Surface Plasmon Resonance Optical Sensor: A Review on Light Source Technology
Abstract
:1. Introduction
2. SPR Sensor Configuration
2.1. Grating
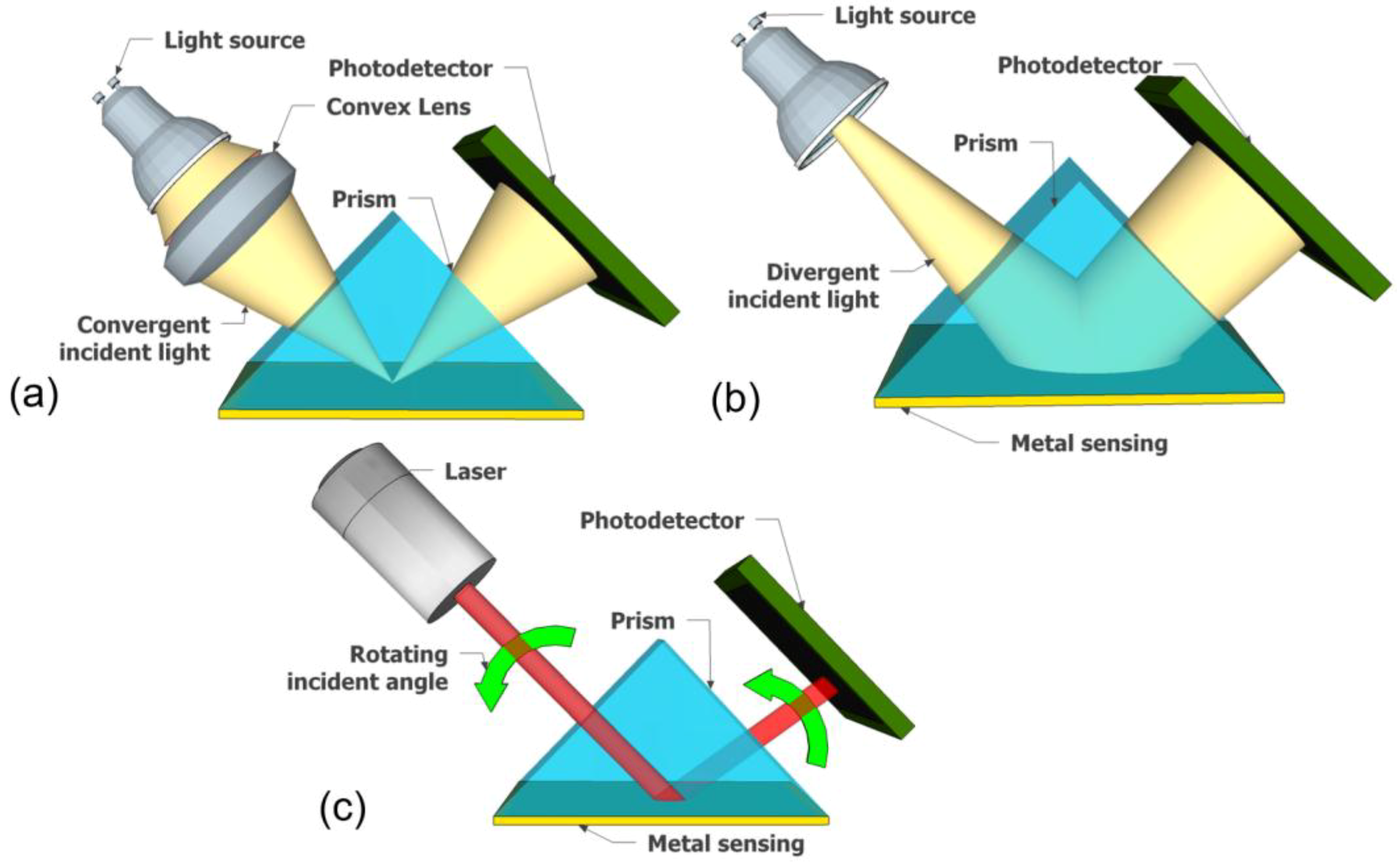
2.2. Prism Coupler
2.3. Waveguide
2.4. Localized SPR
3. SPR Measurement, Methodology and Performance Parameters
4. Light Source and Platform Preferences for SPR Sensor
4.1. Incandescent Lamp
4.2. Laser
4.2.1. Gas Laser
4.2.2. Laser Diode
4.3. Polychromatic Solid State Lighting
4.3.1. Light Emitting Diode
4.3.2. Organic Light-Emitting Diode
4.3.3. Smartphone-Based SPR Sensor
5. The Summary, Future Perspectives, and Challenges
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wood, R.W. On a remarkable case of uneven distribution of light in a diffraction grating spectrum. Philos. Mag. Ser. 6 1902, 4, 396–402. [Google Scholar] [CrossRef]
- Liedberg, B.; Nylander, C.; Lunström, I. Surface plasmon resonance for gas detection and biosensing. Sens. Actuators B 1983, 4, 299–304. [Google Scholar] [CrossRef]
- Šípová, H.; Homola, J. Surface plasmon resonance sensing of nucleic acids: A review. Anal. Chim. Acta 2013, 773, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Piliarik, M.; Párová, L.; Homola, J. High-throughput SPR sensor for food safety. Biosens. Bioelectron. 2009, 24, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Furfari, F.A. A Different Kind of Chemistry: A History of Tungsten Halogen Lamps. IEEE Ind. Appl. Mag. 2001, 7, 10–17. [Google Scholar] [CrossRef]
- Javan, A.; Bennett, W.R.; Herriott, D.R. Population inversion and continuous optical maser oscillation in a gas discharge containing a He-Ne mixture. Phys. Rev. Lett. 1961, 6, 106–110. [Google Scholar] [CrossRef]
- Hecht, J. Short history of laser development. Opt. Eng. 2010, 49, 091002. [Google Scholar] [CrossRef] [Green Version]
- Zheludev, N. The life and times of the LED—A 100-year history. Nat. Photonics 2007, 1, 189–192. [Google Scholar] [CrossRef]
- Nakamura, S.; Mukai, T.; Senoh, M. Candela-class high-brightness InGaN/AlGaN double-heterostructure blue-light-emitting diodes. Appl. Phys. Lett. 1994, 64, 1687–1689. [Google Scholar] [CrossRef]
- Pimputkar, S.; Speck, J.S.; DenBaars, S.P.; Nakamura, S. Prospects for LED lighting. Nat. Photonics 2009, 3, 180–182. [Google Scholar] [CrossRef]
- Lee, S.M.; Kwon, J.H.; Kwon, S.; Choi, K.C. A Review of Flexible OLEDs Toward Highly Durable Unusual Displays. IEEE Trans. Electron Devices 2017, 64, 1922–1931. [Google Scholar] [CrossRef]
- Tang, C.W.; Vanslyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Maindron, T. OLED: Theory and Principles. In OLED Microdisplays: Technology and Applications; Templier, F., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 1–33. ISBN 9781119004745. [Google Scholar]
- Fung, M.-K.; Li, Y.-Q.; Liao, L.-S. Tandem Organic Light-Emitting Diodes. Adv. Mater. 2016, 28, 10381–10408. [Google Scholar] [CrossRef] [PubMed]
- Kooyman, R.P.H.; Schasfoort, R.B.M.; Tudos, A.J. Physics of Surface Plasmon Resonance. In Handbook of Surface Plasmon Resonance; Schasfoort, R.B.M., Tudos, A.J., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2008; p. 403. ISBN 9780854042678. [Google Scholar]
- Maystre, D. Theory of wood’s anomalies. Springer Ser. Opt. Sci. 2012, 167, 39–83. [Google Scholar]
- Homola, J. Surface Plasmon Resonance Based Sensors; Homola, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 9783540339182. [Google Scholar]
- Kretschmann, E.; Raether, H. Radiative decay of non-radiative surface plasmons excited by light. Z. Naturforsch. 1968, 23, 2135–2136. [Google Scholar] [CrossRef]
- Otto, A. Excitation of nonradiative surface plasma waves in silver by the method of frustrated total reflection. Z. Phys. 1968, 216, 398–410. [Google Scholar] [CrossRef]
- Kretschmann, E. Decay of non radiative surface plasmons into light on rough silver films. Comparison of experimental and theoretical results. Opt. Commun. 1972, 6, 185–187. [Google Scholar] [CrossRef]
- Lavers, C.R.; Wilkinson, J.S. A waveguide-coupled surface-plasmon sensor for an aqueous environment. Sens. Actuators B Chem. 1994, 22, 75–81. [Google Scholar] [CrossRef]
- Gartia, M.R.; Hsiao, A.; Pokhriyal, A.; Seo, S.; Kulsharova, G.; Cunningham, B.T.; Bond, T.C.; Liu, G.L. Colorimetric Plasmon Resonance Imaging Using Nano Lycurgus Cup Arrays. Adv. Opt. Mater. 2013, 1, 68–76. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.E.; Anderton, C.R.; Thompson, L.B.; Maria, J.; Gray, S.K.; Rogers, J.A.; Nuzzo, R.G. Nanostructured plasmonic sensors. Chem. Rev. 2008, 108, 494–521. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.J.; Hill, R.T.; Tsai, Y.J.; Chilkoti, A.; Smith, D.R. Probing dynamically tunable localized surface plasmon resonances of film-coupled nanoparticles by evanescent wave excitation. Nano Lett. 2012, 12, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Long, G.L.; Winefordner, J.D. Limit of Detection A Closer Look at the IUPAC Definition. Anal. Chem. 1983, 55, 712A–724A. [Google Scholar] [CrossRef]
- Li, Z.; Ligthart, L.P.; Huang, P.; Lu, W.; Van Der Zwan, W.F. Trade-off between sensitivity and dynamic range in designing digital radar receivers. In Proceedings of the 2008 International Conference on Microwave and Millimeter Wave Technology, Nanjing, China, 21–24 April 2008. [Google Scholar]
- Chen, P.; Shu, X.; Cao, H.; Sugden, K. High-sensitivity and large-dynamic-range refractive index sensors employing weak composite Fabry-Perot cavities. Opt. Lett. 2017, 42, 3145–3148. [Google Scholar] [CrossRef] [PubMed]
- Strutt, J.W. On the Dynamical Theory of Gratings. Proc. R. Soc. Lond. A Math. Phys. Eng. Sci. 1907, 79, 399–416. [Google Scholar] [CrossRef] [Green Version]
- Esteban, O.; Díaz-Herrera, N.; Navarrete, M.-C.; González-Cano, A. Surface plasmon resonance sensors based on uniform-waist tapered fibers in a reflective configuration. Appl. Opt. 2006, 45, 7294–7298. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Tsao, Y.-C.; Tsai, W.-H.; Yang, Y.-W.; Yan, T.-R.; Sheu, B.-C. Development and application of side-polished fiber immunosensor based on surface plasmon resonance for the detection of Legionella pneumophila with halogens light and 850 nm-LED. Sens. Actuators A Phys. 2007, 138, 299–305. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Tsao, Y.; Tsai, W.-H.; Hung, T.-S.; Chen, K.-S.; Liao, S.-C. The enhancement method of optical fiber biosensor based on surface plasmon resonance with cold plasma modification. Sens. Actuators B Chem. 2008, 133, 370–373. [Google Scholar] [CrossRef]
- Singh, B.; Hillier, A.C. Surface plasmon resonance imaging of biomolecular interactions on a grating-based sensor array. Anal. Chem. 2006, 78, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Hastings, J.T.; Guo, J.; Keathley, P.D.; Kumaresh, P.B.; Wei, Y.; Law, S.; Bachas, L.G. Optimal self-referenced sensing using long- and short- range surface plasmons. Opt. Express 2007, 15, 17661–17672. [Google Scholar] [CrossRef] [PubMed]
- Kazuma, E.; Tatsuma, T. Localized surface plasmon resonance sensors based on wavelength-tunable spectral dips. Nanoscale 2013, 6, 2397–2405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.; Kwak, C.H.; Kim, G.; Kim, S.M.; Huh, Y.S.; Jeon, T.J. Identification of genetically modified DNA found in Roundup Ready soybean using gold nanoparticles. Microchim. Acta 2016, 183, 2649–2654. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; da Silva, A.G.M.; de Moura, A.B.L.; Freitas, I.G.; Camargo, P.H.C. Rational design of plasmonic catalysts: matching the surface plasmon resonance with lamp emission spectra for improved performance in AgAu nanorings. RSC Adv. 2016, 6, 62286–62290. [Google Scholar] [CrossRef]
- Ngoc, L.L.T.; Jin, M.; Wiedemair, J.; Van Den Berg, A.; Carlen, E.T. Large Area Metal Nanowire Arrays with Tunable Sub-20 nm Nanogaps. ACS Nano 2013, 7, 5223–5234. [Google Scholar] [CrossRef] [PubMed]
- Slavík, R.; Homola, J. Optical multilayers for LED-based surface plasmon. Appl. Opt. 2006, 45, 3752–3759. [Google Scholar] [CrossRef] [PubMed]
- Kretschmann, E.; Reather, H. Radiative decay of nonradiative surface plasmon excited by light. Z. Naturf. 1968, 23A, 2135–2136. [Google Scholar]
- Simon, H.J.; Mitchell, D.E.; Watson, J.G. Surface plasmons in silver films—a novel undergraduate experiment. Am. J. Phys. 1975, 43, 630–636. [Google Scholar] [CrossRef]
- Siegman, A.; Fauchet, P. Stimulated Wood’s Anomalies on Laser-Illuminated. IEEE J. Quantum Electron. 1986, QE-22, 1384–1403. [Google Scholar] [CrossRef]
- Antes, L.L.; Goldsmith, J.; McMahan, W. Pulsed Helium-Neon Gas Laser Applications. IEEE Trans. Mil. Electron. 1964, 8, 3–12. [Google Scholar] [CrossRef]
- Nylander, C.; Liedberg, B.; Lind, T. Gas detection by means of surface plasmon resonance. Sens. Actuators 1982, 3, 79–88. [Google Scholar] [CrossRef]
- Grigorenko, A.N.; Beloglazov, A.A.; Nikitin, P.I. Dark-field surface plasmon resonance microscopy. Opt. Commun. 2000, 174, 151–155. [Google Scholar] [CrossRef]
- Lin, C.-W.; Chen, K.-P.; Hsiao, C.-N.; Lin, S.; Lee, C.-K. Design and fabrication of an alternating dielectric multi-layer device for surface plasmon resonance sensor. Sens. Actuators B 2006, 113, 169–176. [Google Scholar] [CrossRef]
- Yusmawati, W.Y.; Chuah, H.P.; Mahmood, M.Y.W. Optical Properties and Sugar Content Determination of Commercial Carbonated Drinks using Surface Plasmon Resonance. Am. J. Appl. Sci. 2007, 4, 1–4. [Google Scholar]
- Lesuffleur, A.; Im, H.; Lindquist, N.C.; Lim, K.S.; Oh, S. Laser-illuminated nanohole arrays for multiplex plasmonic microarray sensing. Opt. Express 2008, 16, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Hotta, K.; Teramae, N. Optical Waveguide sensor based on a porous anodic alumina/aluminum multilayer film. Anal. Chem. 2009, 81, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Turker, B.; Guner, H.; Ayas, S.; Ekiz, O.O.; Acar, H.; Guler, M.O.; Dâna, A. Grating coupler integrated photodiodes for plasmon resonance based sensing. Lab Chip 2011, 11, 282–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lertvachirapaiboon, C.; Yamazaki, R.; Pienpinijtham, P.; Baba, A.; Ekgasit, S.; Thammacharoen, C.; Shinbo, K.; Kato, K.; Kaneko, F. Solution-based fabrication of gold grating film for use as a surface plasmon resonance sensor chip. Sens. Actuators B Chem. 2012, 173, 316–321. [Google Scholar] [CrossRef]
- Malachovska, V.; Ribaut, C.; Wattiez, R.; Caucheteur, C. Fiber-Optic SPR Immunosensors Tailored to Target Epithelial Cells through Membrane Receptors. Anal. Chem. 2015, 87, 5957–5965. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, A.; Sharma, A.; Tomar, M.; Gupta, V. Surface plasmon resonance study on the optical sensing properties of tin oxide (SnO2) films to NH3 gas. J. Appl. Phys. 2016, 119, 164502. [Google Scholar] [CrossRef]
- Kaur, G.; Paliwal, A.; Tomar, M.; Gupta, V. Detection of Neisseria meningitidis using surface plasmon resonance based DNA biosensor. Biosens. Bioelectron. 2016, 78, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.; Nguyen, T.T.; Lee, R.; Li, T.; Yun, C.; Ham, Y. Label-Free Quantitative Immunoassay of Fibrinogen in Alzheimer Disease Patient Plasma Using Fiber Optical Surface Plasmon Resonance. J. Electron. Mater. 2016, 45, 2354–2360. [Google Scholar] [CrossRef]
- Galvez, F.; De Lara, D.P.; Spottorno, J.; García, M.A.; Vicent, J.L. Heating effects of low power surface plasmon resonance sensors. Sens. Actuators B Chem. 2017, 243, 806–811. [Google Scholar] [CrossRef]
- Matsubara, K.; Kawata, S.; Minami, S. A Compact Surface Plasmon Resonance Sensor for Measurement of Water in Process. Appl. Spectrosc. 1988, 42, 1375–1379. [Google Scholar] [CrossRef]
- O’Brien, I.I.M.J.; Perez-Luna, V.H.; Brueck, S.R.J.; Lopez, G.P. A surface plasmon resonance array biosensor based on spectroscopic imaging. Biosens. Bioelectron. 2001, 16, 97–108. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Boussaad, S.; Tao, N.J. High-performance differential surface plasmon resonance sensor using quadrant cell photodetector High-performance differential surface plasmon resonance sensor using quadrant cell photodetector. Rev. Sci. Instrum. 2003, 74, 150–153. [Google Scholar] [CrossRef]
- Zybin, A.; Grunwald, C.; Mirsky, V.M.; Wolfbeis, O.S.; Niemax, K. Double-Wavelength Technique for Surface Plasmon Resonance Measurements: Basic Concept and Applications for Single Sensors and Two-Dimensional Sensor Arrays. Anal. Chem. 2005, 77, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Chegel, V.; Whitcombe, M.J.; Turner, N.W.; Piletsky, S.A. Deposition of functionalized polymer layers in surface plasmon resonance immunosensors by in-situ polymerization in the evanescent wave field. Biosens. Bioelectron. 2009, 24, 1270–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herminjard, S.; Sirigu, L.; Herzig, H.P.; Crottini, A.; Pellaux, J.; Gresch, T. Surface Plasmon Resonance sensor showing enhanced sensitivity for CO2 detection in the mid-infrared range. Opt. Express 2009, 17, 221–227. [Google Scholar] [CrossRef]
- Patskovsky, S.; Song, I.; Meunier, M.; Kabashin, A.V.; Montréal, É.P. De Silicon based total internal reflection bio and chemical sensing with spectral phase detection. Opt. Express 2009, 17, 12523–12528. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Min, H.; Jung, Y.; Hyun, B. A new palm-sized surface plasmon resonance (SPR) biosensor based on modulation of a light source by a rotating mirror. Sens. Actuators B Chem. 2010, 150, 1–6. [Google Scholar] [CrossRef]
- Karabchevsky, A.; Karabchevsky, S.; Abdulhalim, I. Fast surface plasmon resonance imaging sensor using Radon transform. Sens. Actuators B Chem. 2011, 155, 361–365. [Google Scholar] [CrossRef]
- Nizamov, S.; Mirsky, V.M. Self-referencing SPR-biosensors based on penetration difference of evanescent waves. Biosens. Bioelectron. 2011, 28, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.J.; Lu, Y.; Wang, M.T.; Wu, B.Q.; Duan, L.C.; Yao, J.Q. Surface Plasmon Resonance Refractive Index Sensor Based on Active Photonic Crystal Fiber. IEEE Photonics J. 2013, 5. [Google Scholar] [CrossRef]
- Daldosso, N.; Pavesi, L. Nanosilicon photonics. Laser Photonics Rev. 2009, 3, 508–534. [Google Scholar] [CrossRef]
- Dupuis, R.D.; Krames, M.R.; Member, S. History, Development, and Applications of High-Brightness Visible Light-Emitting Diodes. J. Lightwave Technol. 2008, 26, 1154–1171. [Google Scholar] [CrossRef]
- Meléndez, J.; Carr, R.; Bartholomew, D.; Taneja, H.; Yee, S.; Jung, C.; Furlong, C. Development of a surface plasmon resonance sensor for commercial applications. Sens. Actuators B Chem. 1997, 39, 375–379. [Google Scholar] [CrossRef]
- Perkins, E.A.; Squirell, D.J. Development of instrumentation to allow the detection of microorganisms using light scattering in combination with surface plasmon resonance. Biosens. Bioelectron. 2000, 14, 853–859. [Google Scholar] [CrossRef]
- Melendez, J.; Carr, R.; Bartholomew, D.U.; Kukanskis, K.; Elkind, J.; Yee, S.; Furlong, C.; Woodbury, R. A commercial solution for surface plasmon sensing. Sens. Actuators B Chem. 1996, 35, 212–216. [Google Scholar] [CrossRef]
- Melendez, J.L.; Carr, R.A.; Keller, R.C. Integrally Formed Surface Plasmon Resonance Sensor. U.S. Patent 5,912,456, 15 June 1999. [Google Scholar]
- Ho, H.P.; Wu, S.Y.; Yang, M.; Cheung, A.C. Application of white light-emitting diode to surface plasmon resonance sensors. Sens. Actuators B 2001, 80, 89–94. [Google Scholar] [CrossRef]
- Akimoto, T.; Wada, S.; Karube, I. A surface plasmon resonance probe without optical fibers as a portable sensing device. Anal. Chim. Acta 2008, 610, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Kondoh, J.; Matsui, Y.; Shiokawa, S.; Suzuki, K. Development of novel optical waveguide surface plasmon resonance (SPR) sensor with dual light emitting diodes. Sens. Actuators B 2005, 106, 383–387. [Google Scholar] [CrossRef]
- Escobedo, C.; Vincent, S.; Choudhury, A.I.K.; Campbell, J.; Brolo, A.G.; Sinton, D.; Gordon, R. Integrated nanohole array surface plasmon resonance sensing device using a dual-wavelength source. J. Micromech. Microeng. 2011, 21, 115001. [Google Scholar] [CrossRef]
- Wilkop, T.; Wang, Z.; Cheng, Q. Analysis of micro-contact printed protein patterns by SPR imaging with a LED light source. Langmuir 2004, 20, 11141–11148. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.P.; Wu, C.M.L.; Wu, S.Y.; Ho, H.P. White-light spectral interferometry for surface plasmon resonance sensing applications. Opt. Express 2011, 19, 4521–4527. [Google Scholar] [CrossRef] [PubMed]
- Sereda, A.; Moreau, J.; Boulade, M.; Olivéro, A.; Canva, M.; Maillart, E. Compact 5-LEDs illumination system for multi-spectral surface plasmon resonance sensing. Sens. Actuators B Chem. 2015, 209, 208–211. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, L.; Zhou, B.; Liu, W.; Ge, J.; Wu, J.; Wang, Y.; Wang, P. Ultrasensitive and selective gold film-based detection of mercury (II) in tap water using a laser scanning confocal imaging-surface plasmon resonance system in real time. Biosens. Bioelectron. 2013, 47, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Bonroy, K.; Reekmans, G.; Laureyn, W.; Verhaegen, K.; De Vlaminck, I.; Lagae, L.; Borghs, G. Localized surface plasmon resonance biosensor integrated with microfluidic chip. Biomed. Microdevices 2009, 11, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Aslan, K.; Geddes, C.D. Wavelength-Ratiometric Plasmon Light Scattering-Based Immunoassays. Plasmonics 2009, 4, 267–272. [Google Scholar] [CrossRef]
- Mitsushio, M.; Higo, M. A gold-deposited surface plasmon resonance-based optical fiber sensor system using various light-emitting diodes. Anal. Sci. 2011, 27, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Yanase, Y.; Araki, A.; Suzuki, H.; Tsutsui, T.; Kimura, T.; Okamoto, K.; Nakatani, T.; Hiragun, T.; Hide, M. Development of an optical fiber SPR sensor for living cell activation. Biosens. Bioelectron. 2010, 25, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.-L.; Chang, C.-C.; Chu-Su, Y.; Wei, S.-C.; Zhao, X.; Hsueh, P.-R.; Lin, C.-W. Disposable surface plasmon resonance aptasensor with membrane-based sample handling design for quantitative interferon-gamma detection. Lab Chip 2014, 14, 2968–2977. [Google Scholar] [CrossRef] [PubMed]
- Cetin, A.E.; Coskun, A.F.; Galarreta, B.C.; Huang, M.; Herman, D.; Ozcan, A.; Altug, H. Handheld high-throughput plasmonic biosensor using computational on-chip imaging. Light Sci. Appl. 2014, 3, e122. [Google Scholar] [CrossRef]
- Shinar, R.; Shinar, J. Organic Electronics in Sensors and Biotechnology; Mc Graw Hill: New York, NY, USA, 2009; ISBN 9780071596763. [Google Scholar]
- Chung, S.; Lee, J.-H.; Jeong, J.; Kim, J.-J.; Hong, Y. Substrate thermal conductivity effect on heat dissipation and lifetime improvement of organic light-emitting diodes. Appl. Phys. Lett. 2009, 94, 253302. [Google Scholar] [CrossRef] [Green Version]
- Frischeisen, J.; Mayr, C.; Reinke, N.A.; Nowy, S.; Brütting, W. Surface plasmon resonance sensor utilizing an integrated organic light emitting diode. Opt. Express 2008, 16, 18426–18436. [Google Scholar] [CrossRef] [PubMed]
- Prabowo, B.A.; Chang, Y.-F.; Lee, Y.-Y.; Su, L.-C.; Yu, C.-J.; Lin, Y.-H.; Chou, C.; Chiu, N.-F.; Lai, H.-C.; Liu, K.C. Application of an OLED integrated with BEF and giant birefringent optical (GBO) film in a SPR biosensor. Sens. Actuators B Chem. 2014, 198, 424–430. [Google Scholar] [CrossRef]
- Prabowo, B.A.; Su, L.-C.; Chang, Y.; Lai, H.; Chiu, N.-F.; Liu, K.-C. Performance of white organic light-emitting diode for portable optical biosensor. Sens. Actuators B 2016, 222, 1058–1065. [Google Scholar] [CrossRef]
- Prabowo, B.A.; Chang, Y.-F.F.; Lai, H.-C.C.; Alom, A.; Pal, P.; Lee, Y.-Y.Y.; Chiu, N.-F.F.; Hatanaka, K.; Su, L.-C.C.; Liu, K.-C.C. Rapid screening of Mycobacterium tuberculosis complex (MTBC) in clinical samples by a modular portable biosensor. Sens. Actuators B Chem. 2018, 254, 742–748. [Google Scholar] [CrossRef]
- Prabowo, B.A.; Alom, A.; Secario, M.K.; Masim, F.C.P.; Lai, H.C.; Hatanaka, K.; Liu, K.C. Graphene-based Portable SPR Sensor for the Detection of Mycobacterium tuberculosis DNA Strain. Procedia Eng. 2016, 168, 541–545. [Google Scholar] [CrossRef]
- Prabowo, B.A.; Wang, R.Y.L.; Secario, M.K.; Ou, P.-T.; Alom, A.; Liu, J.-J.; Liu, K.-C. Rapid detection and quantification of Enterovirus 71 by a portable surface plasmon resonance biosensor. Biosens. Bioelectron. 2017, 92, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Prabowo, B.A.; Alom, A.; Pal, P.; Secario, M.K.; Wang, R.Y.L.; Liu, K.C. Novel Four Layer Metal Sensing in Portable SPR Sensor Platform for Viral Particles Quantification. Proceedings 2017, 1, 528. [Google Scholar] [CrossRef]
- Preechaburana, P.; Gonzalez, M.C.; Suska, A.; Filippini, D. Surface plasmon resonance chemical sensing on cell phones. Angew. Chem. Int. Ed. Engl. 2012, 51, 11585–11588. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Q.; Chen, S.; Cheng, F.; Wang, H.; Peng, W. Surface Plasmon Resonance Biosensor Based on Smart Phone Platforms. Sci. Rep. 2015, 5, 12864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, S.; Saikia, K.; Nath, P. Smartphone based LSPR sensing platform for bio-conjugation detection and quantification. RSC Adv. 2016, 6, 21871–21880. [Google Scholar] [CrossRef]
- Wang, X.; Chang, T.W.; Lin, G.; Gartia, M.R.; Liu, G.L. Self-Referenced Smartphone-Based Nanoplasmonic Imaging Platform for Colorimetric Biochemical Sensing. Anal. Chem. 2017, 89, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Reineke, S.; Lindner, F.; Schwartz, G.; Seidler, N.; Walzer, K.; Lüssem, B.; Leo, K. White organic light-emitting diodes with fluorescent tube efficiency. Nature 2009, 459, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Vaisocherová, H.; Ševců, V.; Adam, P.; Špačková, B.; Hegnerová, K.; de los Santos Pereira, A.; Rodriguez-Emmenegger, C.; Riedel, T.; Houska, M.; Brynda, E.; et al. Functionalized ultra-low fouling carboxy- and hydroxy-functional surface platforms: functionalization capacity, biorecognition capability and resistance to fouling from undiluted biological media. Biosens. Bioelectron. 2014, 51, 150–157. [Google Scholar] [CrossRef]
- Mani, V.; Chikkaveeraiah, B.V.; Patel, V.; Gutkind, J.S.; Rusling, J.F. Ultrasensitive immunosensor for cancer biomarker proteins using gold nanoparticle film electrodes and multienzyme-particle amplification. ACS Nano 2009, 3, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, M.; Yang, J.; Zhao, C.; Hu, R.; Chen, Q.; Chang, Y.; Zheng, J. Synthesis and characterization of antifouling poly(N-acryloylaminoethoxyethanol) with ultralow protein adsorption and cell attachment. Langmuir 2014, 30, 10398–10409. [Google Scholar] [CrossRef] [PubMed]
- Gobi, K.V.; Iwasaka, H.; Miura, N. Self-assembled PEG monolayer based SPR immunosensor for label-free detection of insulin. Biosens. Bioelectron. 2007, 22, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Sin, M.-C.; Chen, S.-H.; Chang, Y. Hemocompatibility of zwitterionic interfaces and membranes. Polym. J. 2014, 46, 436–443. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]










| No | Feature | Incandescent Lamp | Gas Laser | Laser Diode | LED | OLED |
|---|---|---|---|---|---|---|
| 1 | Size | Bulky | Bulky | Small | Small | Small |
| 2 | Color | Polychromatic | Monochromatic | Monochromatic | Polychromatic | Polychromatic |
| 3 | Disposability | No | No | Yes | Yes | Yes |
| 4 | Detection method possibility | λ int., intensity mod. | θ int., intensity mod., phase int. | θ int., intensity mod., phase int. | λ int., intensity mod. | λ int., intensity mod. |
| 5 | Incident light | Convergent, divergent | Convergent, rotating | Convergent, rotating | Convergent, divergent | Convergent, divergent |
| 6 | Component of optical alignment | Lens, fiber optic | Lens, fiber optic Motor | Lens, fiber optic Motor | Lens, fiber optic | Microstructure film |
| 7 | Light source alignment | Hard | Hard | Moderate | Moderate | Simple |
| 8 | Drawback | Self-heating, size, lifetime, stability | Self-heating, size, stability | Self-heating, stability | Self-heating, stability | Technology maturity |
| No | Configuration | Technical Remarks | Target Sample | Results/Performance | Ref. |
|---|---|---|---|---|---|
| 1 | Optical fiber | Uniform-waist tapered optical fibers | Water | Sensitivity: 10−4 RIU | [32] |
| 2 | Optical fiber | Side-polished multimode optical fiber | Water, ethanol, DNA | Sensitivity: 3 × 10−6 RIU | [33] |
| 3 | Optical fiber | Cold plasma modified Side-polished optical fiber | BSA | 5 ng | [34] |
| 4 | Grating | Grating based SPRi | BSA | ∼225 spots cm−2 | [35] |
| 5 | Kretschmann | Optical fiber coupled-prism to enhance long and short range SPR | Streptavidin | LOD: 2.3 × 10−5, RIU: 11 pg/mm2 | [36] |
| 6 | LSPR | Ag nanospheres and nanorods on TiO2 substrate | Streptavidin | LOD: 2.8 × 10−4, RIU: 0.3 µg/mL | [37] |
| 7 | LSPR | Au nanosphere for the detection of DNA mutation in Roundup Ready soybean | DNA hybridization | LOD: 1 nM DNA | [38] |
| 8 | LSPR | LSPR excitation in AgAu nanorings | Methylene blue. | 4.3 and 4.7 fold LSPR enhancement | [39] |
| 9 | LSPR | Large metal nanowire array | Air | SERS enhancement | [40] |
| 10 | LRSPR | Kretschmann, long-range SPR sensing structure (Teflon AF under Au layer). | Water-diethylene glycol | Dynamic range: 8 × 10−3 RIU | [41] |
| No | Laser Type | Technical Remark | Target Sample | Performance | Ref. |
|---|---|---|---|---|---|
| 1 | He-Ne laser | Kretschmann, SPRi, λ = 632.8 nm, microarray sensing membrane | Si coating, SAM | Decay length ~4 µm | [47] |
| 2 | He-Ne laser | Kretschmann, dielectric mirror TiO2/SiO2 sensing structure, λ = 632 | Glucose solution | Res: 1.28 × 10−5 RIU Dynamic range: 1.331–1.50 RIU | [48] |
| 3 | He-Ne laser | Kretschmann, angular int., λ = 632.8 nm | Sugar content in carbonated drink | LOD: 0.01–0.05% | [49] |
| 4 | He-Ne laser | LSPRi, λ = 632.8 nm, nanohole arrays. | SAM | detection sensitivity ~16,6%/RIU | [50] |
| 5 | He-Ne laser | Waveguide, green light (λ = 534.5 nm), PAA/Al sensing structure. | Fe(II) solution | n/a | [51] |
| 6 | He-Ne Laser | Grating, transmission measurement, λ = 632.8 nm, integrated flow cell and detector | NaCl solution | Res: 6.3 × 10−6 RIU/√Hz | [52] |
| 7 | He-Ne laser | Imprinted AuNP Grating, λ = 632.8 nm. | Fe(II)-BTP and PEDOT:PSS | n/a (proof of concept) | [53] |
| 8 | Argon-ion laser | Tilted fiber Bragg grating (TFBG), λ = 1550 nm | Epithelial cells | LOD ~2 × 106 cells/mL | [54] |
| 9 | He-Ne laser | Kretschmann, Au/SnO2 sensing film, angular int., λ = 633 nm | Ammonia gas | Sensitivity 0.055°/ppm (0.5–250 ppm) | [55] |
| 10 | He-Ne laser | Kretschmann, Au/ZnO sensing film, angular int., λ = 633 nm | DNA of N. meningitidis | LOD: 5 ng/μL | [56] |
| 11 | He-Ne laser | Optical fiber coupling, Intensity modulation, λ = 632.8 nm | Fibrinogen on plasma blood | LOD: 20 ng/mL | [57] |
| 12 | He-Ne laser and Laser diode | Kretschmann, angular int., He-Ne laser: 1.5 mW, red, λ = 632.8 nm; Laser diode: 15 mW, green, λ = 543 nm | Air, thermal effects on laser spot area. | Local thermal drift: red laser ∼0.1 K and green laser ∼1 K | [58] |
| 13 | Laser diode | Kretschmann with rotating diffuser, SPRi, CCD camera detector, λ = 633 nm, high throughput and disposable sensing design. | IgG, BSA | Proof of concept for multi-sample detection. | [60] |
| 14 | Laser diode | Kretschmann, linear laser incident, two channels detection area, quadrant cell photodetector. | Pb2+ ions | ~0.2 nM or 0.04 ppb | [61] |
| 15 | Laser diode | Kretschmann, intensity mod. at dual wavelengths references. | DNA hybridization | LOD: 2 × 10−6 RIU | [62] |
| 16 | Laser diode | Integratted in NanoSPR™ | IgG | Sensing regeneration | [63] |
| 17 | Quantum cascade laser | Kretschmann CaF2 prism, TiO2/Au layer on sensing, angular int., λ = 633 nm. | CO2 | 5 times sensitivity improvement | [64] |
| 18 | Laser diode | Kretschmann, Si prism, λ = 1200 nm for air medium, λ = 1500 nm for aqueous medium, phase modulation. | Ar and N2 | LOD: 10−6 RIU | [65] |
| 19 | Laser diode | Portable, Kretschmann, rotating mirror for the incident angle adjustment, powered by battery, 2 channels measurement. | PSA | LOD: 2.5 × 10−6 RIU | [66] |
| 20 | Laser diode | Kretschmann, angular int., diverging laser beam, λ = 637 nm | Ethanol solution | LOD: 5 × 10−6 RIU | [67] |
| 21 | Laser diode | Dual wavelengths, λ = 658 nm and 980 nm, self-referencing | Diluted NaCl on PBS | 20 times reducing noise from bulk RI medium effect | [68] |
| 22 | Laser diode | FBG based coupling, λ = 976 nm | Yb3+ | n/a | [69] |
| No | LED Type | Technical Remark | Target Sample | Performance | Ref. |
|---|---|---|---|---|---|
| 1 | NIR LED | Additional laser diode for scattering enhancement, λ = 820 nm. | Bacterial spores | LOD: 107 mL−1 | [73] |
| 2 | White LED | Kretschmann, wavelength int., 200 µm pin hole to control incident light. | Glycerin solution | Res: 1.98 × 10−4 RIU | [76] |
| 3 | LED | Waveguide coupling using 5 cm cylindrical glass probe (diameter 1.5 mm), photodiode detector, wavelength int. | Glycerin, BSA | Res: 1.2 × 10−5 RIU LOD: 50 ng/mL | [77] |
| 4 | Dual LED | Waveguide coupling using 5 cm flat pyrex glass, intensity mod. At two wavelengths, optional LED for different wavelength. | Ethanol solution, BSA | LOD: 2.3 × 10−5 RIU | [78] |
| 5 | Dual LEDs | Transmission grating based, nanohole array sensing, intensity mod. | Biotin-streptavidin binding | LOD: 6 × 10−4 RIU | [79] |
| 6 | LED | Kretschmann, SPRi, intensity mod., patterned sensing, λ = 648 nm | Cholera toxin (CT), IgG | IgG detection range 0.005 to 0.5 mg/mL | [80] |
| 7 | Warm white light LED | Dual Kretschmann (for reference and target sample), phase int. in preferred wavelength. | NaCl solution | Res: 10−7 RIU | [81] |
| 8 | Five color LEDs | Kretschmann, SPRi, optical fiber waveguide, wavelength int. | DNA hybridization | Res: 3 × 10−6 RIU | [82] |
| 9 | White LED | Kretschmann, wavelength int., integrated with confocal microscope | Mercury ion | LOD: 0.01 ng/mL | [83] |
| 10 | LED | LSPR, integrated with microfluidic device, transmittance measurement, intensity mod. | Glycerol, biotin-antibiotin | LOD: 10−4 RIU LOD: 270 ng/mL | [84] |
| 11 | LED | LSPR, transmittance measurement, intensity mod. | IgG | LOD: 0.05 µg/mL | [85] |
| 12 | LED | Glass fiber waveguide, intensity mod. | Benzyl alcohol in methanol | LOD: 10−4 RIU | [86] |
| 13 | LED | Miniaturize, low-cost, and portable platform, waveguide coupling, wavelength int. | RBL-2H3 cells | LOD: 1.65 × 10−3 RIU | [87] |
| 14 | LED | Portable, integrated with microfluidic chip, low-cost, disposable, aptamer probe. | Interferon-γ | LOD: 10 pM | [88] |
| 15 | LED | Transmission grating, nanohole array | IgG, BSA | Res: 4 × 10−3 RIU | [89] |
| No | Light Source | Technical Remark | Target Sample | Performance (LOD) | Ref. |
|---|---|---|---|---|---|
| 1 | Three colors OLED | Kretschmann, light source attachment on prism, wavelength int. | NaCl solution | 6 × 10−4 RIU | [92] |
| 2 | Red and green OLED | Kretschmann, OLED attachment on prism, red and green OLED, brightness and reflective polarizer enhancement, intensity modulation At dual wavelengths. | Sucrose water, IgG | 3 × 10−6 RIU 40.6 pg/mL | [93] |
| 3 | White color OLED | Kretschmann, OLED attachment on prism, wavelength int., brightness and reflective polarizer enhancement, bimetallic film, intensity modulation at dual wavelengths. | Sucrose water, IgG | 2.6 × 10−6 RIU 40.3 pg/mL | [94] |
| 4 | Tunable color OLED | Kretschmann, integration of intensity modulations. | EV71 viral particle, VP1 protein | 67 vp/mL; 4.8 pg/mL | [97] |
| 5 | Red color OLED | Kretschmann, intensity modulation at dual wavelengths. | IS6110 DNA | 63 pg/mL | [95] |
| 6 | Red color OLED | Four layer structures, Kretschmann, intensity modulations at dual wavelengths. | EV71 viral particle | 43 vp/mL | [98] |
| 7 | Red color OLED | Graphene layer, π-π stacking ssDNA-graphene, AuNP signal enhancement. | DNA hybridization | 28 fM | [96] |
| No | Platform | Technical Remark | Target Sample | Performance | Ref. |
|---|---|---|---|---|---|
| 1 | iPhone | PDMS Kretschmann prism, light source from iPhone screen, the front camera as a photodetector, optional sensing plate using commercial CM5. | β2 microglo-bulin | Res:2.14 × 10−6 RIU LOD: 0.1 mg/mL In serum; 0.25 mg/mL in urine | [99] |
| 2 | Smartphone | Optical fiber coupling, three channels, main camera as photodetector | IgG | Res:7.4 × 10−6 RIU, LOD: 47.4 nM | [100] |
| 3 | Optical module and iPhone integration | White light source, LSPR, transmission illumination, extended optical module, main camera as photodetector, AuNP colloid solution in cuvette. | BSA, Trypsin | LOD: 19.2 µg/mL (BSA); 25.7 µg/mL (Trypsin) | [101] |
| 4 | Optical module and iPhone integration | LED light source, transmission grating, extended optical module, main camera as a photodetector. | Urine, BSA | 0.01 mg/mL | [102] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabowo, B.A.; Purwidyantri, A.; Liu, K.-C. Surface Plasmon Resonance Optical Sensor: A Review on Light Source Technology. Biosensors 2018, 8, 80. https://doi.org/10.3390/bios8030080
Prabowo BA, Purwidyantri A, Liu K-C. Surface Plasmon Resonance Optical Sensor: A Review on Light Source Technology. Biosensors. 2018; 8(3):80. https://doi.org/10.3390/bios8030080
Chicago/Turabian StylePrabowo, Briliant Adhi, Agnes Purwidyantri, and Kou-Chen Liu. 2018. "Surface Plasmon Resonance Optical Sensor: A Review on Light Source Technology" Biosensors 8, no. 3: 80. https://doi.org/10.3390/bios8030080
APA StylePrabowo, B. A., Purwidyantri, A., & Liu, K.-C. (2018). Surface Plasmon Resonance Optical Sensor: A Review on Light Source Technology. Biosensors, 8(3), 80. https://doi.org/10.3390/bios8030080






