1. Introduction
Abnormalities in bone composition, such as loss of bone mass and changes in bone mineral density, are known to occur in patients with chronic kidney disease–mineral bone disorder (CKD–MBD), renal osteodystrophy (ROD), and osteoporosis [
1,
2,
3,
4,
5,
6,
7,
8]. Consequently, an important risk factor for such patients is bone fragility and the associated significant increase in bone fractures [
1,
2,
3,
4,
5,
6,
7,
8]. CKD–MBD is a systemic mineral metabolic disorder associated with CKD that affects bone metabolism and/or the cardiovascular system [
7]. Clinically, the presence of CKD–MBD is determined from abnormal changes in the levels of calcium, phosphorus, parathyroid, and vitamin D metabolism [
6,
7,
8]. Research on ROD, a skeletal disorder component of CKD–MBD, includes studies of bone morphologic changes [
7]. The related problems of osteoporosis, another metabolic bone disorder, concern the deterioration of mechanical properties, such as compromised bone strength.
Healthy bone is a natural composite consisting predominantly of collagen, which acts as a structural framework, and bone mineral (biological apatite, mainly hydroxyapatite) plus other noncollagen constituents, such as proteins, lipids, vascular elements, cells, and trace ions (e.g., potassium, carbonate, sodium, magnesium, fluoride, chloride, and citrate) [
9]. While the bone mineral provides toughness and rigidity to the bone, the collagen assures its tensile strength and flexibility. From biological and clinical perspectives, all of the known abnormalities in bone turnover, mineralization, volume, linear growth, strength, and in vascular and soft tissue calcifications are evaluated under the common terminology of
bone quality [
1,
2,
3,
4,
5,
6,
7,
8]. While changes in mineral metabolism and bone structure can develop early in the course of CKD, they worsen with progressive loss of kidney function; the more severe forms of ROD occur in patients with advanced CKD. Related and similarly problematic bone diseases are hyperparathyroid-mediated high-turnover bone disease or osteitis fibrosa cystica, adynamic bone disease, osteomalacia, and mixed uremic osteodystrophy [
5,
6,
7,
8].
Determining the pathogenesis of ROD and its treatment efficacy is difficult, since many factors potentially affect bone quality, and in a complicated manner. Their mechanisms of action are still not completely elucidated, nor is there a clear distinction between the main causes of key bone lesions associated with CKD [
8,
10]. For example, osteoporosis might be confused with osteomalacia, which is a contributor to ROD. However, detailed investigations have shown that there is a distinct difference between bone characteristics in these two cases, with bone that is porous and brittle in osteoporosis, but soft in osteomalacia. Such variation in bone quality is also associated with the ratio of mineral to organic material, which, in the latter case, is much reduced.
Bone histomorphometry is the technique most employed in assessing bone turnover, mineralization, and volume, and in making in vitro diagnostic discriminations. It is based on analysis of biochemical markers (usually using sample staining) of transiliac bone biopsies [
11,
12]. The invasive nature of the biopsies, the level of experience necessary for pathologists to recognize and classify the abovementioned abnormalities, and the lack of standardized normal values, make obtaining accurate tests still challenging in many laboratories. Therefore, there is still a need for alternative studies in order to achieve improved assessment of bone properties. An appealing approach is the use of optical spectroscopic techniques. One main advantage of such investigations is the fact that they provide information at the molecular level about all constituents at once and without the need for prior staining—a label-free, simultaneous identification of different biomarkers. Therefore, estimations of important bone compositional parameters, such as mineral-to-matrix ratio, carbonate-to-phosphate ratio, mineral crystallinity, the degree to which carbonate replaces the phosphate ions in the mineral lattice, and the amount of collagen crosslinks, can be and were successfully investigated by optical means as reported in the literature [
13,
14,
15,
16,
17,
18,
19]. Such results revealed alteration of the composition of bone tissue, with consequent impaired bone microarchitecture and increased fracture risk in metabolic bone diseases [
13,
14,
15,
16,
17,
18,
19]. However, the majority of these studies were based on Fourier transform infrared optical spectroscopy (FTIR) [
13,
14,
15,
16]. Only recently have they been extended to Raman spectroscopy [
17,
18,
19]. Overall, the literature on such Raman analysis is still quite scarce.
Raman spectroscopy has considerable potential for the development of future optical-fiber-based sensors with better spatial resolution for in vivo ROD detection. One of its immediate advantages is its direct applicability to in vitro measurements of fresh tissue and, consequently, the elimination of the unwanted, potential interference of embedding matrix material (usually polymethyl methacrylate) that is in standard use for FTIR and histomorphometrical sample preparation. In this study, by employing confocal Raman microscopy, we not only identify significant differences in bone composition between healthy (control) bone tissue and ROD bone tissue, but also achieve direct visualization of the main compositional parametric ratios (i.e., mineral-to-matrix and carbonate-to-amide I ratios) and of important constituents such as phenylalanine and calcium. Furthermore, the statistical analysis presented here is very accurate, since it is performed on hundreds of thousands of accumulated Raman spectra. Thus, by combining experimental findings from Raman spectroscopic imaging of biomarkers with statistical analysis, this work presents new ways of obtaining valuable information about bone quality, with the ultimate goal of improving the means of prediction of pathological fracture risk in ROD.
3. Results and Discussion
The overall comparison of differences between all bioconstituents present in healthy (control) bone tissue and ROD bone specimens, which is shown in
Figure 1, reveals an obvious distinction regarding the intensities of phenylalanine vibrational lines at 1005 and 1609 cm
−1. It is known that for patients with a malfunctioning kidney (and/or liver), an increased amount of phenylalanine can be anticipated along with a decrease in tyrosine [
20]. This is a consequence of the fact that the phenylalanine hydroxylase (PAH) enzyme, which is located in both kidney and liver and converts phenylalanine into tyrosine via hydroxylation of the aromatic side chain, fails to be expressed properly; thus, the observed excess of phenylalanine in
Figure 1 for patients diagnosed with ROD. Reaction with PAH is also the primary means by which the body forms tyrosine from ingested phenylalanine, the latter being an essential amino acid due to the body’s inability to produce it.
The Raman spectra presented in
Figure 1 were obtained by averaging multiple integrated spectra of images that were acquired at different locations on the corresponding types of samples, with each integrated Raman image spectrum being the average of 22,500 spectra per image. Thus, these two spectra are outcomes of the averaging of hundreds of thousands of accumulated spectra. Vertical translation and appropriate labeling was applied for easier visualization. Although the raster-scanning employed in acquiring the Raman mapping images for this level of data resolution requires acquisition times of minutes, the results show unprecedented accuracy for detecting potential morphological differences between the ROD and the control specimens. Supporting evidence is the obvious accumulation of phenylalanine in ROD samples reported here, which, to the best of our knowledge, is the first time that this has been observed with Raman spectroscopy. The presence of phosphate, carbonate, proline, and amide bands, which are expected components of the bone structural framework, have also been detected and appropriately labeled. However, no noticeable differences in the respective intensities of these bands are seen for the two types of bone specimens analyzed.
Since phenylalanine can be a potential ROD signature and a biomarker for identification of the disease, we further analyze its signal in
Figure 2a–d, where the locations of 1005 cm
−1 and 1609 cm
−1 vibrations are visually presented through associated confocal Raman images for control (
Figure 2a,c) and ROD (
Figure 2b,d) bone samples, respectively. The bright yellow pseudo-color in these images represents the highest Raman activity associated with the corresponding vibrations, while dark blue corresponds to the lowest Raman activity. Background subtraction and normalization of these images was performed as evidenced by the scaling of the color bars linked to these images. Only the most representative images are presented here. Criteria for this selection were based on patient age and clinical diagnosis as well as prior medical treatment. Besides expected similarities in the images of
Figure 2a,c and those of
Figure 2b,d, much more extensive yellow regions are observed in the images associated with the ROD specimens, corroborating the corresponding higher Raman activity (i.e., intensity) of phenylalanine Raman vibrations seen in
Figure 1 for the ROD samples.
It is also known that for ROD patients a combination of high phosphate levels and low calcium levels, again both attributed to damaged kidney functions, are usually clinically detected. The first are a consequence of the fact that a damaged kidney is unable to excrete phosphate properly, resulting in its systemic accumulation. Furthermore, a damaged kidney is unable to properly convert vitamin D obtained from food (vitamin D3) into its active state of calcitriol, of which the primary function is to increase calcium levels by improving the uptake of calcium in the gut from food and to stimulate kidney reabsorption of calcium. Consequently, the deficit of calcium in blood test analyses of such patients is mainly attributed to kidney malfunction. Since the other potential source of calcium to which the body has access is the bones, lower calcium levels are identified in ROD patients, as the body keeps leeching calcium from the bones and is unable to replenish it at the same rate from external sources (i.e., diet) due to the lack of calcitriol.
With this remark in mind, we analyze in
Figure 3a,b the calcium levels in normal and ROD bone samples, respectively. The υ
2PO
43−/amide III Raman activity ratio, which corresponds to the ratio of the area under the Raman band around 430 cm
−1 (from 395 cm
−1 to 469 cm
−1) to that under the peak around 1275 cm
−1 (from 1215 cm
−1 to 1332 cm
−1) from
Figure 1, was used for such analysis. Roschger et al. reported that this ratio is proportional to the calcium content [
21]. Indeed, based on the overall presence of yellow, green, and light-blue regions, a much lower υ
2PO
43−/amide III Raman activity ratio is observed in the image corresponding to ROD bone (
Figure 3b) than in that of normal bone (
Figure 3a). In addition to visually confirming a lower calcium content in the ROD sample, for a more accurate quantification of this assessment, we also present in
Figure 3c the associated statistical analysis.
The probability
p that the ratios of these Raman features on both samples (control and ROD) have the same average values
μ is calculated via the unpaired
t-test without assuming that the populations have equal variances (Welch’s
t-test, subroutine
t-test 2 in MATLAB
® version r2016a) [
22]. For the images presented in
Figure 3a,b, the corresponding histograms of these ratio values (
Figure 3c), assuming that they are normal distributions, lead to
μa = 0.5358,
σa = 0.0474,
μb = 0.5042,
σb = 0.0456, and the difference of the averages Δ =
μa −
μb = 0.0316 with the 95% confidence interval 0.0308 < Δ < 0.0325. The probability
p that they are sampled from the same distribution (the average ratios are the same in both samples) is negligibly small,
p < 10
−300.
The number of spectra per sample needed to have a detection power
β at a level
α of statistical significance is [
22]:
where
Z is the standard normal distribution, σ
a and σ
b are the standard deviations for the two samples, and Δ =
μa −
μb is the difference between the averages of the samples. Post-hoc power analysis shows that, for a typical detection power of
β = 90% at the level
α = 0.05, only about
n = 46 recorded spectra per sample is sufficient to distinguish between samples.
Another frequently reported measure of bone quality is the relative mineral to organic content, namely, the mineral-to-matrix ratio [
17,
18,
19]. This ratio, which is based on the integrated area under the phosphate peak at 960 cm
−1 (from 907 cm
−1 to 990 cm
−1) and that of the amide I band around 1660 cm
−1 (from 1625 cm
−1 to 1725 cm
−1), represents the amount of mineral per amount of collagen per volume analyzed. While in the literature previously, the assessment of this ratio was achieved by spectral analysis alone, we extend it here to direct visualization through the associated Raman imaging presented in
Figure 4a,b. Since in confocal Raman microscopy the band intensities of some constituents, such as phosphate and collagen amide I bands, are polarization-sensitive [
23,
24,
25], unpolarized recording using a low numerical aperture (NA) objective (i.e., NA = 0.4 of 20X objective) avoids the influence of this effect in addition to that of sample roughness. Furthermore, to minimize the calculation errors, we consider the ratio of areas under the corresponding peaks instead of the ratio of their intensities [
17,
18,
19,
21,
23,
24].
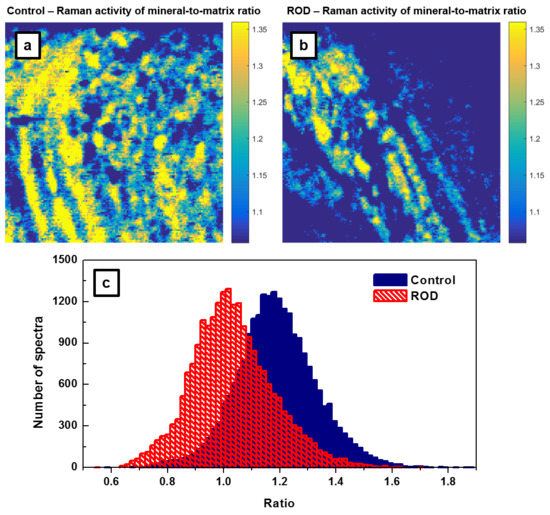
The results presented in
Figure 4a,b demonstrate that bone from patients with ROD exhibited significantly smaller amounts of high mineral-to-matrix ratio than did the normal group (i.e., fewer intense yellow regions are depicted in
Figure 4b of the ROD image). This behavior is expected in ROD patients with hyperparathyroidism, which results in increased bone resorption and turnover, as well as with hyperosteoidosis. The corresponding histograms of these ratios that are shown in
Figure 4c have
μa = 1.1876,
σa = 0.143,
μb = 1.0316,
σb = 0.145, and the difference of the averages Δ =
μa −
μb = 0.156 with the 95% confidence interval 0.1534 < Δ < 0.1587. Again, the
p value is less than 10
−300. Post-hoc power analysis (Equation (1)) shows that for
β = 90% and
α = 0.05, only about
n = 18 recorded spectra per sample is sufficient to distinguish between samples. Furthermore, the ROD histogram has a non-Gaussian shape, with a distribution skewed to the lower side of the mean value. This feature, which was previously observed and reported in the literature, has been attributed to a decrease in bone heterogeneity and mineral properties with age [
17].
Since the carbonate-to-matrix ratio is also a good indicator of bone turnover and remodeling activity, as well as bone cell counts and osteoid production, we present the confocal Raman images of this biomarker in
Figure 5a,b. We were also looking for a potential correlation between the carbonate-to-matrix ratio and the mineral-to-matrix ratio, both being standard parameters usually used for ROD validation. A smaller amount of high CO
32−/amide I ratio is noticed in the ROD image, consistent with a higher turnover remodeling in ROD samples, and supporting the characteristics of the image in
Figure 4b. Areas under the carbonate band around 1074 cm
−1 (from 1033 cm
−1 to 1135 cm
−1) and under the amide I band around 1660 cm
−1 (from 1625 cm
−1 to 1725 cm
−1) were considered for these carbonate to amide I ratios. For further assessment of our findings, the associated statistical analysis of these ratios is presented in
Figure 5c. These histograms have
μa = 1.2023,
σa = 0.0952,
μb = 1.1007,
σb = 0.1016, and the difference of the averages Δ =
μa −
μb = 0.1015 with the 95% confidence interval 0.0997 < Δ < 0.1033 (
p < 10
−300). The number of spectra per sample required to achieve the typical statistical power (i.e.,
β = 90%,
α = 0.05) is only about
n = 20 in this case; this is very similar to that needed for the mineral-to-matrix ratio.
Investigation of the bone collagen matrix in the amide I region in Raman spectroscopy is less precise than in FTIR measurements, with no clear distinction between the 1660 cm
−1 and 1690 cm
−1 bands that are used to measure the nonreducible to reducible collagen crosslink ratio [
15,
17,
23,
25]. Although we are still attempting to assess the collagen quality parameter by using the band centered around 1660 cm
−1, no visible difference in collagen dominance between the control and ROD samples was observed (data not presented here). The statistical results also indicate the necessity of a much larger number of spectra per sample (into the hundreds) for a typical statistical power of
β = 90% and
α = 0.05. Again, as with the mineral-to-matrix ratio, the histograms associated with the collagen matrix were non-Gaussian, especially that of the ROD sample. By analogy, we also associate this behavior with a decrease of collagen content with age.
The computationally estimated values of the ratios of previously analyzed biomarkers along with the number of spectra per sample required to achieve a statistical detection power of
β = 90%, at a level of statistical significance
α = 0.05, are summarized in
Table 1 below.
The mineral-to-matrix and the carbonate-to-matrix ratios are the strongest predictors of bone mechanical properties. Therefore, the results obtained here are of clinical relevance, as accurately distinguishing low-turnover (adynamic) bone from high-turnover bone disease is of importance and not easily done with noninvasive modalities [
26]. Prevention of fractures with medications may then more effectively be considered, especially for patients with prevalent fractures [
7]. Although there are no set published standards, antiresorptive therapy would be an option in high-turnover disease, anabolic treatment with teriparatide in low-turnover disease, and vitamin D uptake in osteomalacia with high turnover [
7]. The confocal Raman microscopy of 150 × 150 spectra recorded per sample and the consequent mapping of the calcium content (
Figure 3a,b), the mineral to organic content (
Figure 4a,b), and the carbonate-to-matrix ratio (
Figure 5a,b) presented in this work allow for clear distinction between normal and ROD bone. Even more important, since a relatively low number of spectra per sample is required for the usual statistical power to distinguish between normal and ROD bone (about 20 for these two cases), this raises the possibility of future development and use of in vivo Raman microsensors to determine the status of the bone. The correlation with the results of the “gold standard” method currently used for ROD assessment, histomorphometry, which might be of interest to the medical community, is not presented here, as it would be unlikely that one could acquire both types of measurements at the same location. More significantly, the advantage of multiplexing by using a label-free optical method for such investigations would be lost, in addition to the fact that the labeling required in histomorphometry would definitely influence the S/N of Raman measurements.