Applications of Nanomaterials for Immunosensing
Abstract
:1. Introduction
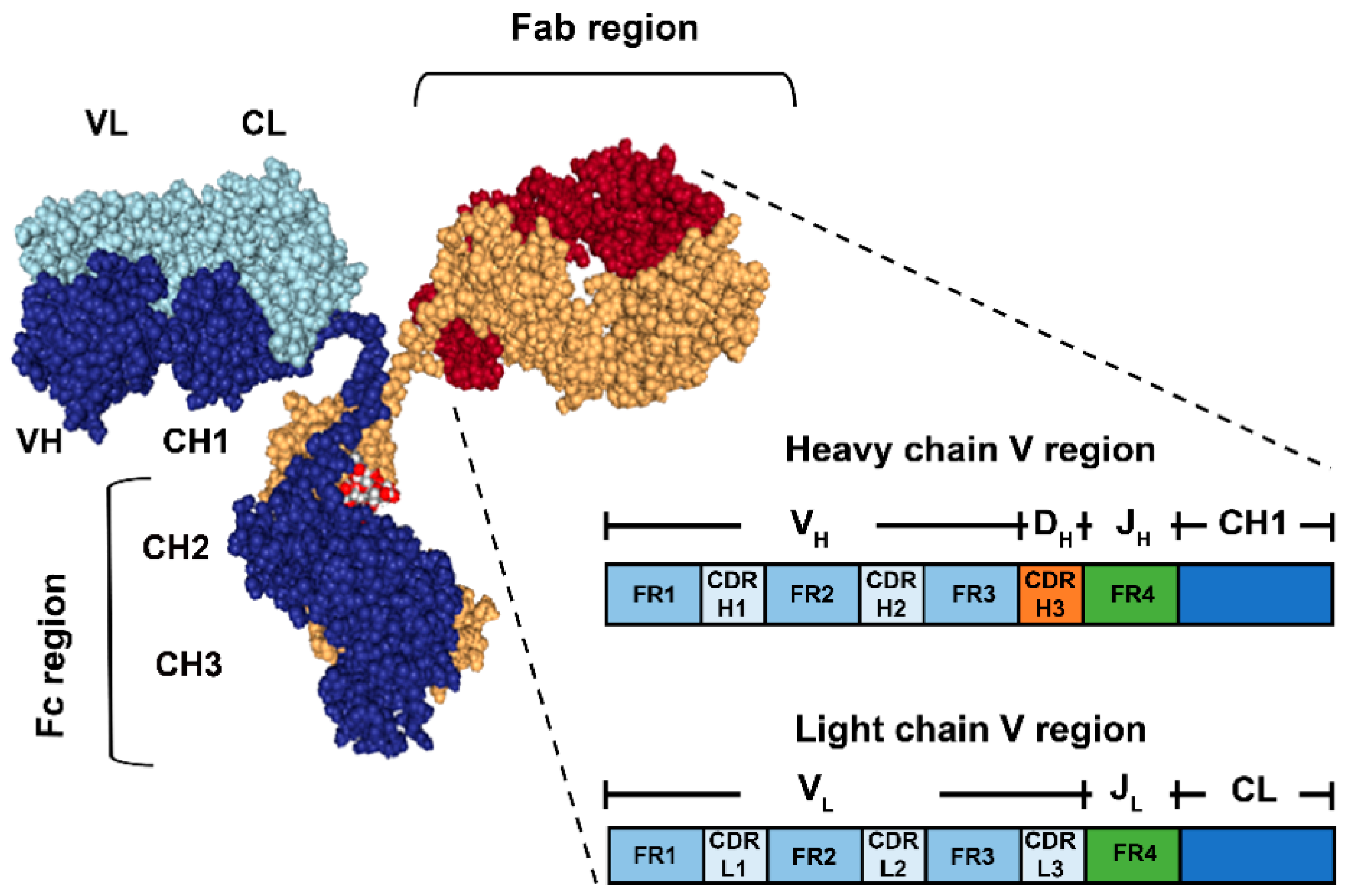
1.1. Immune Complex: Antibody-Antigen Interactions
1.2. Nanoparticles for Biomarker Immunosensing
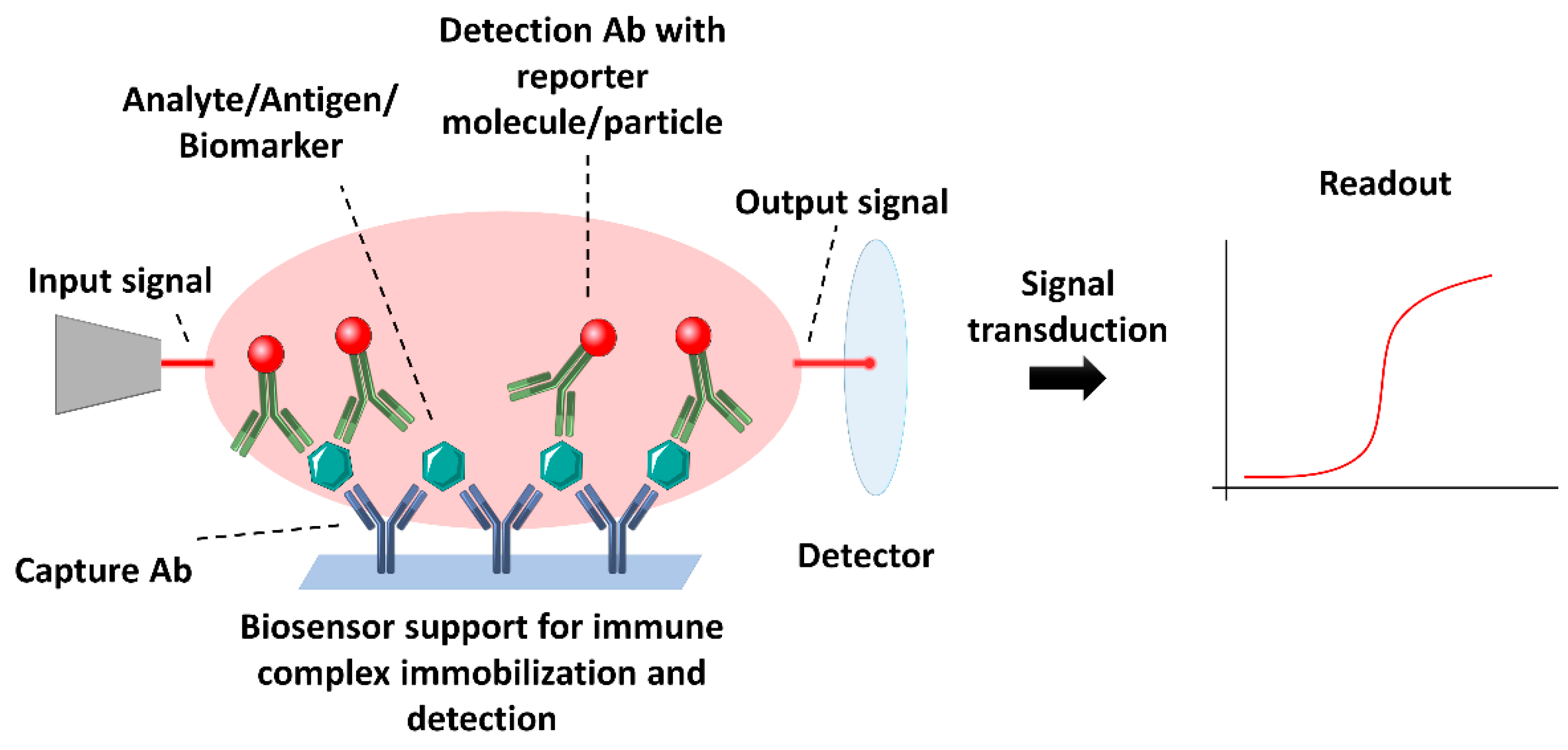
2. Nanomaterials for Immunosensing
2.1. Immobilization of Antibodies
2.2. Types of Nanomaterials for Immunosensing
2.2.1. Carbon-Based Nanomaterials
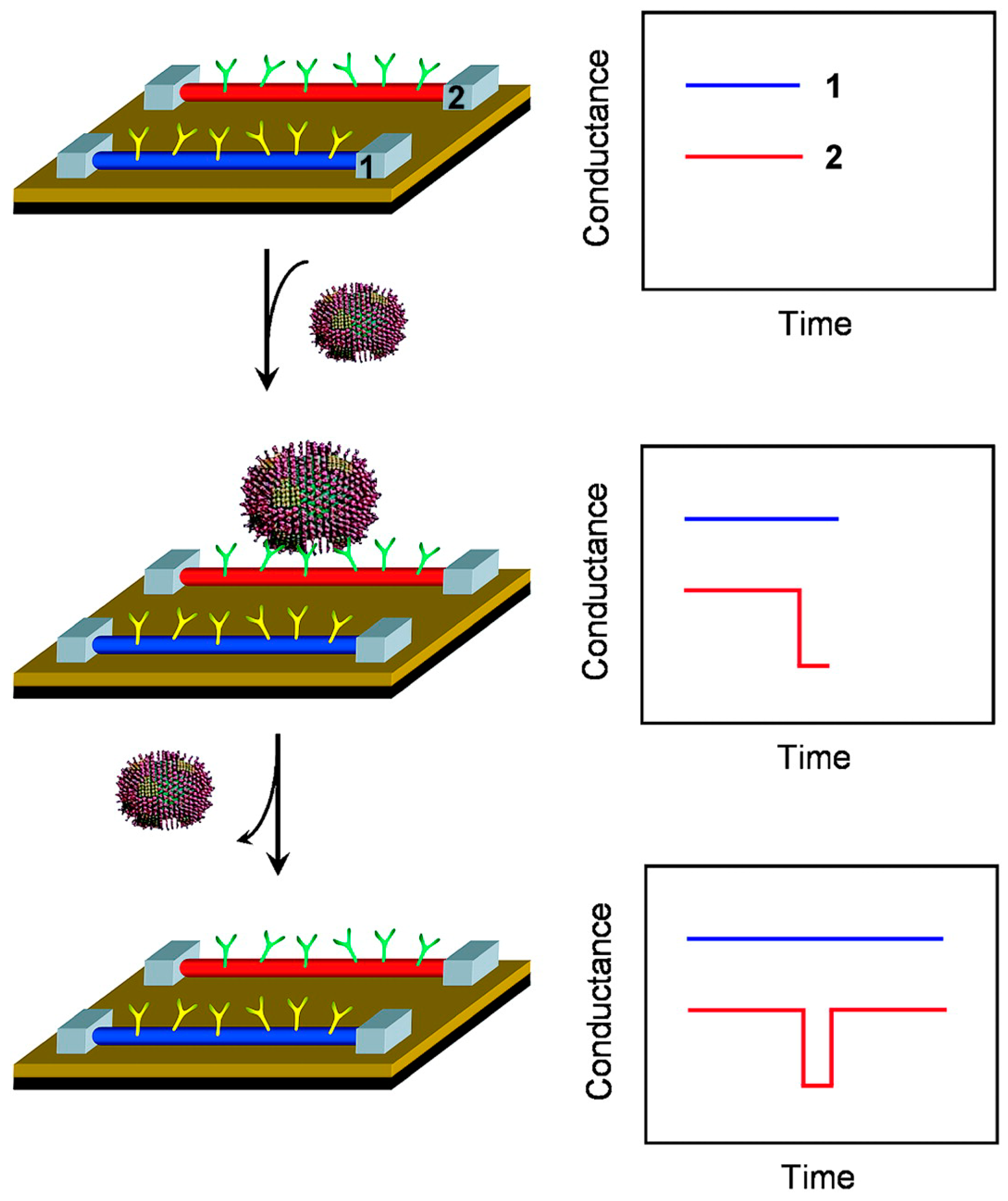
2.2.2. Metallic Nanomaterials
2.2.3. Silicon Nanomaterials
3. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- EU Commission. Commission Recommendation of 18 October 2011 on the Definition of Nanomaterial (2011/696/EU). Official Journal of the European Communities, 20 October 2011. [Google Scholar]
- Kim, B.H.; Hackett, M.J.; Park, J.; Hyeon, T. Synthesis, Characterization, and Application of Ultrasmall Nanoparticles. Chem. Mater. 2014, 26, 59–71. [Google Scholar] [CrossRef]
- Chou, L.Y.T.; Ming, K.; Chan, W.C.W. Strategies for the intracellular delivery of nanoparticles. Chem. Soc. Rev. 2011, 40, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Grzelczak, M.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L.M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Hristov, D.R.; Rocks, L.; Kelly, P.M.; Thomas, S.S.; Pitek, A.S.; Verderio, P.; Mahon, E.; Dawson, K.A. Tuning of nanoparticle biological functionality through controlled surface chemistryandcharacterisation at the bioconjugated nanoparticle surface. Sci. Rep. 2015, 5, 17040. [Google Scholar] [CrossRef] [PubMed]
- Hühn, J.; Carrillo-Carrion, C.; Soliman, M.G.; Pfeiffer, C.; Valdeperez, D.; Masood, A.; Chakraborty, I.; Zhu, L.; Gallego, M.; Yue, Z.; et al. Selected Standard Protocols for the Synthesis, Phase Transfer, and Characterization of Inorganic Colloidal Nanoparticles. Chem. Mater. 2017, 29, 399–461. [Google Scholar] [CrossRef]
- Gao, Y.; Xie, X.; Li, F.; Lu, Y.; Li, T.; Lian, S.; Zhang, Y.; Zhang, H.; Mei, H.; Jia, L. A novel nanomissile targeting two biomarkers and accurately bombing CTCs with doxorubicin. Nanoscale 2017, 9, 5624–5640. [Google Scholar] [CrossRef] [PubMed]
- Chowdhuri, A.R.; Bhattacharya, D.; Sahu, S.K. Magnetic nanoscale metal organic frameworks for potential targeted anticancer drug delivery, imaging and as an MRI contrast agent. Dalton Trans. 2016, 45, 2963–2973. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Palma, M.; Hanahan, D. The biology of personalized cancer medicine: Facing individual complexities underlying hallmark capabilities. Mol. Oncol. 2012, 6, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Borrebaeck, C.A.K. Precision diagnostics: Moving towards protein biomarker signatures of clinical utility in cancer. Nat. Rev. Cancer 2017, 17, 199. [Google Scholar] [CrossRef] [PubMed]
- Neves, H.; Kwok, H.F. Recent advances in the field of anti-cancer immunotherapy. BBA Clin. 2015, 3, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.J. Potent antibody therapeutics by design. Nat. Rev. Immunol. 2006, 6, 343. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.R.; Delgado, T.; Rodriguez, J.A.; Helguera, G.; Penichet, M.L. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin. Immunol. 2006, 121, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Regulation of Adaptive Immunity by the Innate Immune System. Science 2010, 327, 291. [Google Scholar] [CrossRef] [PubMed]
- Hudson, P.J.; Souriau, C. Engineered antibodies. Nat. Med. 2003, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Sela-Culang, I.; Kunik, V.; Ofran, Y. The Structural Basis of Antibody-Antigen Recognition. Front. Immunol. 2013, 4, 302. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Davis, M.M. Diversity in the CDR3 Region of VH Is Sufficient for Most Antibody Specificities. Immunity 2000, 13, 37–45. [Google Scholar] [CrossRef]
- Boyd, S.D.; Crowe, J.E. Deep sequencing and human antibody repertoire analysis. Curr. Opin. Immunol. 2016, 40, 103–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgiou, G.; Ippolito, G.C.; Beausang, J.; Busse, C.E.; Wardemann, H.; Quake, S.R. The promise and challenge of high-throughput sequencing of the antibody repertoire. Nat. Biotechnol. 2014, 32, 158–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanehsazzadeh, S.; Gruettner, C.; Lahooti, A.; Mahmoudi, M.; Allen, B.J.; Ghavami, M.; Daha, F.J.; Oghabian, M.A. Monoclonal antibody conjugated magnetic nanoparticles could target MUC-1-positive cells in vitro but not in vivo. Contrast Media Mol. Imaging 2015, 10, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495. [Google Scholar] [CrossRef] [PubMed]
- Holliger, P.; Hudson, P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 2005, 23, 1126. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, V.S.P.K.; Das, A.B.; Saxena, U. Recent advances in biosensor development for the detection of cancer biomarkers. Biosens. Bioelectron. 2017, 91, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kalubowilage, M.; Covarrubias-Zambrano, O.; Malalasekera, A.P.; Wendel, S.O.; Wang, H.; Yapa, A.S.; Chlebanowski, L.; Toledo, Y.; Ortega, R.; Janik, K.E.; et al. Early detection of pancreatic cancers in liquid biopsies by ultrasensitive fluorescence nanobiosensors. Nanomedicine 2018, 14, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Chinen, A.B.; Guan, C.M.; Ferrer, J.R.; Barnaby, S.N.; Merkel, T.J.; Mirkin, C.A. Nanoparticle Probes for the Detection of Cancer Biomarkers, Cells, and Tissues by Fluorescence. Chem. Rev. 2015, 115, 10530–10574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, H.; Chung, J.; Balaj, L.; Charest, A.; Bigner, D.D.; Carter, B.S.; Hochberg, F.H.; Breakefield, X.O.; Weissleder, R.; Lee, H. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat. Med. 2012, 18, 1835. [Google Scholar] [CrossRef] [PubMed]
- Henry, N.L.; Hayes, D.F. Cancer biomarkers. Mol. Oncol. 2012, 6, 140–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrier, A.; Gligorov, J.; Lefèvre, G.; Boissan, M. The extracellular domain of Her2 in serum as a biomarker of breast cancer. Lab. Investig. 2018, 98, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Triulzi, T.; Bianchi, G.V.; Tagliabue, E. Predictive biomarkers in the treatment of HER2-positive breast cancer: An ongoing challenge. Future Oncol. 2016, 12, 1413–1428. [Google Scholar] [CrossRef] [PubMed]
- Andree, K.C.; Mentink, A.; Zeune, L.L.; Terstappen, L.W.; Stoecklein, N.H.; Neves, R.P.; Driemel, C.; Lampignano, R.; Yang, L.; Neubauer, H. Towards a real liquid biopsy in metastatic breast and prostate cancer: Diagnostic LeukApheresis increases CTC yields in a European prospective multi-center study (CTCTrap). Int. J. Cancer 2018. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Naranjo, J.C.; Wu, H.-J.; Ugaz, V.M. Microfluidics for exosome isolation and analysis: Enabling liquid biopsy for personalized medicine. Lab Chip 2017, 17, 3558–3577. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Moxley, K.; Ruskin, R.; Dhanasekaran, D.N.; Zhao, Y.D.; Ramesh, R. A Non-invasive Liquid Biopsy Screening of Urine-Derived Exosomes for miRNAs as Biomarkers in Endometrial Cancer Patients. AAPS J. 2018, 20, 82. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Chia, D.; Wei, F.; Wong, D. Liquid biopsy for detection of actionable oncogenic mutations in human cancers and electric field induced release and measurement liquid biopsy (eLB). Analyst 2016, 141, 393–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pazos, E.; Garcia-Algar, M.; Penas, C.; Nazarenus, M.; Torruella, A.; Pazos-Perez, N.; Guerrini, L.; Vázquez, M.E.; Garcia-Rico, E.; Mascareñas, J.L. Surface-enhanced raman scattering surface selection rules for the proteomic liquid biopsy in real samples: Efficient detection of the oncoprotein c-MYC. J. Am. Chem. Soc. 2016, 138, 14206–14209. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Lv, Z.; Liu, J.; Bai, W.; Yang, S.; Chen, A. Colorimetric Aptasensor Using Unmodified Gold Nanoparticles for Homogeneous Multiplex Detection. PLoS ONE 2014, 9, e109263. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Zhang, B.; Tang, J.; Liu, B.; Lai, W.; Tang, D. Sandwich-type immunosensors and immunoassays exploiting nanostructure labels: A review. Anal. Chim. Acta 2013, 758, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.-H.; Kuo, S.-H.; Lin, T.-Y.; Lin, C.-W.; Fang, P.-Y.; Yang, H.-W. An electrochemical biosensor to simultaneously detect VEGF and PSA for early prostate cancer diagnosis based on graphene oxide/ssDNA/PLLA nanoparticles. Biosens. Bioelectron. 2017, 89, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A.J.A.; Chemistry, B. Lateral flow (immuno)assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.A.; Sánchez, J.L.A.; O’Sullivan, C.K.; Abbas, M.N. DNA biosensors based on gold nanoparticles-modified graphene oxide for the detection of breast cancer biomarkers for early diagnosis. Bioelectrochemistry 2017, 118, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howes, P.D.; Chandrawati, R.; Stevens, M.M. Colloidal nanoparticles as advanced biological sensors. Science 2014, 346. [Google Scholar] [CrossRef] [PubMed]
- Bakhori, N.M.; Yusof, N.A.; Abdullah, J.; Wasoh, H.; Noor, S.S.M.; Raston, N.H.A.; Mohammad, F. ImmunoNanosensor for the Ultrasensitive Naked Eye Detection of Tuberculosis. Sensors 2018, 18, 1932. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Z.; Zhang, P.; Zhang, X.; Fu, A. An ultra-sensitive chemiluminescence immunosensor of carcinoembryonic antigen using HRP-functionalized mesoporous silica nanoparticles as labels. Sens. Actuators B 2011, 155, 557–561. [Google Scholar] [CrossRef]
- Huang, Y.; Kannan, P.; Zhang, L.; Chen, T.; Kim, D.-H. Concave gold nanoparticle-based highly sensitive electrochemical IgG immunobiosensor for the detection of antibody–antigen interactions. RSC Adv. 2015, 5, 58478–58484. [Google Scholar] [CrossRef]
- Suresh, L.; Brahman, P.K.; Reddy, K.R.; Bondili, J. Development of an electrochemical immunosensor based on gold nanoparticles incorporated chitosan biopolymer nanocomposite film for the detection of prostate cancer using PSA as biomarker. Enzyme Microb. Technol. 2018, 112, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Patolsky, F.; Zheng, G.; Hayden, O.; Lakadamyali, M.; Zhuang, X.; Lieber, C.M. Electrical detection of single viruses. Proc. Natl. Acad. Sci. USA 2004, 101, 14017–14022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, R.; Singh, J.; Solanki, P.R.; Manaka, T.; Iwamoto, M.; Basu, T.; Malhotra, B.D. Label-free piezoelectric immunosensor decorated with gold nanoparticles: Kinetic analysis and biosensing application. Sens. Actuators B 2016, 222, 804–814. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Wolfbeis, O.S.; Hahn, Y.-B.; Alshareef, H.N.; Torsi, L.; Salama, K.N. Deposition of nanomaterials: A crucial step in biosensor fabrication. Mater. Today Commun. 2018, 17, 289–321. [Google Scholar] [CrossRef]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Byrne, H.; O’Kennedy, R.J. Antibodies and antibody-derived analytical biosensors. Essays Biochem. 2016, 60, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parolo, C.; de la Escosura-Muñiz, A.; Polo, E.; Grazú, V.; de la Fuente, J.M.; Merkoçi, A. Design, Preparation, and Evaluation of a Fixed-Orientation Antibody/Gold-Nanoparticle Conjugate as an Immunosensing Label. ACS Appl. Mater. Interfaces 2013, 5, 10753–10759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biju, V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef] [PubMed]
- Herda, L.M.; Hristov, D.R.; Lo Giudice, M.C.; Polo, E.; Dawson, K.A. Mapping of Molecular Structure of the Nanoscale Surface in Bionanoparticles. J. Am. Chem. Soc. 2017, 139, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, G.; Wu, Y.; Hao, P.; Zhou, W.; Zhang, Z. Gold nanoparticle amplified optical microfiber evanescent wave absorption biosensor for cancer biomarker detection in serum. Talanta 2014, 120, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Li, Q.; Ji, W.; Bin, X.; Song, J. Highly Sensitive Homogeneous Immunoassays Based on Construction of Silver Triangular Nanoplates-Quantum Dots FRET System. Sci. Rep. 2016, 6, 26534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara, S.; Perez-Potti, A.; Herda, L.M.; Adumeau, L.; Dawson, K.A.; Yan, Y. Differential Recognition of Nanoparticle Protein Corona and Modified Low-Density Lipoprotein by Macrophage Receptor with Collagenous Structure. ACS Nano 2018, 12, 4930–4937. [Google Scholar] [CrossRef] [PubMed]
- Kavosi, B.; Navaee, A.; Salimi, A. Amplified fluorescence resonance energy transfer sensing of prostate specific antigen based on aggregation of CdTe QDs/antibody and aptamer decorated of AuNPs-PAMAM dendrimer. J. Lumin. 2018, 204, 368–374. [Google Scholar] [CrossRef]
- Richards, D.A.; Maruani, A.; Chudasama, V. Antibody fragments as nanoparticle targeting ligands: A step in the right direction. Chem. Sci. 2017, 8, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Bolley, J.; Guenin, E.; Lievre, N.; Lecouvey, M.; Soussan, M.; Lalatonne, Y.; Motte, L. Carbodiimide versus Click Chemistry for Nanoparticle Surface Functionalization: A Comparative Study for the Elaboration of Multimodal Superparamagnetic Nanoparticles Targeting αvβ3 Integrins. Langmuir 2013, 29, 14639–14647. [Google Scholar] [CrossRef] [PubMed]
- Demay-Drouhard, P.; Nehlig, E.; Hardouin, J.; Motte, L.; Guénin, E. Nanoparticles under the Light: Click Functionalization by Photochemical Thiol-yne Reaction, Towards Double Click Functionalization. Chemistry 2013, 19, 8388–8392. [Google Scholar] [CrossRef] [PubMed]
- Bugiel, M.; Fantana, H.; Bormuth, V.; Trushko, A.; Schiemann, F.; Howard, J.; Schäffer, E.; Jannasch, A. Versatile microsphere attachment of GFP-labeled motors and other tagged proteins with preserved functionality. J. Biol. Chem. 2015, 2. [Google Scholar] [CrossRef]
- Sheng, S.; Kong, F. Separation of antigens and antibodies by immunoaffinity chromatography. Pharm. Biol. 2012, 50, 1038–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene, M.K.; Richards, D.A.; Nogueira, J.C.F.; Campbell, K.; Smyth, P.; Fernández, M.; Scott, C.J.; Chudasama, V. Forming next-generation antibody–nanoparticle conjugates through the oriented installation of non-engineered antibody fragments. Chem. Sci. 2018, 9, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Ruoff, R.S.; Bielawski, C.W. From Conception to Realization: An Historial Account of Graphene and Some Perspectives for Its Future. Angew. Chem. 2010, 49, 9336–9344. [Google Scholar] [CrossRef] [PubMed]
- Cheeveewattanagul, N.; Morales-Narváez, E.; Hassan, A.-R.H.A.; Bergua, J.F.; Surareungchai, W.; Somasundrum, M.; Merkoçi, A. Straightforward Immunosensing Platform Based on Graphene Oxide-Decorated Nanopaper: A Highly Sensitive and Fast Biosensing Approach. Adv. Funct. Mater. 2017, 27, 1702741. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Jeon, E.A.; Lee, D.Y. Cell surface-camouflaged graphene oxide immunosensor for identifying immune reactions. Ind. Eng. Chem. 2018, 59, 28–34. [Google Scholar] [CrossRef]
- Rauf, S.; Mishra, G.K.; Azhar, J.; Mishra, R.K.; Goud, K.Y.; Nawaz, M.A.H.; Marty, J.L.; Hayat, A. Carboxylic group riched graphene oxide based disposable electrochemical immunosensor for cancer biomarker detection. Anal. Biochem. 2018, 545, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Hong, S.; Jang, J. Label-free Detection of Influenza Viruses using a Reduced Graphene Oxide-based Electrochemical Immunosensor Integrated with a Microfluidic Platform. Sci. Rep. 2017, 7, 42771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallavicini, P.; Cabrini, E.; Borzenkov, M.; Sironi, L.; Chirico, G. Applications of Gold Nanostars: Nanosensing, Thermal Therapy, Delivery Systems. In Gold Nanostars. SpringerBriefs in Materials; Springer International Publishing: Cham, Switzerland, 2015; pp. 43–59. [Google Scholar]
- Potenza, M.A.C.; Krpetić, Ž.; Sanvito, T.; Cai, Q.; Monopoli, M.; de Araújo, J.M.; Cella, C.; Boselli, L.; Castagnola, V.; Milani, P.; et al. Detecting the shape of anisotropic gold nanoparticles in dispersion with single particle extinction and scattering. Nanoscale 2017, 9, 2778–2784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dondapati, S.K.; Sau, T.K.; Hrelescu, C.; Klar, T.A.; Stefani, F.D.; Feldmann, J. Label-free Biosensing Based on Single Gold Nanostars as Plasmonic Transducers. ACS Nano 2010, 4, 6318–6322. [Google Scholar] [CrossRef] [PubMed]
- Ben Haddada, M.; Hu, D.; Salmain, M.; Zhang, L.; Peng, C.; Wang, Y.; Liedberg, B.; Boujday, S. Gold nanoparticle-based localized surface plasmon immunosensor for staphylococcal enterotoxin A (SEA) detection. Anal. Bioanal. Chem. 2017, 409, 6227–6234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Andresen, H.; Ghadiali, J.E.; Stevens, M.M. Kinase-Actuated Immunoaggregation of Peptide-Conjugated Gold Nanoparticles. Small 2010, 6, 1509–1513. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-R.; Shin, D.-M.; Song, H.; Lee, D.; Kim, K. Current achievements of nanoparticle applications in developing optical sensing and imaging techniques. Nano Converg. 2016, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, L.-J.; Jiang, J.-H. Surface-Enhanced Raman Spectroscopy-Based, Homogeneous, Multiplexed Immunoassay with Antibody-Fragments-Decorated Gold Nanoparticles. Anal. Chem. 2013, 85, 9213–9220. [Google Scholar] [CrossRef] [PubMed]
- Pantaleo, G.; Harari, A. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat. Rev. Immunol. 2006, 6, 417. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yao, D.; Knoll, W. Surface Plasmon Field-Enhanced Fluorescence Spectroscopy Studies of the Interaction between an Antibody and Its Surface-Coupled Antigen. Anal. Chem. 2003, 75, 2610–2617. [Google Scholar] [CrossRef] [PubMed]
- Bu, D.; Zhuang, H.; Yang, G.; Ping, X. An immunosensor designed for polybrominated biphenyl detection based on fluorescence resonance energy transfer (FRET) between carbon dots and gold nanoparticles. Sens. Actuators B Chem. 2014, 195, 540–548. [Google Scholar] [CrossRef]
- Lee, J.-H.; Cho, H.-Y.; Choi, H.; Lee, J.-Y.; Choi, J.-W. Application of Gold Nanoparticle to Plasmonic Biosensors. Int. J. Mol. Sci. 2018, 19, 2021. [Google Scholar] [CrossRef] [PubMed]
- Alarfaj, N.A.; El-Tohamy, M.F. A label-free electrochemical immunosensor based on gold nanoparticles and graphene oxide for the detection of tumor marker calcitonin. New J. Chem. 2017, 41, 11029–11035. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.-J.; Li, L.; Yang, Y.; Mao, L.-G.; Peng, Z. A label-free electrochemical immunosensor based on gold nanoparticles for direct detection of atrazine. Sens. Actuators B Chem. 2014, 191, 408–414. [Google Scholar] [CrossRef]
- Zeinabad, H.A.; Ghourchian, H.; Falahati, M.; Fathipour, M.; Azizi, M.; Boutorabi, S.M. Ultrasensitive interdigitated capacitance immunosensor using gold nanoparticles. Nanotechnology 2018, 29, 265102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Pradeep, T. Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnol. Bioeng. 2005, 90, 59–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sriram, M.; Zong, K.; Vivekchand, S.; Gooding, J. Single Nanoparticle Plasmonic Sensors. Sensors 2015, 15, 25774. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Ma, Y.; Yang, H.; Ding, Y.; Luo, X. A label-free biosensor based on silver nanoparticles array for clinical detection of serum p53 in head and neck squamous cell carcinoma. Int. J. Nanomed. 2011, 6, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Suwa, H.; Ohshio, G.; Okada, N.; Wang, Z.; Fukumoto, M.; Imamura, T.; Imamura, M. Clinical significance of serum p53 antigen in patients with pancreatic carcinomas. Gut 1997, 40, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, D.; Wu, D.; Zhang, Y.; Ma, H.; Du, B.; Wei, Q. Simple synthesis of silver nanoparticles functionalized cuprous oxide nanowires nanocomposites and its application in electrochemical immunosensor. Sens. Actuators B Chem. 2016, 236, 241–248. [Google Scholar] [CrossRef]
- Ortega, F.G.; Fernández-Baldo, M.A.; Serrano, M.J.; Messina, G.A.; Lorente, J.A.; Raba, J. Epithelial cancer biomarker EpCAM determination in peripheral blood samples using a microfluidic immunosensor based in silver nanoparticles as platform. Sens. Actuators B Chem. 2015, 221, 248–256. [Google Scholar] [CrossRef]
- Zhao, L.-J.; Yu, R.-J.; Ma, W.; Han, H.-X.; Tian, H.; Qian, R.-C.; Long, Y.-T. Sensitive detection of protein biomarkers using silver nanoparticles enhanced immunofluorescence assay. Theranostics 2017, 7, 876–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamieson, T.; Bakhshi, R.; Petrova, D.; Pocock, R.; Imani, M.; Seifalian, A.M. Biological applications of quantum dots. Biomaterials 2007, 28, 4717–4732. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Hildebrandt, N. Semiconductor quantum dots for in vitro diagnostics and cellular imaging. Trends Biotechnol. 2012, 30, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Cui, G.; Chen, X.; Yin, H.; Yong, Q.; Xu, L.; Peng, C.; Wang, L.; Xu, C. A One-Step Homogeneous Sandwich Immunosensor for Salmonella Detection Based on Magnetic Nanoparticles (MNPs) and Quantum Dots (QDs). Int. J. Mol. Sci. 2013, 14, 8603. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, M.C.; Herda, L.M.; Polo, E.; Dawson, K.A. In situ characterization of nanoparticle biomolecular interactions in complex biological media by flow cytometry. Nat. Commun. 2016, 7, 13475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Giudice, M.C.; Meder, F.; Polo, E.; Thomas, S.S.; Alnahdi, K.; Lara, S.; Dawson, K.A. Constructing bifunctional nanoparticles for dual targeting: Improved grafting and surface recognition assessment of multiple ligand nanoparticles. Nanoscale 2016, 8, 16969–16975. [Google Scholar] [CrossRef] [PubMed]
- Lara, S.; Alnasser, F.; Polo, E.; Garry, D.; Lo Giudice, M.C.; Hristov, D.R.; Rocks, L.; Salvati, A.; Yan, Y.; Dawson, K.A. Identification of Receptor Binding to the Biomolecular Corona of Nanoparticles. ACS Nano 2017, 11, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.; Dennis, A. Förster Resonance Energy Transfer between Quantum Dot Donors and Quantum Dot Acceptors. Sensors 2015, 15, 13288. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mamedova, N.; Kotov, N.A.; Chen, W.; Studer, J. Antigen/Antibody Immunocomplex from CdTe Nanoparticle Bioconjugates. Nano Lett. 2002, 2, 817–822. [Google Scholar] [CrossRef]
- Peng, F.; Su, Y.; Zhong, Y.; Fan, C.; Lee, S.-T.; He, Y. Silicon Nanomaterials Platform for Bioimaging, Biosensing, and Cancer Therapy. Acc. Chem. Res. 2014, 47, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Madu, C.O.; Lu, Y. Novel diagnostic biomarkers for prostate cancer. J. Cancer 2010, 1, 150–177. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Chu, X.; Shen, G.; Yu, R. A novel electrochemical immunosensor based on colabeled silica nanoparticles for determination of total prostate specific antigen in human serum. Talanta 2008, 76, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors—A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, H.; Javadi, A.; Gong, S. Multifunctional mesoporous silica nanoparticles as labels for the preparation of ultrasensitive electrochemical immunosensors. Biomaterials 2010, 31, 3281–3286. [Google Scholar] [CrossRef] [PubMed]
- Namdari, P.; Daraee, H.; Eatemadi, A. Recent Advances in Silicon Nanowire Biosensors: Synthesis Methods, Properties, and Applications. Nanoscale Res. Lett. 2016, 11, 406. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara, S.; Perez-Potti, A. Applications of Nanomaterials for Immunosensing. Biosensors 2018, 8, 104. https://doi.org/10.3390/bios8040104
Lara S, Perez-Potti A. Applications of Nanomaterials for Immunosensing. Biosensors. 2018; 8(4):104. https://doi.org/10.3390/bios8040104
Chicago/Turabian StyleLara, Sandra, and André Perez-Potti. 2018. "Applications of Nanomaterials for Immunosensing" Biosensors 8, no. 4: 104. https://doi.org/10.3390/bios8040104
APA StyleLara, S., & Perez-Potti, A. (2018). Applications of Nanomaterials for Immunosensing. Biosensors, 8(4), 104. https://doi.org/10.3390/bios8040104





