B.; Kellner, P.; Baumann, A.; Fiebach, J.B.; Villringer, K.; et al. Prehospital thrombolysis in acute stroke: Results of the phantom-s pilot study. Neurology 2013, 80, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Handschu, R.; Littmann, R.; Reulbach, U.; Gaul, C.; Heckmann, J.G.; Neundorfer, B.; Scibor, M. Telemedicine in emergency evaluation of acute stroke: Interrater agreement in remote video examination with a novel multimedia system. Stroke 2003, 34, 2842–2846. [Google Scholar] [CrossRef] [PubMed]
- Demaerschalk, B.M.; Vargas, J.E.; Channer, D.D.; Noble, B.N.; Kiernan, T.E.; Gleason, E.A.; Vargas, B.B.; Ingall, T.J.; Aguilar, M.I.; Dodick, D.W.; et al. Smartphone teleradiology application is successfully incorporated into a telestroke network environment. Stroke 2012, 43, 3098–3101. [Google Scholar] [CrossRef] [PubMed]
- Amadi-Obi, A.; Gilligan, P.; Owens, N.; O'Donnell, C. Telemedicine in pre-hospital care: A review of telemedicine applications in the pre-hospital environment. Intern. J. Emerg. Med. 2014, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Raman, R.; Ernstrom, K.; Claycomb, R.J.; Meyer, D.M.; Hemmen, T.M.; Modir, R.F.; Kachhi, P.; Meyer, B.C. Accuracy of stroke diagnosis in telestroke-guided tissue plasminogen activator patients. J. Stroke Cerebrovasc. Dis. 2016, 25, 2942–2946. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.B.; Ryoo, S.M.; Lee, D.H.; Kwon, S.U.; Jang, S.; Lee, E.J.; Lee, S.H.; Han, J.H.; Yoon, M.J.; Jeong, S.; et al. Multidisciplinary approach to decrease in-hospital delay for stroke thrombolysis. J. Stroke 2017, 19, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Vecchiato, A. Can we imagine a survival chain also for ischemic stroke? Available online: http://www.sciencedirect.com/science/article/pii/S0735675717304370 (accessed on 3 August 2017).
- Walter, S.; Kostopoulos, P.; Haass, A.; Keller, I.; Lesmeister, M.; Schlechtriemen, T.; Roth, C.; Papanagiotou, P.; Grunwald, I.; Schumacher, H.; et al. Diagnosis and treatment of patients with stroke in a mobile stroke unit versus in hospital: A randomised controlled trial. Lancet. Neurol. 2012, 11, 397–404. [Google Scholar] [CrossRef]
- Fassbender, K.; Walter, S.; Liu, Y.; Muehlhauser, F.; Ragoschke, A.; Kuehl, S.; Mielke, O. “Mobile stroke unit” for hyperacute stroke treatment. Stroke 2003, 34, e44. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.A.; Bowry, R.; Wu, T.C.; Noser, E.A.; Jackson, K.; Richardson, L.; Persse, D.; Grotta, J.C. Establishing the first mobile stroke unit in the united states. Stroke 2015, 46, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Ebinger, M.; Rozanski, M.; Waldschmidt, C.; Weber, J.; Wendt, M.; Winter, B.; Kellner, P.; Baumann, A.M.; Malzahn, U.; Heuschmann, P.U.; et al. Phantom-s: The prehospital acute neurological therapy and optimization of medical care in stroke patients-study. Int. J. Stroke 2012, 7, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Wendt, M.; Ebinger, M.; Kunz, A.; Rozanski, M.; Waldschmidt, C.; Weber, J.E.; Winter, B.; Koch, P.M.; Freitag, E.; Reich, J.; et al. Improved prehospital triage of patients with stroke in a specialized stroke ambulance: Results of the pre-hospital acute neurological therapy and optimization of medical care in stroke study. Stroke 2015, 46, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.A.; Ahrens, C.L.; Hussain, M.S.; Winners, S.; Rasmussen, P.A.; Uchino, K. Prehospital reversal of warfarin-related coagulopathy in intracerebral hemorrhage in a mobile stroke treatment unit. Stroke 2015, 46, e118–e120. [Google Scholar] [CrossRef] [PubMed]
- Rizos, T.; Herweh, C.; Jenetzky, E.; Lichy, C.; Ringleb, P.A.; Hacke, W.; Veltkamp, R. Point-of-care international normalized ratio testing accelerates thrombolysis in patients with acute ischemic stroke using oral anticoagulants. Stroke 2009, 40, 3547–3551. [Google Scholar] [CrossRef] [PubMed]
- Nusa, D.; Harvey, I.; Almansouri, A.Y.; Wright, S.; Neeman, T.; Ahmad, O.; Hughes, A.R.; Lueck, C.J. Assessment of point-of-care measurement of international normalised ratio using the coaguchek xs plus system in the setting of acute ischaemic stroke. Intern. Med. J. 2013, 43, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Green, T.L.; Mansoor, A.; Newcommon, N.; Stephenson, C.; Stewart, E.; Hill, M.D. Reliability of point-of-care testing of inr in acute stroke. Can. J. Neurol. Sci. 2008, 35, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Thorne, K.; McNaughton, H.; Weatherall, M. An audit of coagulation screening in patients presenting to the emergency department for potential stroke thrombolysis. Intern. Med. J. 2017, 47, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.; Kostopoulos, P.; Haass, A.; Lesmeister, M.; Grasu, M.; Grunwald, I.; Keller, I.; Helwig, S.; Becker, C.; Geisel, J.; et al. Point-of-care laboratory halves door-to-therapy-decision time in acute stroke. Ann. Neurol. 2011, 69, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Briggs, C.; Kunka, S.; Pennaneach, C.; Forbes, L.; Machin, S.J. Performance evaluation of a new compact hematology analyzer, the sysmex poch-100i. Lab. Hematol. 2003, 9, 225–233. [Google Scholar] [PubMed]
- Drescher, M.J.; Spence, A.; Rockwell, D.; Staff, I.; Smally, A.J. Point-of-care testing for coagulation studies in a stroke protocol: A time-saving innovation. Am. J. Emerg. Med. 2011, 29, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, S.; Tayal, A.H.; Hegde, G.G.; Shang, J.; Venkat, A. An analysis of discrepancy between point-of-care and central laboratory international normalized ratio testing in ED patients with cerebrovascular disease. Am. J. Emerg. Med. 2012, 30, 2025–2029. [Google Scholar] [CrossRef] [PubMed]
- Abbott, Inc. A single, Integrated Point-of-care Testing Solution. Available online: https://www.pointofcare.abbott/int/en/offerings/istat/istat-handheld (accessed on 2 August 2017).
- Chaudhuri, J.R.; Sharma, V.K.; Mridula, K.R.; Balaraju, B.; Bandaru, V.C. Association of plasma brain natriuretic peptide levels in acute ischemic stroke subtypes and outcome. J. Stroke Cerebrovasc. Dis. 2015, 24, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Clerico, A.; Franzini, M.; Masotti, S.; Prontera, C.; Passino, C. State of the art of immunoassay methods for b-type natriuretic peptides: An update. Crit. Rev. Clin. Lab. Sci. 2015, 52, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Kawase, S.; Kowa, H.; Suto, Y.; Fukuda, H.; Kusumi, M.; Nakayasu, H.; Nakashima, K. Plasma brain natriuretic peptide is a marker of prognostic functional outcome in non-cardioembolic infarction. J. Stroke Cerebrovasc. Dis. 2015, 24, 2285–2290. [Google Scholar] [CrossRef] [PubMed]
- Llombart, V.; Antolin-Fontes, A.; Bustamante, A.; Giralt, D.; Rost, N.S.; Furie, K.; Shibazaki, K.; Biteker, M.; Castillo, J.; Rodriguez-Yanez, M.; et al. B-type natriuretic peptides help in cardioembolic stroke diagnosis: Pooled data meta-analysis. Stroke 2015, 46, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Balion, C.; McKelvie, R.; Don-Wauchope, A.C.; Santaguida, P.L.; Oremus, M.; Keshavarz, H.; Hill, S.A.; Booth, R.A.; Ali, U.; Brown, J.A.; et al. B-type natriuretic peptide-guided therapy: A systematic review. Heart Fail. Rev. 2014, 19, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Kara, K.; Gronewold, J.; Neumann, T.; Mahabadi, A.A.; Weimar, C.; Lehmann, N.; Berger, K.; Kalsch, H.I.; Bauer, M.; Broecker-Preuss, M.; et al. B-type natriuretic peptide predicts stroke of presumable cardioembolic origin in addition to coronary artery calcification. Eur. J. Neurol. 2014, 21, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Shiga, T.; Iijima, M.; Moriya, S.; Mizuno, S.; Toi, S.; Arai, K.; Ashihara, K.; Abe, K.; Uchiyama, S. Brain natriuretic peptide in acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2014, 23, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Qihong, G.; Zhixin, W.; Mingfeng, H.; Lianhong, Y.; Wenchong, X. Experiences and the use of bnp poct platform on suspected stroke patients by a chinese emergency department. Annals Indian Acad. Neurol. 2014, 17, 243–244. [Google Scholar]
- Yang, H.L.; Lin, Y.P.; Long, Y.; Ma, Q.L.; Zhou, C. Predicting cardioembolic stroke with the b-type natriuretic peptide test: A systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 2014, 23, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, L.; Guo, Q.; He, M.; Li, K. Experiences and the use of bnp poct platform on suspected ischemic stroke patients in the emergency department setting. Clin. Neurol. Neurosurg. 2014, 123, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Shibazaki, K.; Kimura, K.; Aoki, J.; Sakai, K.; Saji, N.; Uemura, J. Brain natriuretic peptide level on admission predicts recurrent stroke after discharge in stroke survivors with atrial fibrillation. Clin. Neurol. Neurosurg. 2014, 127, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Shibazaki, K.; Kimura, K.; Aoki, J.; Sakai, K.; Saji, N.; Uemura, J. Plasma brain natriuretic peptide as a predictive marker of early recurrent stroke in cardioembolic stroke patients. J. Stroke Cerebrovasc. Dis. 2014, 23, 2635–2640. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, I.M.; Cojocaru, M.; Sapira, V.; Ionescu, A.; Barlan, S.; Tacu, N. Could pro-bnp, uric acid, bilirubin, albumin and transferrin be used in making the distinction between stroke subtypes? Rom. J. Intern. Med. 2013, 51, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Hajsadeghi, S.; Kashani Amin, L.; Bakhshandeh, H.; Rohani, M.; Azizian, A.R.; Jafarian Kerman, S.R. The diagnostic value of n-terminal pro-brain natriuretic peptide in differentiating cardioembolic ischemic stroke. J. Stroke Cerebrovasc. Dis. 2013, 22, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Shibazaki, K.; Kimura, K.; Aoki, J.; Kobayashi, K.; Fujii, S.; Okada, Y. Brain natriuretic peptide as a predictor of cardioembolism in acute ischemic stroke patients: Brain natriuretic peptide stroke prospective study. Eur. Neurol. 2013, 69, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Berrocoso, T.; Giralt, D.; Bustamante, A.; Etgen, T.; Jensen, J.K.; Sharma, J.C.; Shibazaki, K.; Saritas, A.; Chen, X.; Whiteley, W.N.; et al. B-type natriuretic peptides and mortality after stroke: A systematic review and meta-analysis. Neurology 2013, 81, 1976–1985. [Google Scholar] [CrossRef] [PubMed]
- Jickling, G.C.; Foerch, C. Predicting stroke mortality: Bnp could it be? Neurology 2013, 81, 1970–1971. [Google Scholar] [CrossRef] [PubMed]
- Shibazaki, K.; Kimura, K.; Sakai, K.; Fujii, S.; Aoki, J.; Saji, N. Brain natriuretic peptide on admission as a biological marker of long-term mortality in ischemic stroke survivors. Eur. Neurol. 2013, 70, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhan, X.; Chen, M.; Lei, H.; Wang, Y.; Wei, D.; Jiang, X. The prognostic value of combined nt-pro-bnp levels and nihss scores in patients with acute ischemic stroke. Intern. Med. 2012, 51, 2887–2892. [Google Scholar] [CrossRef] [PubMed]
- Montaner, J.; Garcia-Berrocoso, T.; Mendioroz, M.; Palacios, M.; Perea-Gainza, M.; Delgado, P.; Rosell, A.; Slevin, M.; Ribo, M.; Molina, C.A.; et al. Brain natriuretic peptide is associated with worsening and mortality in acute stroke patients but adds no prognostic value to clinical predictors of outcome. Cerebrovasc. Dis. 2012, 34, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Shibazaki, K.; Kimura, K.; Iguchi, Y.; Okada, Y.; Inoue, T. Plasma brain natriuretic peptide can be a biological marker to distinguish cardioembolic stroke from other stroke types in acute ischemic stroke. Intern. Med. 2009, 48, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Shibazaki, K.; Kimura, K.; Okada, Y.; Iguchi, Y.; Uemura, J.; Terasawa, Y.; Aoki, J. Plasma brain natriuretic peptide as an independent predictor of in-hospital mortality after acute ischemic stroke. Intern. Med. 2009, 48, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.K.; Atar, D.; Kristensen, S.R.; Mickley, H.; Januzzi, J.L., Jr. Usefulness of natriuretic peptide testing for long-term risk assessment following acute ischemic stroke. Am. J. Cardiol. 2009, 104, 287–291. [Google Scholar] [CrossRef] [PubMed]
- United States Government; Development of stroke point of care immunoassay for cellular fibronectin. Available online: https://www.sbir.gov/sbirsearch/detail/280110 (accessed on 3 August 2017).
- Mäkikallio, A.M.; Mäkikallio, T.H.; Korpelainen, J.T.; Vuolteenaho, O.; Tapanainen, J.M.; Ylitalo, K.; Sotaniemi, K.A.; Huikuri, H.V.; Myllylä, V.V. Natriuretic peptides and mortality after stroke. Stroke 2005, 36, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wu, Z.; Li, Y.; Lei, J. A new algorithm of suspected stroke patient management with brain natriuretic peptide/n-terminal pro-brain natriuretic peptide point of care testing platform in the emergency department. Annals Indian Acad. Neurol. 2017, 20, 81–82. [Google Scholar]
- Cohen, R.; Lata, J.P.; Lee, Y.; Hernández, J.C.C.; Nishimura, N.; Schaffer, C.B.; Mukai, C.; Nelson, J.L.; Brangman, S.A.; Agrawal, Y.; et al. Use of tethered enzymes as a platform technology for rapid analyte detection. Available online: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0142326. (accessed on 3 August 2017).
- Dash, P.K.; Zhao, J.; Hergenroeder, G.; Moore, A.N. Biomarkers for the diagnosis, prognosis, and evaluation of treatment efficacy for traumatic brain injury. Neurotherapeutics 2010, 7, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, O.; Wardlaw, J.; Whiteley, W.N. Correlation of levels of neuronal and glial markers with radiological measures of infarct volume in ischaemic stroke: A systematic review. Cerebrovasc. Dis. 2012, 33, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, M.T.; Lins, H.; Skalej, M.; Wallesch, C.W.; Goertler, M. Neuron-specific enolase and tau protein as neurobiochemical markers of neuronal damage are related to early clinical course and long-term outcome in acute ischemic stroke. Clin. Neurol. Neurosurg. 2006, 108, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Wevers, R.A.; Jacobs, A.A.; Hommes, O.R. A bioluminescent assay for enolase (ec 4.2.1.11) activity in human serum and cerebrospinal fluid. Clin. Chim. Acta. 1983, 135, 159–168. [Google Scholar] [CrossRef]
- Viallard, J.L.; Murthy, M.R.; Dastugue, B. An ultramicro bioluminescence assay of enolase: Application to human cerebrospinal fluid. Neurochem. Res. 1985, 10, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Wevers, R.A.; Theunisse, A.W.; Rijksen, G. An immunobioluminescence assay for gamma-gamma enolase activity in human serum and cerebrospinal fluid. Clin. Chim. Acta. 1988, 178, 141–150. [Google Scholar] [CrossRef]
- Valtari Bio™ Inc. Overview. Available online: http://valtaribio.com/executive-overview/ (accessed on 1 August 2017).
- Karlinski, M.; Gluszkiewicz, M.; Czlonkowska, A. The accuracy of prehospital diagnosis of acute cerebrovascular accidents: An observational study. AMS 2015, 11, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Brandler, E.S.; Sharma, M.; Sinert, R.H.; Levine, S.R. Prehospital stroke scales in urban environments: A systematic review. Neurology 2014, 82, 2241–2249. [Google Scholar] [CrossRef] [PubMed]
- Sarissa Biomedical Ltd. Sarissa’s stroke diagnostic: SMARTChip, wins SBRI grant award. Available online: http://www.sarissa-biomedical.com/news/sarissa%E2%80%99s-stroke-diagnostic-smartchip,-wins-sbri-grant-award.aspx (accessed on 1 August 2017).
- Harrison, P.; Segal, H.; Blasbery, K.; Furtado, C.; Silver, L.; Rothwell, P.M. Screening for aspirin responsiveness after transient ischemic attack and stroke. Comparison of 2 Point-of-Care Platelet Function Tests With Optical. Aggregometry 2005, 36, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Antithrombotic Trialists' Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002, 324, 71–86.
- Howard, P.A. Aspirin resistance. Ann. Pharmacother. 2002, 36, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- McKee, S.A.; Sane, D.C.; Deliargyris, E.N. Aspirin resistance in cardiovascular disease: A review of prevalence, mechanisms, and clinical significance. Thromb. Haemost. 2002, 88, 711–715. [Google Scholar] [PubMed]
- Altman, R.; Luciardi, H.L.; Muntaner, J.; Herrera, R.N. The antithrombotic profile of aspirin. Aspirin resistance, or simply failure? Thromb. J. 2004, 2, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hankey, G.J.; Eikelboom, J.W. Aspirin resistance. BMJ 2004, 328, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, J.W.; Hankey, G.J. Failure of aspirin to prevent atherothrombosis: Potential mechanisms and implications for clinical practice. Am. J. Cardiovasc. Drugs 2004, 4, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Gum, P.A.; Kottke-Marchant, K.; Poggio, E.D.; Gurm, H.; Welsh, P.A.; Brooks, L.; Sapp, S.K.; Topol, E.J. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am. J. Cardiol. 2001, 88, 230–235. [Google Scholar] [CrossRef]
- Helgason, C.M.; Tortorice, K.L.; Winkler, S.R.; Penney, D.W.; Schuler, J.J.; McClelland, T.J.; Brace, L.D. Aspirin response and failure in cerebral infarction. Stroke 1993, 24, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Patrono, C. Aspirin resistance: Definition, mechanisms and clinical read-outs. JTH 2003, 1, 1710–1713. [Google Scholar] [CrossRef] [PubMed]
- Gum, P.A.; Kottke-Marchant, K.; Welsh, P.A.; White, J.; Topol, E.J. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J. Am. Coll. Cardiol. 2003, 41, 961–965. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Hankey, G.J. Aspirin resistance: A new independent predictor of vascular events? J. Am. Coll. Cardiol. 2003, 41, 966–968. [Google Scholar] [CrossRef]
- Rand, M.L.; Leung, R.; Packham, M.A. Platelet function assays. Transfus. Apheresis Sci. 2003, 28, 307–317. [Google Scholar] [CrossRef]
- Baigent, C.; Blackwell, L.; Collins, R.; Emberson, J.; Godwin, J.; Peto, R.; Buring, J.; Hennekens, C.; Kearney, P.; Meade, T.; et al. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009, 373, 1849–1860. [Google Scholar] [PubMed]
- Harrison, P. Progress in the assessment of platelet function. Br. J. Haematol. 2000, 111, 733–744. [Google Scholar] [PubMed]
- Lordkipanidzé, M.; Pharand, C.; Schampaert, E.; Turgeon, J.; Palisaitis, D.A.; Diodati, J.G. A comparison of six major platelet function tests to determine the prevalence of aspirin resistance in patients with stable coronary artery disease. Eur. Heart J. 2007, 28, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Algra, A.; Chen, Z.; Diener, H.C.; Norrving, B.; Mehta, Z. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: Time-course analysis of randomised trials. Lancet 2016, 388, 365–375. [Google Scholar] [CrossRef]
- Pearson, C.; Przyklenk, K.; Mika, V.H.; Ayaz, S.I.; Ellis, M.; Varade, P.; Tolomello, R.; Welch, R.D. Utility of point of care assessment of platelet reactivity (using the PFA-100(r)) to aid in diagnosis of stroke. Am. J. Emerg. Med. 2017, 35, 802.e1–802.e5. [Google Scholar] [CrossRef] [PubMed]
- Drescher, M.J.; Spence, A.; Rockwell, D.; Smally, A.J. Point-of-care testing for coagulation studies in an emergency department stroke protocol: A time-saving innovation. Annals Emerg. Med. 2007, 50, S25. [Google Scholar] [CrossRef]
- Mortezabeigi, H.R.; Taghizadeh, A.; Talebi, M.; Amini, K.; Goldust, M. ABCD2 score and BNP level in patients with TIA and cerebral stroke. Pak. J. Biolog. Sciences 2013, 16, 1393–1397. [Google Scholar]
- Yokobori, S.; Hosein, K.; Burks, S.; Sharma, I.; Gajavelli, S.; Bullock, R. Biomarkers for the clinical differential diagnosis in traumatic brain injury--a systematic review. CNS. Neurosci. Ther. 2013, 19, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Cata, J.P.; Abdelmalak, B.; Farag, E. Neurological biomarkers in the perioperative period. Br. J. Anaesth. 2011, 107, 844–858. [Google Scholar] [CrossRef] [PubMed]
- AP., D. Biomarkers: Coming of age for environmental health and risk assessment. Environ. Sci. Technol. 1997, 31, 1837–1848. [Google Scholar]
- Monbailliu, T.; Goossens, J.; Hachimi-Idrissi, S. Blood protein biomarkers as diagnostic tool for ischemic stroke: A systematic review. Biomark. Med. 2017, 11, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Ehrenreich, H. Brain derived proteins as markers of acute stroke: Their relation to pathophysiology, outcome prediction and neuroprotective drug monitoring. Restorative Neurol. Neurosci. 2003, 21, 177–190. [Google Scholar]
- Herrmann, M.; Vos, P.; Wunderlich, M.T.; de Bruijn, C.H.; Lamers, K.J. Release of glial tissue-specific proteins after acute stroke: A comparative analysis of serum concentrations of protein s-100b and glial fibrillary acidic protein. Stroke 2000, 31, 2670–2677. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, M.T.; Ebert, A.D.; Kratz, T.; Goertler, M.; Jost, S.; Herrmann, M. Early neurobehavioral outcome after stroke is related to release of neurobiochemical markers of brain damage. Stroke 1999, 30, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Kapural, M.; Krizanac-Bengez, L.; Barnett, G.; Perl, J.; Masaryk, T.; Apollo, D.; Rasmussen, P.; Mayberg, M.R.; Janigro, D. Serum s-100beta as a possible marker of blood-brain barrier disruption. Brain Res. 2002, 940, 102–104. [Google Scholar] [CrossRef]
- Gazzolo, D.; Abella, R.; Frigiola, A.; Giamberti, A.; Tina, G.; Nigro, F.; Florio, P.; Colivicchi, M.; Temporini, F.; Ricotti, A.; et al. Neuromarkers and unconventional biological fluids. J. Maternal-fetal Neonatal Med. 2010, 23, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Mir, I.N.; Chalak, L.F. Serum biomarkers to evaluate the integrity of the neurovascular unit. Early. Hum. Dev. 2014, 90, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Brouns, R.; De Vil, B.; Cras, P.; De Surgeloose, D.; Marien, P.; De Deyn, P.P. Neurobiochemical markers of brain damage in cerebrospinal fluid of acute ischemic stroke patients. Clin. Chem. 2010, 56, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Lian, T.; Qu, D.; Zhao, X.; Yu, L.; Gao, B. Identification of site-specific stroke biomarker candidates by laser capture microdissection and labeled reference peptide. Int. J. Mol. Sci. 2015, 16, 13427–13441. [Google Scholar] [CrossRef] [PubMed]
- Pelinka, L.E.; Kroepfl, A.; Leixnering, M.; Buchinger, W.; Raabe, A.; Redl, H. Gfap versus s100b in serum after traumatic brain injury: Relationship to brain damage and outcome. J. Neurotrauma 2004, 21, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, F.; Haberer, I.; Sitzer, M.; Foerch, C. Characterisation of the diagnostic window of serum glial fibrillary acidic protein for the differentiation of intracerebral haemorrhage and ischaemic stroke. Cerebrovasc. Dis. 2009, 27, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, K.K. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, C.E.; Dijkstra, C.; Polman, C. Biological markers in csf and blood for axonal degeneration in multiple sclerosis. Lancet. Neurol. 2005, 4, 32–41. [Google Scholar] [CrossRef]
- Shibata, D.; Cain, K.; Tanzi, P.; Zierath, D.; Becker, K. Myelin basic protein autoantibodies, white matter disease and stroke outcome. J. Neuroimmunol. 2012, 252, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Zierath, D.; Kunze, A.; Fecteau, L.; Becker, K. Promiscuity of autoimmune responses to mbp after stroke. J. Neuroimmunol. 2015, 285, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.J.; Tanzi, P.; Zierath, D.; Buckwalter, M.S. Antibodies to myelin basic protein are associated with cognitive decline after stroke. J. Neuroimmunol. 2016, 295, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.M.; Fusi, C.; Conti, A.A.; Paniccia, R.; Trefoloni, G.; Pracucci, G.; Di Carlo, A.; Noferi, D.; Carbonetto, F.; Pretelli, P.; et al. S-100 protein and neuron-specific enolase as markers of subclinical cerebral damage after cardiac surgery: Preliminary observation of a 6-month follow-up study. Eur. Neurol. 2001, 45, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Karkela, J.; Bock, E.; Kaukinen, S. Csf and serum brain-specific creatine kinase isoenzyme (ck-bb), neuron-specific enolase (nse) and neural cell adhesion molecule (ncam) as prognostic markers for hypoxic brain injury after cardiac arrest in man. J. Neurol. Sci. 1993, 116, 100–109. [Google Scholar] [CrossRef]
- Liu, M.C.; Akinyi, L.; Scharf, D.; Mo, J.; Larner, S.F.; Muller, U.; Oli, M.W.; Zheng, W.; Kobeissy, F.; Papa, L.; et al. Ubiquitin c-terminal hydrolase-l1 as a biomarker for ischemic and traumatic brain injury in rats. Eur. J. Neurosci. 2010, 31, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; Akinyi, L.; Liu, M.C.; Pineda, J.A.; Tepas, J.J.; Oli, M.W.; Zheng, W.; Robinson, G.; Robicsek, S.A.; Gabrielli, A.; et al. Ubiquitin c-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit. Care Med. 2010, 38, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zoltewicz, S.; Guingab-Cagmat, J.; Anagli, J.; Gao, M.; Hafeez, A.; Li, N.; Cao, J.; Geng, X.; Kobeissy, F.; et al. Different expression of ubiquitin c-terminal hydrolase-l1 and alphaii-spectrin in ischemic and hemorrhagic stroke: Potential biomarkers in diagnosis. Brain Res. 2013, 1540, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Tongaonkar, P.; Chen, L.; Lambertson, D.; Ko, B.; Madura, K. Evidence for an interaction between ubiquitin-conjugating enzymes and the 26s proteasome. Mol. Cell. Biol. 2000, 20, 4691–4698. [Google Scholar] [CrossRef] [PubMed]
- Vaagenes, P.; Urdal, P.; Melvoll, R.; Valnes, K. Enzyme level changes in the cerebrospinal fluid of patients with acute stroke. Arch. Neurol. 1986, 43, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Ingebrigtsen, T.; Romner, B. Biochemical serum markers for brain damage: A short review with emphasis on clinical utility in mild head injury. Restor. Neurol. Neurosci. 2003, 21, 171–176. [Google Scholar] [PubMed]
- Atisha, D.; Bhalla, M.A.; Morrison, L.K.; Felicio, L.; Clopton, P.; Gardetto, N.; Kazanegra, R.; Chiu, A.; Maisel, A.S. A prospective study in search of an optimal b-natriuretic peptide level to screen patients for cardiac dysfunction. Am. Heart J. 2004, 148, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, A.; Papageorgiou, N.; Falconer, D.; Rehal, O.; Sewart, E.; Zacharia, E.; Toutouzas, K.; Vlachopoulos, C.; Siasos, G.; Tsioufis, C.; et al. Biomarkers associated with stroke risk in atrial fibrillation. Curr. Med. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, A.; Lopez-Cancio, E.; Pich, S.; Penalba, A.; Giralt, D.; Garcia-Berrocoso, T.; Ferrer-Costa, C.; Gasull, T.; Hernandez-Perez, M.; Millan, M.; et al. Blood biomarkers for the early diagnosis of stroke: The stroke-chip study. Stroke 2017. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, M.; Leira, R.; Serena, J.; Pumar, J.M.; Lizasoain, I.; Castillo, J.; Davalos, A. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke 2003, 34, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Sood, R.; Yang, Y.; Taheri, S.; Candelario-Jalil, E.; Estrada, E.Y.; Walker, E.J.; Thompson, J.; Rosenberg, G.A. Increased apparent diffusion coefficients on mri linked with matrix metalloproteinases and edema in white matter after bilateral carotid artery occlusion in rats. J. Cereb. Blood Flow MeTable 2009, 29, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Laskowitz, D.T.; Kasner, S.E.; Saver, J.; Remmel, K.S.; Jauch, E.C. Clinical usefulness of a biomarker-based diagnostic test for acute stroke: The biomarker rapid assessment in ischemic injury (brain) study. Stroke 2009, 40, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Zhang, Z.M.; Yin, X.L.; Zhang, K.; Cai, H.; Ling, F. Exploring the optimal operation time for patients with hypertensive intracerebral hemorrhage: Tracking the expression and progress of cell apoptosis of prehematomal brain tissues. Chin. Med. J. 2010, 123, 1246–1250. [Google Scholar] [PubMed]
- Taurino, M.; Raffa, S.; Mastroddi, M.; Visco, V.; Rizzo, L.; Torrisi, M.R.; Faraglia, V. Metalloproteinase expression in carotid plaque and its correlation with plasma levels before and after carotid endarterectomy. Vascular Endovasc. Surg. 2007, 41, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Tayal, V.; Kalra, B.S. Cytokines and anti-cytokines as therapeutics--an update. Eur. J. Pharmacol. 2008, 579, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rostene, W.; Dansereau, M.A.; Godefroy, D.; Van Steenwinckel, J.; Reaux-Le Goazigo, A.; Melik-Parsadaniantz, S.; Apartis, E.; Hunot, S.; Beaudet, N.; Sarret, P. Neurochemokines: A menage a trois providing new insights on the functions of chemokines in the central nervous system. J. Neurochem. 2011, 118, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Di Raimondo, D.; Pecoraro, R.; Arnao, V.; Pinto, A.; Licata, G. Inflammation in ischemic stroke subtypes. Curr. Pharm. Des. 2012, 18, 4289–4310. [Google Scholar] [CrossRef] [PubMed]
- Licata, G.; Tuttolomondo, A.; Di Raimondo, D.; Corrao, S.; Di Sciacca, R.; Pinto, A. Immuno-inflammatory activation in acute cardio-embolic strokes in comparison with other subtypes of ischaemic stroke. Thromb. Haemostasis 2009, 101, 929–937. [Google Scholar] [CrossRef] [Green Version]
- Abbott, N.J.; Ronnback, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.L.; Carpentier, P.A.; McMahon, E.J.; Begolka, W.S.; Miller, S.D. Innate and adaptive immune responses of the central nervous system. Crit. Rev. Immunol. 2006, 26, 149–188. [Google Scholar] [CrossRef] [PubMed]
- Vela, J.M.; Molina-Holgado, E.; Arevalo-Martin, A.; Almazan, G.; Guaza, C. Interleukin-1 regulates proliferation and differentiation of oligodendrocyte progenitor cells. Mol. Cell. Neurosci. 2002, 20, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Yanez, M.; Castillo, J. Role of inflammatory markers in brain ischemia. Current Opin. Neurol. 2008, 21, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Gokhan, S.; Ozhasenekler, A.; Mansur Durgun, H.; Akil, E.; Ustundag, M.; Orak, M. Neutrophil lymphocyte ratios in stroke subtypes and transient ischemic attack. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 653–657. [Google Scholar] [PubMed]
- Huang, G.; Zhong, X.N.; Zhong, B.; Chen, Y.Q.; Liu, Z.Z.; Su, L.; Ling, Z.Y.; Cao, H.; Yin, Y.H. Significance of white blood cell count and its subtypes in patients with acute coronary syndrome. Eur. J. Clin. Investig. 2009, 39, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.; Emdin, M.; Passino, C.; Michelassi, C.; Battaglia, D.; Cocci, F. Predictive value of elevated neutrophil-lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin. Chim. Acta. 2008, 395, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Cook, E.J.; Walsh, S.R.; Farooq, N.; Alberts, J.C.; Justin, T.A.; Keeling, N.J. Post-operative neutrophil-lymphocyte ratio predicts complications following colorectal surgery. Intern. J. Surg. 2007, 5, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Karabinos, I.; Koulouris, S.; Kranidis, A.; Pastromas, S.; Exadaktylos, N.; Kalofoutis, A. Neutrophil count on admission predicts major in-hospital events in patients with a non-st-segment elevation acute coronary syndrome. Clin. Cardiol. 2009, 32, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Buck, B.H.; Liebeskind, D.S.; Saver, J.L.; Bang, O.Y.; Yun, S.W.; Starkman, S.; Ali, L.K.; Kim, D.; Villablanca, J.P.; Salamon, N.; et al. Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke 2008, 39, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Elkind, M.S.; Sciacca, R.R.; Boden-Albala, B.; Rundek, T.; Paik, M.C.; Sacco, R.L. Relative elevation in baseline leukocyte count predicts first cerebral infarction. Neurology 2005, 64, 2121–2125. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A. Neurofilament phosphoforms: Surrogate markers for axonal injury, degeneration and loss. J. Neurol. Sci. 2005, 233, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Meaney, D.F.; Xu, B.N.; Nonaka, M.; McIntosh, T.K.; Wolf, J.A.; Saatman, K.E.; Smith, D.H. Evolution of neurofilament subtype accumulation in axons following diffuse brain injury in the pig. J. Neuropathol. Experim. Neurol. 1999, 58, 588–596. [Google Scholar] [CrossRef]
- Jafari, S.S.; Maxwell, W.L.; Neilson, M.; Graham, D.I. Axonal cytoskeletal changes after non-disruptive axonal injury. J. Neurocytol. 1997, 26, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Ost, M.; Nylen, K.; Csajbok, L.; Ohrfelt, A.O.; Tullberg, M.; Wikkelso, C.; Nellgard, P.; Rosengren, L.; Blennow, K.; Nellgard, B. Initial csf total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology 2006, 67, 1600–1604. [Google Scholar] [CrossRef] [PubMed]
- Folkerts, M.M.; Berman, R.F.; Muizelaar, J.P.; Rafols, J.A. Disruption of map-2 immunostaining in rat hippocampus after traumatic brain injury. J. Neurotrauma 1998, 15, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Zemlan, F.P.; Jauch, E.C.; Mulchahey, J.J.; Gabbita, S.P.; Rosenberg, W.S.; Speciale, S.G.; Zuccarello, M. C-tau biomarker of neuronal damage in severe brain injured patients: Association with elevated intracranial pressure and clinical outcome. Brain Res. 2002, 947, 131–139. [Google Scholar] [CrossRef]
- Zemlan, F.P.; Mulchahey, J.J.; Gudelsky, G.A. Quantification and localization of kainic acid-induced neurotoxicity employing a new biomarker of cell death: Cleaved microtubule-associated protein-tau (c-tau). Neuroscience 2003, 121, 399–409. [Google Scholar] [CrossRef]
- Olmsted, J.B. Microtubule-associated proteins. Ann. Rev. Cell Biol. 1986, 2, 421–457. [Google Scholar] [CrossRef] [PubMed]
- Matus, A. Neurofilament protein phosphorylation--where, when and why. Trends Neurosci. 1988, 11, 291–292. [Google Scholar] [CrossRef]
- Garner, C.C.; Tucker, R.P.; Matus, A. Selective localization of messenger RNA for cytoskeletal protein MAP2 in dendrites. Nature 1988, 336, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Kobeissy, F.H.; Sadasivan, S.; Oli, M.W.; Robinson, G.; Larner, S.F.; Zhang, Z.; Hayes, R.L.; Wang, K.K. Neuroproteomics and systems biology-based discovery of protein biomarkers for traumatic brain injury and clinical validation. Proteomics. Clin. Appl. 2008, 2, 1467–1483. [Google Scholar] [CrossRef] [PubMed]
- Buki, A.; Siman, R.; Trojanowski, J.Q.; Povlishock, J.T. The role of calpain-mediated spectrin proteolysis in traumatically induced axonal injury. J. Neuropathol. Experim. Neurol. 1999, 58, 365–375. [Google Scholar] [CrossRef]
- Reeves, T.M.; Greer, J.E.; Vanderveer, A.S.; Phillips, L.L. Proteolysis of submembrane cytoskeletal proteins ankyrin-g and alphaii-spectrin following diffuse brain injury: A role in white matter vulnerability at nodes of ranvier. Brain Pathol. 2010, 20, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.K.; Posmantur, R.; Nath, R.; McGinnis, K.; Whitton, M.; Talanian, R.V.; Glantz, S.B.; Morrow, J.S. Simultaneous degradation of alphaii- and betaii-spectrin by caspase 3 (cpp32) in apoptotic cells. J. Biol. Chem. 1998, 273, 22490–22497. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.D.; West, E.J.; Liu, M.C.; Wang, K.K.; Hayes, R.L.; Lyeth, B.G. Dicyclomine, an m1 muscarinic antagonist, reduces biomarker levels, but not neuronal degeneration, in fluid percussion brain injury. J. Neurotrauma 2008, 25, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Adam, S.S.; Key, N.S.; Greenberg, C.S. D-dimer antigen: Current concepts and future prospects. Blood 2009, 113, 2878–2887. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.; Xia, J.; Wu, Y.; Ke, J.; Li, J.; Pan, J.; Chen, W.; Shao, Y.; Yang, Z.; Luo, S.; et al. Clinical presentation and imaging characteristics of occult lung cancer associated ischemic stroke. J. Clin. Neurosci. 2015, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, J.H. Risk factors and biomarkers of ischemic stroke in cancer patients. J. Stroke 2014, 16, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.B.; Li, M.; Zhuo, W.Y.; Zhang, Y.S.; Xu, A.D. The role of hs-crp, d-dimer and fibrinogen in differentiating etiological subtypes of ischemic stroke. PLoS. One 2015, 10, e0118301. [Google Scholar] [CrossRef] [PubMed]
- Zi, W.J.; Shuai, J. Plasma d-dimer levels are associated with stroke subtypes and infarction volume in patients with acute ischemic stroke. PLoS One 2014, 9, e86465. [Google Scholar] [CrossRef] [PubMed]
- Zecca, B.; Mandelli, C.; Maino, A.; Casiraghi, C.; Bolla, G.; Consonni, D.; Santalucia, P.; Torgano, G. A bioclinical pattern for the early diagnosis of cardioembolic stroke. Emerg. Med. Intern. 2014, 2014, 242171. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Shi, Z.H. The relationship between plasma d-dimer levels and outcome of chinese acute ischemic stroke patients in different stroke subtypes. J. Neur. Transm. 2014, 121, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, S.; Marlborough, F.; Doubal, F.; Webb, D.J.; Wardlaw, J. Blood markers of coagulation, fibrinolysis, endothelial dysfunction and inflammation in lacunar stroke versus non-lacunar stroke and non-stroke: Systematic review and meta-analysis. Cerebrovasc. Dis. 2014, 37, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Yamamoto, Y.; Yoda, K.; Nagahiro, S. The ratio of d-dimer to brain natriuretic peptide may help to differentiate between cerebral infarction with and without acute aortic dissection. J. Neurol. Sci. 2014, 340, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Isenegger, J.; Meier, N.; Lammle, B.; Alberio, L.; Fischer, U.; Nedeltchev, K.; Gralla, J.; Kohler, H.P.; Mattle, H.P.; Arnold, M. D-dimers predict stroke subtype when assessed early. Cerebrovasc. Dis. 2010, 29, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, D.; Ozbabalik, D.; Gulcan, E.; Ozdemir, O.; Gulbacs, Z. Evaluation of platelet activation, coagulation, and fibrinolytic activation in patients with symptomatic lacunar stroke. Neurologist 2010, 16, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Haapaniemi, E.; Tatlisumak, T. Is d-dimer helpful in evaluating stroke patients? A systematic review. Acta Neurol. Scand. 2009, 119, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Montaner, J.; Perea-Gainza, M.; Delgado, P.; Ribo, M.; Chacon, P.; Rosell, A.; Quintana, M.; Palacios, M.E.; Molina, C.A.; Alvarez-Sabin, J. Etiologic diagnosis of ischemic stroke subtypes with plasma biomarkers. Stroke 2008, 39, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Dougu, N.; Takashima, S.; Sasahara, E.; Taguchi, Y.; Toyoda, S.; Hirai, T.; Nozawa, T.; Tanaka, K.; Inoue, H. Differential diagnosis of cerebral infarction using an algorithm combining atrial fibrillation and d-dimer level. Eur. J. Neurol. 2008, 15, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Squizzato, A.; Ageno, W.; Finazzi, S.; Mera, V.; Romualdi, E.; Bossi, A.; Venco, A. D-dimer is not a long-term prognostic marker following acute cerebral ischemia. Blood Coagul. Fibrinol. 2006, 17, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Squizzato, A.; Ageno, W. D-dimer testing in ischemic stroke and cerebral sinus and venous thrombosis. Semin. Vasc. Med. 2005, 5, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.J.; Horn, M.; Bogdahn, U.; Ickenstein, G.W. The relationship between plasma d-dimer concentrations and acute ischemic stroke subtypes. J. Stroke Cerebrovasc. Dis. 2005, 14, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Ageno, W.; Finazzi, S.; Steidl, L.; Biotti, M.G.; Mera, V.; Melzi D'Eril, G.; Venco, A. Plasma measurement of d-dimer levels for the early diagnosis of ischemic stroke subtypes. Arch. Intern. Med. 2002, 162, 2589–2593. [Google Scholar] [CrossRef]
- Takano, K.; Yamaguchi, T.; Uchida, K. Markers of a hypercoagulable state following acute ischemic stroke. Stroke 1992, 23, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Elkind, M.S.; Tai, W.; Coates, K.; Paik, M.C.; Sacco, R.L. High-sensitivity c-reactive protein, lipoprotein-associated phospholipase a2, and outcome after ischemic stroke. Arch. Intern. Med. 2006, 166, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.W.; Weir, C.J.; Alwan, W.; Squire, I.B.; Lees, K.R. C-reactive protein and outcome after ischemic stroke. Stroke 1999, 30, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Audebert, H.J.; Rott, M.M.; Eck, T.; Haberl, R.L. Systemic inflammatory response depends on initial stroke severity but is attenuated by successful thrombolysis. Stroke 2004, 35, 2128–2133. [Google Scholar] [CrossRef] [PubMed]
- Suwanwela, N.C.; Chutinet, A.; Phanthumchinda, K. Inflammatory markers and conventional atherosclerotic risk factors in acute ischemic stroke: Comparative study between vascular disease subtypes. J. Med. Assoc. Thail. 2006, 89, 2021–2027. [Google Scholar]
- Masotti, L.; Ceccarelli, E.; Forconi, S.; Cappelli, R. Prognostic role of c-reactive protein in very old patients with acute ischaemic stroke. J. Intern. Med. 2005, 258, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Takashima, S.; Dougu, N.; Taguchi, Y.; Nukui, T.; Konishi, H.; Toyoda, S.; Kitajima, I.; Tanaka, K. Study of hemostatic biomarkers in acute ischemic stroke by clinical subtype. J. Stroke Cerebrovasc. Dis. 2012, 21, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Iskra, T.; Turaj, W.; Slowik, A.; Zwolinska, G.; Strojny, J.; Szczudlik, A. [hemostatic markers of endothelial injury in ischaemic stroke caused by large or small vessel disease]. Pol. Merkur. Lekarski. 2006, 21, 429–433. [Google Scholar] [PubMed]
- Bang, O.Y.; Saver, J.L.; Liebeskind, D.S.; Pineda, S.; Ovbiagele, B. Association of serum lipid indices with large artery atherosclerotic stroke. Neurology 2008, 70, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Slowik, A.; Iskra, T.; Turaj, W.; Hartwich, J.; Dembinska-Kiec, A.; Szczudlik, A. Ldl phenotype b and other lipid abnormalities in patients with large vessel disease and small vessel disease. J. Neurol. Sci. 2003, 214, 11–16. [Google Scholar] [CrossRef]
- Iskra, T.; Turaj, W.; Hartwich, J.; Slowik, A.; Pankiewicz, J.; Dembinska-Kiec, A.; Szczudlik, A. [ldl phenotype a and b in ischemic stroke]. Prz. Lekarski 2002, 59, 7–10. [Google Scholar]
- Zimmermann-Ivol, C.G.; Burkhard, P.R.; Le Floch-Rohr, J.; Allard, L.; Hochstrasser, D.F.; Sanchez, J.C. Fatty acid binding protein as a serum marker for the early diagnosis of stroke: A pilot study. MCP. 2004, 3, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.A.; Scott, M.G. Clinical utility of biochemical analysis of cerebrospinal fluid. Clin. Chem. 1995, 41, 343–360. [Google Scholar] [PubMed]
- Sun, G.J.; Ding, S.C.; Ling, W.Y.; Wang, F.; Yang, X.P. Cerebrospinal fluid free fatty acid levels are associated with stroke subtypes and severity in chinese patients with acute ischemic stroke. World Neurosurg. 2015, 84, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Zambrelli, E.; Emanuele, E.; Marcheselli, S.; Montagna, L.; Geroldi, D.; Micieli, G. Apo(a) size in ischemic stroke: Relation with subtype and severity on hospital admission. Neurology 2005, 64, 1366–1370. [Google Scholar] [CrossRef] [PubMed]
- Iskra, T.; Turaj, W.; Slowik, A.; Szczudlik, A.; Dembinska-Kiec, A. [lipoprotein (a) in stroke patients with large and small vessel disease]. Prz. Lekarski 2002, 59, 877–880. [Google Scholar]
- Saidi, S.; Slamia, L.B.; Ammou, S.B.; Mahjoub, T.; Almawi, W.Y. Association of apolipoprotein E gene polymorphism with ischemic stroke involving large-vessel disease and its relation to serum lipid levels. J. Stroke Cerebrovasc. Dis. 2007, 16, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Lampl, Y.; Paniri, Y.; Eshel, Y.; Sarova-Pinhas, I. Cerebrospinal fluid lactate dehydrogenase levels in early stroke and transient ischemic attacks. Stroke 1990, 21, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Perez, F.J.; Castelo-Branco, M.; Alvarez-Sabin, J. Albumin level and stroke. Potential association between lower albumin level and cardioembolic aetiology. Int. J. Neurosci. 2011, 121, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Z.; Yang, Z.G.; Zhang, Y.H.; Zhang, Y.W.; Hong, B.; Liu, J.M. Dynamic reduction of plasma decorin following ischemic stroke: A pilot study. Neurochem. Res. 2012, 37, 1843–1848. [Google Scholar] [CrossRef] [PubMed]
- Drain, P.K.; Hyle, E.P.; Noubary, F.; Freedberg, K.A.; Wilson, D.; Bishai, W.R.; Rodriguez, W.; Bassett, I.V. Diagnostic point-of-care tests in resource-limited settings. Lancet. Infect. Dis. 2014, 14, 239–249. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Ali, M.A.; Anand, P.; Agrawal, V.V.; John, R.; Maji, S.; Malhotra, B.D. Microfluidic-integrated biosensors: Prospects for point-of-care diagnostics. Biotechnol. J. 2013, 8, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Eltzov, E.; Cohen, A.; Marks, R.S. Bioluminescent liquid light guide pad biosensor for indoor air toxicity monitoring. Anal. Chem. 2015, 87, 3655–3661. [Google Scholar] [CrossRef] [PubMed]
- Eltzov, E.; Guttel, S.; Low Yuen Kei, A.; Sinawang, P.D.; Ionescu, R.E.; Marks, R.S. Lateral flow immunoassays – from paper strip to smartphone technology. Electroanalysis 2015, 27, 2116–2130. [Google Scholar] [CrossRef]
- Eltzov, E.; Marks, R.S. Colorimetric stack pad immunoassay for bacterial identification. Biosens. Bioelectron. 2016, 87, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Eltzov, E.; Marks, R.S. Miniaturized flow stacked immunoassay for detecting escherichia coli in a single step. Anal. Chem. 2016, 88, 6441–6449. [Google Scholar] [CrossRef] [PubMed]
- Algaar, F.; Eltzov, E.; Vdovenko, M.M.; Sakharov, I.Y.; Fajs, L.; Weidmann, M.; Mirazimi, A.; Marks, R.S. Fiber-optic immunosensor for detection of crimean-congo hemorrhagic fever igg antibodies in patients. Anal. Chem. 2015, 87, 8394–8398. [Google Scholar] [CrossRef] [PubMed]
- Eltzov, E.; Cosnier, S.; Marks, R.S. Biosensors based on combined optical and electrochemical transduction for molecular diagnostics. Exp. Rev.Molec. Diagn. 2011, 11, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Eltzov, E.; Marks, R.S. Fiber-optic based cell sensors. In Whole Cell Sensing Systems I; Springer: Berlin/Heidelberg, Germany, 2010; pp. 131–154. [Google Scholar]
- Thevenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eltzov, E.; Marks, R.S. Whole-cell aquatic biosensors. Anal. Bioanal. Chem. 2011, 400, 895–913. [Google Scholar] [CrossRef] [PubMed]
- Collings, A.F.; Caruso, F. Biosensors: Recent advances. Rep. Prog. Phys. 1997, 60, 1397–1445. [Google Scholar] [CrossRef]
- Eltzov, E.; Kushmaro, A.; Marks, R.S. Biosensors and related techniques for endocrine disruptors. In Endocrine Disrupting Chemicals in Food, Snow, I., Ed.; Woodhead: Cambridge, UK, 2009. [Google Scholar]
- Marks, R.S.; Cullen, D.C.; Karube, I.; Lowe, C.R.; Weetall, H.H. Handbook of Biosensors and Biochips; Wiley-Blackwell: Tokyo, Japan, 2007. [Google Scholar]
- Chin, C.D.; Linder, V.; Sia, S.K. Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip 2012, 12, 2118–2134. [Google Scholar] [CrossRef] [PubMed]
- Sinawang, P.D.; Rai, V.; Ionescu, R.E.; Marks, R.S. Electrochemical lateral flow immunosensor for detection and quantification of dengue ns1 protein. Biosens. Bioelectron. 2016, 77, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Sinawang, P.D.; Harpaz, D.; Fajs, L.; Seet, R.C.S.; Tok, A.I.Y.; Marks, R.S. Electrochemical impedimetric detection of stroke biomarker nt-probnp using disposable screen-printed gold electrodes. EuroBiotech J. 2017, 5, 165–176. [Google Scholar] [CrossRef]
- Stephenson, D.; Hu, M.T.; Romero, K.; Breen, K.; Burn, D.; Ben-Shlomo, Y.; Bhattaram, A.; Isaac, M.; Venuto, C.; Kubota, K.; et al. Precompetitive data sharing as a catalyst to address unmet needs in parkinson's disease1. J. Parkinsons. Dis. 2015, 5, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Easton, J.F.; Stephens, C.R.; Angelova, M. Risk factors and prediction of very short term versus short/intermediate term post-stroke mortality: A data mining approach. Comput. Biol. Med. 2014, 54, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Tian, Y.; Li, P.F.; Tian, L.L.; Qian, Y.M.; Li, J.S. Design and development of a medical big data processing system based on hadoop. J. Med. Syst. 2015, 39, 23. [Google Scholar] [CrossRef] [PubMed]
- Davidoff, F.; Haynes, B.; Sackett, D.; Smith, R. Evidence based medicine. BMJ 1995, 310, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Llatas, C.; García-Gómez, J.M. (Eds.) Data Mining in Clinical Medicine; Humana Press: New York, NY, USA, 2015. [Google Scholar]


| Technology | Name | Application | Clinical Value | Reference |
|---|---|---|---|---|
| Imaging Technique which uses computerized x-ray imaging | Computed Tomography (CT) | 3D scan of body tissues. Shows evidence of early ischemia and rules out haemorrhage. Can be performed with contrast agent for better visualization | Ischemic stroke diagnosis and admission of thrombolytic therapy | [2,30,31] |
| Multi-detector Computed Tomography (MDCT) | 2D array of detector elements which enables multiple slices simultaneously, and faster image acquisition. High resolution and long range scans | Ischemic stroke diagnosis and admission of thrombolytic therapy | [2] | |
| SPECT Computed Tomography (SPECT-CT) | This technology uses radioisotopes. Shows cross-sectional image of the target organ. The patient either swallows or is injected with a radioisotope, which travels to a target organ. The radioisotope emits radiation, which is detected. Does not reliably distinguish between hemorrhage and infarction | Determine if a specific area of the body is receiving adequate blood flow | [32] | |
| XENON-Contrast Computed Tomography (XENON-CT) | This technology uses the inert gas xenon to measure cerebral blood flow (CBF) in various brain regions. The patient inhales a mixture of xenon and oxygen over a period of a few minutes, allowing measurement of an increase in their density caused by their presence in the brain tissue | Determine local cerebral blood flow in small area | [32] | |
| Positron Emission Tomography (PET) | Measures related changes in cortical function by tracking the chemical changes which occur in tissues. Detect biochemical changes in an organ or tissue that can identify the stroke onset before anatomical changes can be seen with other imaging processes | Guide decision making for brain surgical planning | [33] | |
| Carotid Angiography (CA) | This technology uses dye to show the inside of human carotid arteries. A small tube (catheter) is put into an artery, usually in the groin (upper thigh), then moved upwards into one of the carotid arteries | Identification of extracranial vessel disease | [2] | |
| Imaging Technique which uses magnetic fields and radio waves | Magnetic Resonance Imaging (MRI) | Show the slowing of water movement through the injured brain tissue, which is caused by ischemia and ruling out haemorrhage. Can detect a variety of changes in the brain and blood vessels and visualize blockages in the arteries | Ischemic stroke diagnosis and admission of thrombolytic therapy | [2,34,35,36,37] |
| Magnetic Resonance Arteriogram (MRA) | Permits the visualization of blood flow in vessels and allows rapid characterization of the cervical and cephalic large vessels. Detects and grades cervical internal carotid stenosis with an accuracy of 85% to 96% compared to digital subtraction angiography | Identification of extracranial vessel disease | [34,35,38] | |
| Diffusion-Weighted Imaging (DWI-MRI) | Can render ischemic fields visible within minutes of ischemia onset and detects the effects of stroke on caudal motor pathways in the recovery process. Can also predict lasting motor impairment in stroke recovery | Early visualization of site and extent of ischemia | [35,38,39] | |
| Perfusion-Weighted Imaging (PWI-MRI) | Used to assess cerebral blood flow and blood volume in various brain regions. Usually performed by injecting a contrast agent and then obtaining a rapid series of MRIs using an ultrafast technique | Early visualization of site and extent of ischemia | [35] | |
| Magnetic Resonance Spectroscopy (MRS) | Spectroscopy measurement, enabling measurement of ATP, lactate levels, and pH at discrete locations within the brain. Can distinguish areas that have no viable neurons from areas that may be salvageable | Identify areas in the brain that may be salvageable | [32] | |
| Functional Magnetic Resonance Imaging (f-MRI) | Measurement of brain activity by detecting the changes in blood oxygenation and flow. Can measure differences in cognitive reserve | Monitoring patient recovery | [2,33,40,41,42,43] | |
| Magnetic Resonance Imaging Diffusion Tensor (MRI-DTI) | Provide information on white matter damage. Correlate better with cognition than conventional MRI measures | Monitoring damage progression | [40,44,45,46] | |
| Imaging Technique which uses sound waves | Carotid Ultrasound (CU) | Show the inside of human carotid arteries and detect whether plaque has narrowed or blocked carotid arteries. Can include Doppler to show the speed and direction of blood flow through the blood vessels | Show the condition of the carotid arteries in the neck and/or intracranial vessels | [2] |
| B-Mode Carotid Ultrasound (B-CU) | Provides images of various levels, or planes, enabling the creation of a three-dimensional image of the carotid artery wall and surrounding structures. Provides information on the type and extent of arterial damage, though blood clots sometimes do not appear and the method cannot distinguish a narrowed from a completely occluded artery | Show the condition of the carotid arteries wall and structure | [2] | |
| Duplex-Carotid Ultrasound (D-CU) | Show the human carotid arteries and detect their condition. Combines B-mode imaging and pulsed Doppler ultrasound to provide more detail on the condition of arteries | Show the condition of the carotid arteries | [47] | |
| Transcranial Doppler (TCD) | Probe is placed over areas on the head to detect blood velocity and pressure in certain arteries at various depths in the brain. Allows the assessment of the location and extent of occlusions or atheromatous plaques in extracranial carotid and large intracranial vessels | Show the condition of the carotid arteries and location of occlusions | [48] | |
| Echo-Cardiography (ECHO) | Show images of the human heart, gives information on the size, shape and function of the organ. Can also detect possible blood clots inside the heart, and problems with the aorta | Heart pathologies diagnosis | [2] | |
| Other | Electro-Cardiogram (ECG) | Records the heart’s electrical activity, showing how fast the heart is beating, and its rhythm (steady or irregular). Can detect heart problems that may lead to a stroke and can also record the strength and timing of electrical signals which pass through the heart | Atrial fibrillation or previous heart attack diagnosis | [2] |
| Blood Tests (BT) | Includes: Glucose test, Platelets count and PT/PTT. Low glucose levels can cause symptoms similar to stroke and abnormal platelet levels can be a sign of bleeding/thrombotic disorder. Can also test whether the blood is clotting normally | Bleeding/Thrombotic disorders diagnosis | [2] |
| Description | Stroke Subtypes | Strengths | Weaknesses | Reference |
|---|---|---|---|---|
| Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification | ||||
| Since 1993, the most clinically used method for ischemic stroke subtyping is TOAST, which is mainly based on clinical symptoms. |
| Reliability has been improved by the use of a computerized algorithm | Stroke from undetermined cause is the most heterogeneous group in the TOAST system, as well as in the Stroke Data Bank. Once a patient matches more than one possible cause, he is equally grouped as a patient with a no cause identified or an incomplete investigation. This weakness could flaw the medical decision-making process. | [38,66,67,68,69] |
| National Institute of Neurological Disorders and Stroke (NINDS) Classification | ||||
| Derived from the Harvard Stroke Registry classification. |
| The best option in the search of new causes of stroke amongst patients with no known causes or with another disease not causally related to the stroke event. | Most of the currently used diagnostic tools were not available at that time, such as modern MRI with diffusion-weighted imaging, transesophageal echocardiography, TEE, magnetic resonance angiography, MRA, duplex ultrasound examination, and transcranial Doppler | [70] |
| Oxford Community Stroke Project (OCSP, Bamford/Oxford) classification | ||||
| Relies on the initial symptoms and based on their extent. |
The type of stroke is then coded by adding a final letter to the above:
| Patients are easily classified into groups based on clinical grounds and CT scanning, which are usually done in all stroke patients. The outcome of the stroke event is driven strongly by the severity of the stroke, which is well reflected in this classification, without addressing the cause of the stroke. | The extent and site of the brain infarct is unlikely to be specific to a particular stroke etiology. Patients classified as having a LAC infarct may have a missed cardiac source of embolism. In addition, this classification should no longer be used to investigate potential risk factors or causes of stroke. | [71,72] |
| POCT Device | Description | Application | Clinical Value | Reference |
|---|---|---|---|---|
| Mobile Stroke Unit (MSU) | Imaging and a variety of Blood-tests. Integration of CT scanners and POCTs in ambulances, IV-tPA treatment can be started on-site | Consists of a registered nurse, paramedic, emergency medical technician, and a CT technologist, in addition, POCT are used, which includes coagulation profile, complete blood count, and blood chemistry | Pre-hospital: Improves stroke diagnosis and reduces time-to-IV-tPA admission | [79,86,87,88,89,90,91] |
| CoaguChek® (Roche) | Test strips with electro-chemical detection. Based on amperometric (electrochemical) determination of the PT time after activation of the coagulation with human recombinant thromboplastin, results are obtained within 1 min | Convenient, portable and user-friendly device for monitoring oral anticoagulation therapy which can determine the INR value from a drop of capillary whole blood | Pre/In-hospital: Improves stroke diagnosis and reduces time-to-IV-tPA admission | [91,92,93,94,95] |
| Hemochron® Junior (ITC) | Optical detection. Micro-coagulation system, results within minutes | POCTs monitoring of: (1) ACT-LR, (2) ACT, (3) PT, (4) Citrate PT, (5) APTT and (6) Citrate APTT | Pre/In-hospital: Improves stroke diagnosis and reduces time-to-IV-tPA admission | [96] |
| PocH-100i hematology analyzer (Sysmex) | Micro-fluidics. WBCs, RBCs and PLTs are counted using the direct current detection method with hydrodynamic focusing technology. Hemoglobin analysis is conducted using a non-cyanide method | Provides a full blood count and a 3-part differential leukocyte count | Pre/In-hospital: Improves stroke diagnosis and reduces time-to-IV-tPA admission | [91,96,97] |
| i-STAT (Abbott) | Micro-fluidics. Based on advanced microfluidic and deliver fast, reliable lab accurate results within 2 min | Bedside care tests such as blood gases, electrolytes, metabolites and coagulation | Pre/In-hospital: Improves stroke diagnosis and reduces time-to-IV-tPA admission | [91,98,99,100] |
| Reflotron® plus analyzer (Roche, Cobas series) | Test strips with optical detection (Reflectance photometry). Single-test clinical chemistry system which is able to measure whole blood, plasma or serum for: liver and pancreas enzymes, metabolites and blood lipids. Results within 2–3 mins | Used for blood clinical-chemistry parameters measurement, such as c-glutamyltransferase, p-amylase, and glucose | Pre/In-hospital: Improves stroke diagnosis and reduces time-to-IV-tPA admission | [96] |
| Abbott AxSYM® BNP/Alere Triage® BNP/i-STAT BNP | Optical Detection (AxSYM)/Fluorescent detection (Triage)/Electro-chemical sensor on a silicon chip (i-STAT). BNP POCT. | BNP elevated serum levels in stroke patients show (1) correlation with CEI stroke, (2) increased mortality and (3) indication on second stroke recurrence | In/Post-hospital: Improves stroke prognostic (correlation to CEI) and stroke recovery (indication on second stroke reoccurrence) | [88,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126] |
| Cornell University, State University of New York and the New York Presbyterian Hospital | Luminescent detection. NSE POCT based on enzymes tethered to nanoparticles | NSE elevated serum levels in stroke patients assist in distinguishing stroke from mimics, an important first step in expediting the diagnostic process | In-hospital: Improves stroke diagnosis and reduces time-to-IV-tPA admission | [127,128,129,130,131,132,133] |
| Prediction Sciences LLC | Lateral flow POCT for the proteomic marker cellular fibronectin (c-Fn) | Fibronectin (c-Fn) elevated serum levels in stroke patients at IV-tPA admission can identify if the patient is at high or low risk for a subsequent hemorrhage | In-hospital: Reduces time-to-IV-tPA admission | [124] |
| ReST™ (Valtari Bio™ Inc.) | Rapid evaluation stroke triage POCT for the measurement of blood brain-specific biomarkers associated with immune responses, results within 10 min | Following stroke, the immune system is activated. The degree and direction of the immune system activation allow the accurate identification of acute stroke from non-stroke | In-hospital: Initial stroke versus no stroke diagnosis | [134,135,136] |
| SMARTChip (Sarissa Biomedical) | Micro-electrode POCT device for stroke diagnosis that measures purines from a drop of whole blood and give the reading within minutes | Can be used by paramedics, which will allow faster identification of stoke victims at the point of injury | In-hospital: Stroke diagnosis | [137] |
| PFA-100®, Platelet Function Analyzer (Dade Behring) | High-shear force dynamic flow system POCT that assesses platelet aggregation under high shear, mimicking platelet-rich thrombus formation after injury to a small vessel wall under flow conditions | Rapid and reliable identification of aspirin non-responsive patients, without the requirement of a specialized laboratory | Post-hospital: Prevention of second stroke recurrence | [138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155] |
| Ultegra-RPFA VerifyNow Aspirin® test (Accumetrics) | Optical detection. POCT based on turbidimetric optical detection of platelet aggregation in whole blood. As aggregation occurs, the system converts luminosity transmittance results into ‘Aspirin Reaction Units’ | Rapid and reliable identification of aspirin non-responsive patients, without the requirement of a specialized laboratory | Post-hospital: Prevention of second stroke recurrence | [138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154] |
| Family | Biomarker | Expression Association | Stroke Clinical Value | Reference |
|---|---|---|---|---|
| Glial cells origin | S100-Beta | Associated with blood–brain barrier (BBB) dysfunction | Diagnosis | [157,158,161,162,163,164,165] |
| Glial Fibrillary Acidic Protein (GFAP) | Associated with the size of brain lesions, the neurological status and short-term functional outcome | Prognosis: outcome prediction | [157,161,162,166,167,168,169,170,171] | |
| Myelin Basic Protein (MBP) | Associated with worsened outcome | Prognosis: outcome prediction | [172,173,174,175] | |
| Neuronal cells origin | Neuron-Specific Enolase (NSE) | Associated with neurological outcomes and infarct volume | Prognosis: outcome prediction | [129,157,158,161,162,163,166,176,177] |
| Ubiquitin Carboxyl-terminal Hydrolase L1 (UCH-L1) | Associated with the extent of the neuronal injury | Prognosis: outcome prediction | [157,158,178,179,180,181] | |
| Creatinine Kinase-BB (CKBB) | Associated with the extent and severity of the brain damage and the recovery potential | Prognosis and recovery | [182,183] | |
| Heart muscle cells (cardio-myocytes) origin | B-Type Natriuretic Peptide (BNP) | Associated with CEI ischemic stroke, increased mortality and second stroke indication | Prognosis: subtype classification and recovery: second stroke prevention | [87,100,101,102,103,104,105,106,107,108,110,111,112,113,114,115,116,117,118,119,120,121,122,124,156,184,185,186] |
| Blood vessels cells (myocytes) origin | Matrix Metallo-Proteinase 9 (MMP-9) | Associated with blood–brain barrier (BBB) dysfunction and second stroke indication | Diagnosis and recovery: second stroke prevention | [187,188,189,190,191] |
| General inflammatory cytokines and proteins | Interleukin-6 (IL-6), interleukin-1b (IL-1b), tumor necrosis factor-α (TNF-α) | Associated with CEI ischemic stroke subtype | Prognosis: subtype classification | [166,192,193,194,195,196,197,198,199] |
| Neutrophil Lymphocyte Ratios (NLR) | Associated with increased mortality and LAA ischemic stroke subtype | Prognosis: subtype classification and recovery | [199,200,201,202,203,204,205,206] | |
| Cytoskeleton proteins | Neurofilaments (NF) | Associated with disconnected axons | Prognosis | [207,208,209] |
| Cleaved-tau (C-tau) | Associated with neuronal degeneration and disease progression | Prognosis | [157,158,172,210,211,212,213] | |
| Microtubule-associated protein 2 (MAP2) | Associated with dendritic injury | Prognosis | [214,215,216,217] | |
| Alpha-II spectrin break-down products (SBDPs) | Associated with apoptosis and necrotic neuronal death | Prognosis | [157,218,219,220,221] | |
| Hemostatic proteins | D-dimer (DD) | Associated with CEI ischemic stroke subtype and recurrence strokes | Prognosis: subtype classification | [11,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240] |
| C-reactive protein (CRP) | Associated with AIS diagnosis, stroke severity and LAA ischemic stroke subtype | Prognosis: subtype classification | [11,241,242,243,244,245] | |
| Fibrin monomer complex (FMC) | Associated with stroke early recognition and CEI ischemic stroke subtype | Diagnosis and prognosis: subtype classification | [246] | |
| Soluble fibrin (SF) | Associated with stroke early recognition and CEI ischemic stroke subtype | Diagnosis and prognosis: subtype classification | [246] | |
| Fibrinogen | Associated with stroke early recognition | Diagnosis | [246,247] | |
| Fibrin/fibrinogen degradation products (FDPs) | Associated with stroke early recognition | Diagnosis | [246] | |
| Von willebrand factor (vWF) | Associated with stroke early recognition and LAC ischemic stroke subtype | Diagnosis and prognosis: subtype classification | [247] | |
| Lipids origin | Triglycerides | Associated with LAA ischemic stroke subtype | Prognosis: subtype classification | [248,249,250] |
| Low density lipoprotein (LDL)/High density lipoprotein (HDL) | Associated with LAA ischemic stroke subtype | Prognosis: subtype classification | [248,249,250] | |
| Heart fatty acid binding protein (H-FABP) | Associated with early diagnosis of stroke | Diagnosis | [251,252] | |
| Free fatty acid (FFA) | Associated with CEI ischemic stroke subtype | Prognosis: subtype classification | [253] | |
| ApoA | Associated with LAA ischemic stroke subtype, and stroke severity | Prognosis: subtype classification and outcome prediction | [254,255] | |
| ApoE4 | Associated with ischemic stroke diagnosis (vs. hemorrhagic stroke) and with LAC and LAA ischemic stroke subtypes | Diagnosis and prognosis: subtype classification | [249,256] | |
| Metabolic proteins | lactate dehydrogenase (LD) | Associated with the extent and severity of the brain damage and the recovery potential | Prognosis and recovery | [182,257] |
| Albumin | Associated with CEI ischemic stroke subtype | Prognosis: subtype classification | [258] | |
| Others | Decorin | Associated with LAA ischemic stroke subtype | Prognosis: subtype classification | [259] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harpaz, D.; Eltzov, E.; Seet, R.C.S.; Marks, R.S.; Tok, A.I.Y. Point-of-Care-Testing in Acute Stroke Management: An Unmet Need Ripe for Technological Harvest. Biosensors 2017, 7, 30. https://doi.org/10.3390/bios7030030
Harpaz D, Eltzov E, Seet RCS, Marks RS, Tok AIY. Point-of-Care-Testing in Acute Stroke Management: An Unmet Need Ripe for Technological Harvest. Biosensors. 2017; 7(3):30. https://doi.org/10.3390/bios7030030
Chicago/Turabian StyleHarpaz, Dorin, Evgeni Eltzov, Raymond C.S. Seet, Robert S. Marks, and Alfred I.Y. Tok. 2017. "Point-of-Care-Testing in Acute Stroke Management: An Unmet Need Ripe for Technological Harvest" Biosensors 7, no. 3: 30. https://doi.org/10.3390/bios7030030
APA StyleHarpaz, D., Eltzov, E., Seet, R. C. S., Marks, R. S., & Tok, A. I. Y. (2017). Point-of-Care-Testing in Acute Stroke Management: An Unmet Need Ripe for Technological Harvest. Biosensors, 7(3), 30. https://doi.org/10.3390/bios7030030






