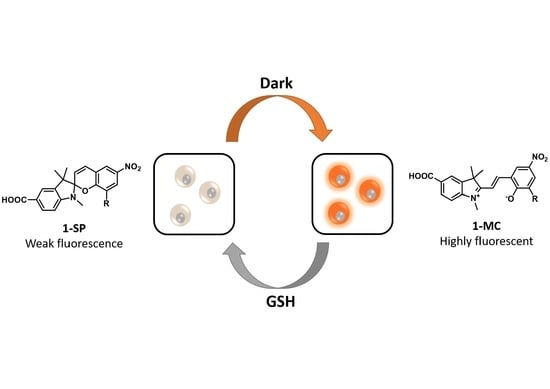
A Rationally Designed Reversible ‘Turn-Off’ Sensor for Glutathione
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Chemical Syntheses
2.2.1. 3,3′-(((2S,5R)-2,5-Dimethylpiperazine-1,4-diyl)bis(methylene))bis(2-hydroxy-5-nitrobenzaldehyde) (4a)
2.2.2. 3,3′-(((2S,5R)-2,5-Dimethylpiperazine-1,4-diyl)bis(methylene))bis(2-hydroxy-5-methylbenzaldehyde) (4b)
2.2.3. 8,8′′-(((2R,5S)-2,5-Dimethylpiperazine-1,4-diyl)bis(methylene))bis(1′,3′,3′-trimethyl-6-nitrospiro[chromene-2,2′-indoline]-5′-carboxylic acid) (1)
2.2.4. 8,8′′-(((2R,5S)-2,5-Dimethylpiperazine-1,4-diyl)bis(methylene))bis(1′,3′,3′,6-tetramethylspiro[chromene-2,2′-indoline]-5′-carboxylic acid) (6)
2.3. Spectroscopic Analyses of Sensors 1 and 6
2.3.1. Absorbance
2.3.2. In-Solution Photoswitching of Sensor 1 in the Presence and Absence of GSH
2.3.3. Determining the Rate Constant and Half-Life of Sensor 1 upon Reaction with GSH
2.3.4. Fluorescence of Sensor 1 and 6 with Increasing Concentrations of GSH
2.3.5. Quantum Yield Calculation
2.3.6. Selectivity of Sensor 1
2.4. Cell-Based Experiments
2.4.1. Cell Viability Assay
2.4.2. Confocal Cell Imaging
3. Results and Discussion
3.1. Design and Synthesis of Sensor 1
3.2. Spectroscopic Analysis of Sensor 1
3.3. Sensing of Intracellular GSH Using Sensor 1
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E. Glutathione: An overview of biosynthesis and modulation. Chem. Biol. Interact. 1998, 111–112, 1–14. [Google Scholar] [CrossRef]
- Hansen, J.M.; Harris, C. Glutathione during embryonic development. Biochim. Biophys. Acta 2015, 1850, 1527–1542. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.V.; Jones, D.P.; Guenthner, T.M.; Lash, L.H.; Lauterburg, B.H. Compartmentation of glutathione: Implications for the study of toxicity and disease. Toxicol. Appl. Pharmacol. 1996, 140, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Filomeni, G.; Rotilio, G.; Ciriolo, M.R. Cell signalling and the glutathione redox system. Biochem. Pharmacol. 2002, 64, 1057–1064. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Glutathione and modulation of cell apoptosis. Biochim. Biophys. Acta 2012, 1823, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Tipple, T.E.; Rogers, L.K. Methods for the determination of plasma or tissue glutathione levels. Methods Mol. Biol. 2012, 889, 315–324. [Google Scholar] [PubMed]
- Tsikas, D.; Hanff, E.; Kayacelebi, A.A.; Böhmer, A. Gas chromatographic-mass spectrometric analysis of the tripeptide glutathione in the electron-capture negative-ion chemical ionization mode. Amino Acids 2016, 48, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Harfield, J.C.; Batchelor-McAuley, C.; Compton, R.G. Electrochemical determination of glutathione: A review. Analyst 2012, 137, 2285–2296. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shu, H.; Zhou, B.; Geng, Y.; Bao, X.; Zhu, J. Design and synthesis of a new rhodamine b-based fluorescent probe for selective detection of glutathione and its application for live cell imaging. Sens. Actuators B Chem. 2016, 237, 431–442. [Google Scholar] [CrossRef]
- Miao, Q.; Li, Q.; Yuan, Q.; Li, L.; Hai, Z.; Liu, S.; Liang, G. Discriminative fluorescence sensing of biothiols in vitro and in living cells. Anal. Chem. 2015, 87, 3460–3466. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, K.; Yoshida, M.; Kamiya, M.; Yamasoba, T.; Urano, Y. Rational design of reversible fluorescent probes for live-cell imaging and quantification of fast glutathione dynamics. Nat. Chem. 2017, 9, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Checa, J.; Kaplowitz, N. The use of monochlorobimane to determine hepatic gsh levels and synthesis. Anal. Biochem. 1990, 190, 212–219. [Google Scholar] [CrossRef]
- Dai, X.; Du, Z.-F.; Wang, L.-H.; Miao, J.-Y.; Zhao, B.-X. A quick response fluorescent probe based on coumarin and quinone for glutathione and its application in living cells. Anal. Chim. Acta 2016, 922, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Peng, H.; Wang, B. Recent advances in thiol and sulfide reactive probes. J. Cell. Biochem. 2014, 115, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Y.; Peng, X.; Yoon, J. Fluorescent and colorimetric probes for detection of thiols. Chem. Soc. Rev. 2010, 39, 2120–2135. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids 2012, 2012, 26. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.L.; Teismann, P. Glutathione—A review on its role and significance in Parkinson’s disease. FASEB J. 2009, 23, 3263–3272. [Google Scholar] [CrossRef] [PubMed]
- Mischley, L.K.; Conley, K.E.; Shankland, E.G.; Kavanagh, T.J.; Rosenfeld, M.E.; Duda, J.E.; White, C.C.; Wilbur, T.K.; De La Torre, P.U.; Padowski, J.M. Central nervous system uptake of intranasal glutathione in Parkinson’s disease. NPJ Park. Dis. 2016, 2, 16002. [Google Scholar] [CrossRef] [PubMed]
- Balendiran, G.K.; Dabur, R.; Fraser, D. The role of glutathione in cancer. Cell Biochem. Funct. 2004, 22, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Estrela, J.M.; Ortega, A.; Obrador, E. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 2006, 43, 143–181. [Google Scholar] [CrossRef] [PubMed]
- Damy, T.; Kirsch, M.; Khouzami, L.; Caramelle, P.; Le Corvoisier, P.; Roudot-Thoraval, F.; Dubois-Randé, J.-L.; Hittinger, L.; Pavoine, C.; Pecker, F. Glutathione deficiency in cardiac patients is related to the functional status and structural cardiac abnormalities. PLoS ONE 2009, 4, e4871. [Google Scholar] [CrossRef] [PubMed]
- Saharan, S.; Mandal, P.K. The emerging role of glutathione in alzheimer’s disease. J. Alzheimers Dis. 2014, 40, 519–529. [Google Scholar] [PubMed]
- Ballatori, N.; Krance, S.M.; Notenboom, S.; Shi, S.; Tieu, K.; Hammond, C.L. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 2009, 390, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.; Jin, J.; Wang, H.; Zheng, J.; Yang, R.; Chan, W.; Abliz, Z. Design of bis-spiropyran ligands as dipolar molecule receptors and application to in vivo glutathione fluorescent probes. J. Am. Chem. Soc. 2010, 132, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Sunamoto, J.; Iwamoto, K.; Mohri, Y.; Kominato, T. Liposomal membranes. 13. Transport of an amino acid across liposomal bilayers as mediated by a photoresponsive carrier. J. Am. Chem. Soc. 1982, 104, 5502–5504. [Google Scholar] [CrossRef]
- Heng, S.; McDevitt, C.A.; Kostecki, R.; Morey, J.R.; Eijkelkamp, B.A.; Ebendorff-Heidepriem, H.; Monro, T.M.; Abell, A.D. Microstructured optical fiber-based biosensors: Reversible and nanoliter-scale measurement of zinc ions. ACS Appl. Mater. Interfaces 2016, 8, 12727–12732. [Google Scholar] [CrossRef] [PubMed]
- Heng, S.; Nguyen, M.-C.; Kostecki, R.; Monro, T.M.; Abell, A.D. Nanoliter-scale, regenerable ion sensor: Sensing with a surface functionalized microstructured optical fibre. RSC Adv. 2013, 3, 8308–8317. [Google Scholar] [CrossRef]
- Baldrighi, M.; Locatelli, G.; Desper, J.; Aakeröy, C.B.; Giordani, S. Probing metal ion complexation of ligands with multiple metal binding sites: The case of spiropyrans. Chem. Eur. J. 2016, 22, 13976–13984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Heng, S.; Abell, A.D. Photoregulation of α-chymotrypsin activity by spiropyran-based inhibitors in solution and attached to an optical fiber. Chem. Eur. J. 2015, 21, 10703–10713. [Google Scholar] [CrossRef] [PubMed]
- LeBel, R.G.; Goring, D.A.I. Density, viscosity, refractive index, and hygroscopicity of mixtures of water and dimethyl sulfoxide. J. Chem. Eng. Data 1962, 7, 100–101. [Google Scholar] [CrossRef]
- Magde, D.; Rojas, G.E.; Seybold, P.G. Solvent dependence of the fluorescence lifetimes of xanthene dyes. Photochem. Photobiol. 1999, 70, 737–744. [Google Scholar] [CrossRef]
- Pei, J.V.; Kourghi, M.; De Ieso, M.L.; Campbell, E.M.; Dorward, H.S.; Hardingham, J.E.; Yool, A.J. Differential inhibition of water and ion channel activities of mammalian aquaporin-1 by two structurally related bacopaside compounds derived from the medicinal plant Bacopa monnieri. Mol. Pharmacol. 2016, 90, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Kohl-Landgraf, J.; Braun, M.; Özçoban, C.; Gonçalves, D.P.N.; Heckel, A.; Wachtveitl, J. Ultrafast dynamics of a spiropyran in water. J. Am. Chem. Soc. 2012, 134, 14070–14077. [Google Scholar] [CrossRef] [PubMed]
- Timm, M.; Saaby, L.; Moesby, L.; Hansen, E.W. Considerations regarding use of solvents in in vitro cell based assays. Cytotechnology 2013, 65, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, X.; Miao, Y.; Hu, Y.; Kwon, N.; Wu, X.; Yoon, J. A reversible fluorescent probe for real-time quantitative monitoring of cellular glutathione. Angew. Chem. Int. Ed. 2017, 56, 5812–5816. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, X.; Carroll, S.L.; Huang, J.; Wang, J. Theoretical and experimental investigation of thermodynamics and kinetics of thiol-michael addition reactions: A case study of reversible fluorescent probes for glutathione imaging in single cells. Org. Lett. 2015, 17, 5978–5981. [Google Scholar] [CrossRef] [PubMed]
- Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 2013, 43, 148–184. [Google Scholar] [CrossRef] [PubMed]
- Sumiya, S.; Doi, T.; Shiraishi, Y.; Hirai, T. Colorimetric sensing of cyanide anion in aqueous media with a fluorescein-spiropyran conjugate. Tetrahedron 2012, 68, 690–696. [Google Scholar] [CrossRef]
- Perrin, D.D. Dissociation Constants of Organic Bases in Aqueous Solution; Butterworths: London, UK, 1965. [Google Scholar]
- Tang, S.-S.; Chang, G.-G. Kinetic characterization of the endogenous glutathione transferase activity of octopus lens s-crystallin. J. Biochem. 1996, 119, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, A.A.; Dmitrenko, V.V. HEK293 in cell biology and cancer research: Phenotype, karyotype, tumorigenicity, and stress-induced genome-phenotype evolution. Gene 2015, 569, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Boone, M.; Meuris, L.; Lemmens, I.; Van Roy, N.; Soete, A.; Reumers, J.; Moisse, M.; Plaisance, S.; Drmanac, R.; et al. Genome dynamics of the human embryonic kidney 293 lineage in response to cell biology manipulations. Nat. Commun. 2014, 5, 4767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz-Alfayate, G.; Obeso, A.; Agapito, M.T.; González, C. Reduced to oxidized glutathione ratios and oxygen sensing in calf and rabbit carotid body chemoreceptor cells. J. Physiol. 2001, 537, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K.D.; Simon, M.C.; Keith, B. Hypoxic reduction in cellular glutathione levels requires mitochondrial reactive oxygen species. J. Appl. Physiol. 2004, 97, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.; Sobotta, M.C.; Dick, T.P. Measuring EGSH and H2O2 with roGFP2-based redox probes. Free Radic. Biol. Med. 2011, 51, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Movia, D.; Prina-Mello, A.; Volkov, Y.; Giordani, S. Determination of spiropyran cytotoxicity by high content screening and analysis for safe application in bionanosensing. Chem. Res. Toxicol. 2010, 23, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Sutton-McDowall, M.L.; Wang, X.; Sugimura, S.; Thompson, J.G.; Gilchrist, R.B. Extending prematuration with camp modulators enhances the cumulus contribution to oocyte antioxidant defence and oocyte quality via gap junctions. Hum. Reprod. 2016, 31, 810–821. [Google Scholar] [CrossRef] [PubMed]









© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heng, S.; Zhang, X.; Pei, J.; Abell, A.D. A Rationally Designed Reversible ‘Turn-Off’ Sensor for Glutathione. Biosensors 2017, 7, 36. https://doi.org/10.3390/bios7030036
Heng S, Zhang X, Pei J, Abell AD. A Rationally Designed Reversible ‘Turn-Off’ Sensor for Glutathione. Biosensors. 2017; 7(3):36. https://doi.org/10.3390/bios7030036
Chicago/Turabian StyleHeng, Sabrina, Xiaozhou Zhang, Jinxin Pei, and Andrew D. Abell. 2017. "A Rationally Designed Reversible ‘Turn-Off’ Sensor for Glutathione" Biosensors 7, no. 3: 36. https://doi.org/10.3390/bios7030036
APA StyleHeng, S., Zhang, X., Pei, J., & Abell, A. D. (2017). A Rationally Designed Reversible ‘Turn-Off’ Sensor for Glutathione. Biosensors, 7(3), 36. https://doi.org/10.3390/bios7030036