Self-Organized Nanostructure Modified Microelectrode for Sensitive Electrochemical Glutamate Detection in Stem Cells-Derived Brain Organoids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Biosensor Design and Fabrication
2.4. Enzyme Immobilization on Microelectrodes
2.5. Cell Culture
2.6. Immunoassay Analysis
2.7. HPLC Analysis
3. Results and Discussion
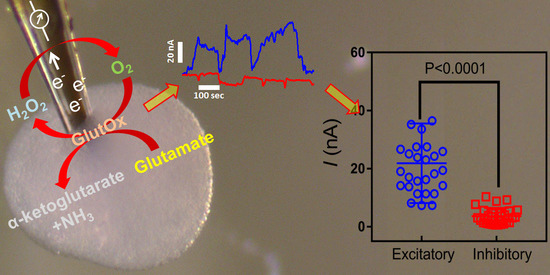
3.1. Principle of the Amperometric Glutamate Biosensor
3.2. Glutamate Biosensor Characterisation, Sensitivity and Selectivity Analysis
3.3. Measurement of Glutamate within hESC-Derived Organoids
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hu, Y.; Mitchell, K.M.; Albahadily, F.N.; Michaelis, E.K.; Wilson, G.S. Direct measurement of glutamate release in the brain using a dual enzyme-based electrochemical sensor. Brain Res. 1994, 659, 117–125. [Google Scholar] [CrossRef]
- Camacho, A.; Massieu, L. Role of glutamate transporters in the clearance and release of glutamate during ischemia and its relation to neuronal death. Arch. Med. Res. 2006, 37, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Ferrarese, C.; Zoia, C.; Pecora, N.; Piolti, R.; Frigo, M.; Bianchi, G.; Sala, G.; Begni, B.; Riva, R.; Frattola, L. Reduced platelet glutamate uptake in Parkinson’s disease. J. Neural Transm. 1999, 106, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Niswender, C.M.; Jones, C.K.; Lin, X.; Bubser, M.; Thompson Gray, A.; Blobaum, A.L.; Engers, D.W.; Rodriguez, A.L.; Loch, M.T.; Daniels, J.S.; et al. Development and Antiparkinsonian Activity of VU0418506, a Selective Positive Allosteric Modulator of Metabotropic Glutamate Receptor 4 Homomers without Activity at mGlu2/4 Heteromers. ACS Chem. Neurosci. 2016, 7, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Hol, E.M.; Roelofs, R.F.; Moraal, E.; Sonnemans, M.A.F.; Sluijs, J.A.; Proper, E.A.; de Graan, P.N.E.; Fischer, D.F.; van Leeuwen, F.W. Neuronal expression of GFAP in patients with Alzheimer pathology and identification of novel GFAP splice forms. Mol. Psychiatry 2003, 8, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Walton, H.S.; Dodd, P.R. Glutamate—Glutamine cycling in Alzheimer’s disease. Neurochem. Int. 2007, 50, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Babb, T.L.; Ying, Z.; Hadam, J.; Penrod, C. Glutamate receptor mechanisms in human epileptic dysplastic cortex. Epilepsy Res. 1998, 32, 24–33. [Google Scholar] [CrossRef]
- Coulter, D.A.; Eid, T. Astrocytic regulation of glutamate homeostasis in epilepsy. Glia 2012, 60, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Maragakis, N.J.; Rothstein, J.D. Glutamate transporters: Animal models to neurologic disease. Neurobiol. Dis. 2004, 15, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Hires, S.A.; Zhu, Y.; Tsien, R.Y. Optical measurement of synaptic glutamate spillover and reuptake by linker optimized glutamate-sensitive fluorescent reporters. Proc. Natl. Acad. Sci. USA 2008, 105, 4411–4416. [Google Scholar] [CrossRef] [PubMed]
- Cordek, J.; Wang, X.; Tan, W. Direct immobilization of glutamate dehydrogenase on optical fiber probes for ultrasensitive glutamate detection. Anal. Chem. 1999, 71, 1529–1533. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Parpura, V. History of Electrophysiology and the Patch Clamp; Springer: Berlin, Germany, 2014; Volume 1183, ISBN 9781493910953. [Google Scholar]
- Van der Zeyden, M.; Oldenziel, W.H.; Rea, K.; Cremers, T.I.; Westerink, B.H. Microdialysis of GABA and glutamate: Analysis, interpretation and comparison with microsensors. Pharmacol. Biochem. Behav. 2008, 90, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Akagi, Y.; Hashigasako, A.; Degenaar, P.; Iwabuchi, S.; Hasan, Q.; Morita, Y.; Tamiya, E. Enzyme-Linked Sensitive Fluorometric Imaging of Glutamate Release from Cerebral Neurons of Chick Embryos. J. Biochem. 2003, 134, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Kang, J.; Jaiswal, J.K.; Simon, S.M.; Lin, J.H.-C.; Yu, Y.; Li, Y.; Yang, J.; Dienel, G.; Zielke, H.R.; et al. Receptor-mediated glutamate release from volume sensitive channels in astrocytes. Proc. Natl. Acad. Sci. USA 2005, 102, 16466–16471. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Stein, K. Flow injection methods for determination of l-glutamate using glutamate decarboxylase and glutamate dehydrogenase reactors with spectrophotometric detection. Analyst 1996, 121, 1305–1309. [Google Scholar] [CrossRef]
- Khampha, W.; Meevootisom, V.; Wiyakrutta, S. Spectrophotometric enzymatic cycling method using l-glutamate dehydrogenase and d-phenylglycine aminotransferase for determination of l-glutamate in foods. Anal. Chim. Acta 2004, 520, 133–139. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Nayuk, P.; Xia, C.; Alshareef, H.N. Novel amperometric glucose biosensor based on MXene nanocomposite. Sci. Rep. 2016, 6, 36422. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, Z.; Wu, C.; Xu, P.; Wang, X. Amperometric inhibitive biosensor based on horseradish peroxidase-nanoporous gold for sulfide determination. Sci. Rep. 2016, 6, 30905. [Google Scholar] [CrossRef] [PubMed]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured metal oxide-based biosensors. NPG Asia Mater. 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Burmeister, J.J.; Davis, V.A.; Quintero, J.E.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A. Glutaraldehyde cross-linked glutamate oxidase coated microelectrode arrays: Selectivity and resting levels of glutamate in the CNS. ACS Chem. Neurosci. 2013, 4, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Kergoat, L.; Piro, B.; Simon, D.T.; Pham, M.C.; Noël, V.; Berggren, M. Detection of glutamate and acetylcholine with organic electrochemical transistors based on conducting polymer/platinum nanoparticle composites. Adv. Mater. 2014, 26, 5658–5664. [Google Scholar] [CrossRef] [PubMed]
- Sirca, D.; Vardeu, A.; Pinna, M.; Diana, M.; Enrico, P. A robust, state-of-the-art amperometric microbiosensor for glutamate detection. Biosens. Bioelectron. 2014, 61, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Abeyrathne, C.D.; Huynh, D.H.; McIntire, T.W.; Nguyen, T.C.; Nasr, B.; Zantomio, D.; Chana, G.; Abbott, I.; Choong, P.; Catton, M.; et al. Lab on a chip sensor for rapid detection and antibiotic resistance determination of Staphylococcus aureus. Analyst 2016, 141, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Nasr, B.; Chana, G.; Lee, T.T.; Nguyen, T.; Abeyrathne, C.; D’Abaco, G.M.; Dottori, M.; Skafidas, E. Vertical Nanowire Electrode Arrays as Novel Electrochemical Label-Free Immunosensors. Small 2015, 11, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Wang, J.; Wang, L.; Du, D.; Timchalk, C.; Barry, R.; Lin, Y. A Novel Nanoparticle-Based Disposable Electrochemical Immunosensor for Diagnosis of Exposure to Toxic Organophosphorus Agents. Adv. Funct. Mater. 2011, 21, 4371–4378. [Google Scholar] [CrossRef]
- Stern, E.; Klemic, J.F.; Routenberg, D.A.; Wyrembak, P.N.; Turner-Evans, D.B.; Hamilton, A.D.; LaVan, D.A.; Fahmy, T.M.; Reed, M.A. Label-free immunodetection with CMOS-compatible semiconducting nanowires. Nature 2007, 445, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Chikkaveeraiah, B.V.; Bhirde, A.A.; Morgan, N.Y.; Eden, H.S.; Chen, X. Electrochemical immunosensors for detection of cancer protein biomarkers. ACS Nano 2012, 6, 6546–6561. [Google Scholar] [CrossRef] [PubMed]
- Lilienthal, K.; Fischer, M.; Stubenrauch, M.; Schober, A. Self-organized nanostructures in silicon and glass for MEMS, MOEMS and BioMEMS. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2010, 169, 78–84. [Google Scholar] [CrossRef]
- Gorton, L. Biosensors and Modern Biospecific Analytical Techniques, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2005; Volume 44, ISBN 9780444507150. [Google Scholar]
- Denham, M.; Dottori, M. Neural Differentiation of Induced Pluripotent Stem Cells. In Neurodegeneration: Methods and Protocols; Manfredi, G., Kawamata, H., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 99–110. ISBN 978-1-61779-328-8. [Google Scholar]
- Dottori, M.; Pera, M.F. Neural differentiation of human embryonic stem cells. In Methods in Molecular Biology (Clifton, N.J.); Springer: Berlin, Germany, 2008; Volume 438, pp. 19–30. ISBN 7065830071. [Google Scholar]
- Neal, A.; Yuen, T.; Bjorksten, A.R.; Kwan, P.; O’Brien, T.J.; Morokoff, A. Peritumoural glutamate correlates with post-operative seizures in supratentorial gliomas. J. Neurooncol. 2016, 129, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Yuen, T.I.; Morokoff, A.P.; Bjorksten, A.; D’Abaco, G.; Paradiso, L.; Finch, S.; Wong, D.; Reid, C.A.; Powell, K.L.; Drummond, K.J.; et al. Glutamate is associated with a higher risk of seizures in patients with gliomas. Neurology 2012, 79, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Liubinas, S.V.; Drummond, K.J.; Desmond, P.M.; Bjorksten, A.; Morokoff, A.P.; Kaye, A.H.; O’Brien, T.J.; Moffat, B.A. Glutamate quantification in patients with supratentorial gliomas using chemical shift imaging. NMR Biomed. 2014, 27, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Van Den Heuvel, R.H.H.; Svergun, D.I.; Petoukhov, M.V.; Coda, A.; Curti, B.; Ravasio, S.; Vanoni, M.A.; Mattevi, A. The active conformation of glutamate synthase and its binding to ferredoxin. J. Mol. Biol. 2003, 330, 113–128. [Google Scholar] [CrossRef]
- Arima, J.; Sasaki, C.; Sakaguchi, C.; Mizuno, H.; Tamura, T.; Kashima, A.; Kusakabe, H.; Sugio, S.; Inagaki, K. Structural characterization of l-glutamate oxidase from Streptomyces sp. X-119-6. FEBS J. 2009, 276, 4318–4327. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, J.J.; Gerhardt, G.A. Self-referencing ceramic-based multisite microelectrodes for the detection and elimination of interferences from the measurement of l-glutamate and other analytes. Anal. Chem. 2001, 73, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Geise, R.J.; Adams, J.M.; Barone, N.J.; Yacynych, A.M. Electropolymerized films to prevent interferences and electrode fouling in biosensors. Biosens. Bioelectron. 1991, 6, 151–160. [Google Scholar] [CrossRef]
- Wahono, N.; Qin, S.; Oomen, P.; Cremers, T.I.F.; de Vries, M.G.; Westerink, B.H.C. Evaluation of permselective membranes for optimization of intracerebral amperometric glutamate biosensors. Biosens. Bioelectron. 2012, 33, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Khudaish, E.; Hart, A. Electrochemical oxidation of hydrogen peroxide at platinum electrodes. Part II: Effect of potential. Electrochim. Acta 1998, 43, 2015–2024. [Google Scholar] [CrossRef]
- Cai, L.T.; Chen, H.Y. Electrocatalytic reduction of hydrogen peroxide at platinum microparticles dispersed in a poly(o-phenylenediamine) film. Sens. Actuators B Chem. 1999, 55, 14–18. [Google Scholar] [CrossRef]
- McMahon, C.P.; Rocchitta, G.; Serra, P.A.; Kirwan, S.M.; Lowry, J.P.; O’Neill, R.D. The efficiency of immobilised glutamate oxidase decreases with surface enzyme loading: An electrostatic effect, and reversal by a polycation significantly enhances biosensor sensitivity. Analyst 2006, 131, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Weltin, A.; Kieninger, J.; Enderle, B.; Gellner, A.K.; Fritsch, B.; Urban, G.A. Polymer-based, flexible glutamate and lactate microsensors for in vivo applications. Biosens. Bioelectron. 2014, 61, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Alshawaf, A.J.; Antonic, A.; Skafidas, E.; Ng, D.C.H.; Dottori, M. WDR62 Regulates Early Neural and Glial Progenitor Specification of Human Pluripotent Stem Cells. Stem Cells Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Birey, F.; Andersen, J.; Makinson, C.D.; Islam, S.; Wei, W.; Huber, N.; Fan, H.C.; Metzler, K.R.C.; Panagiotakos, G.; Thom, N.; et al. Assembly of functionally integrated human forebrain spheroids. Nature 2017, 545, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; O’Mahony, S.; Malone, G.; Dinan, T.G. An isocratic high performance liquid chromatography method for the determination of GABA and glutamate in discrete regions of the rodent brain. J. Neurosci. Methods 2007, 160, 223–230. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasr, B.; Chatterton, R.; Yong, J.H.M.; Jamshidi, P.; D’Abaco, G.M.; Bjorksten, A.R.; Kavehei, O.; Chana, G.; Dottori, M.; Skafidas, E. Self-Organized Nanostructure Modified Microelectrode for Sensitive Electrochemical Glutamate Detection in Stem Cells-Derived Brain Organoids. Biosensors 2018, 8, 14. https://doi.org/10.3390/bios8010014
Nasr B, Chatterton R, Yong JHM, Jamshidi P, D’Abaco GM, Bjorksten AR, Kavehei O, Chana G, Dottori M, Skafidas E. Self-Organized Nanostructure Modified Microelectrode for Sensitive Electrochemical Glutamate Detection in Stem Cells-Derived Brain Organoids. Biosensors. 2018; 8(1):14. https://doi.org/10.3390/bios8010014
Chicago/Turabian StyleNasr, Babak, Rachael Chatterton, Jason Hsien Ming Yong, Pegah Jamshidi, Giovanna Marisa D’Abaco, Andrew Robin Bjorksten, Omid Kavehei, Gursharan Chana, Mirella Dottori, and Efstratios Skafidas. 2018. "Self-Organized Nanostructure Modified Microelectrode for Sensitive Electrochemical Glutamate Detection in Stem Cells-Derived Brain Organoids" Biosensors 8, no. 1: 14. https://doi.org/10.3390/bios8010014
APA StyleNasr, B., Chatterton, R., Yong, J. H. M., Jamshidi, P., D’Abaco, G. M., Bjorksten, A. R., Kavehei, O., Chana, G., Dottori, M., & Skafidas, E. (2018). Self-Organized Nanostructure Modified Microelectrode for Sensitive Electrochemical Glutamate Detection in Stem Cells-Derived Brain Organoids. Biosensors, 8(1), 14. https://doi.org/10.3390/bios8010014