Raman Spectroscopy and Microscopy Applications in Cardiovascular Diseases: From Molecules to Organs
Abstract
:1. Introduction
2. Raman Scattering Applications for Cardiac Studies
2.1. Principle of Raman Scattering
2.2. Raman Imaging Applications
2.2.1. Raman Spectroscopy for Cardiac Biomarker Detection
Cardiac Biomarkers
2.2.2. Raman Spectroscopy for Cardiac Cells and Cardiac Stem Cells
2.2.3. Raman Spectroscopy for Cardiac Tissues
2.2.4. Raman Spectroscopy for Whole Heart (Organ)
3. Challenges and Future Perspective
Funding
Acknowledgments
Conflicts of Interest
References
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L. Using Raman spectroscopy to characterize biological materials. Nat. Protoc. 2016, 11, 664–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pence, I.; Mahadevan-Jansen, A. Clinical instrumentation and applications of Raman spectroscopy. Chem. Soc. Rev. 2016, 45, 1958–1979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talari, A.C.S.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2015, 50, 46–111. [Google Scholar] [CrossRef]
- Camp, C.H., Jr.; Cicerone, M.T. Chemically sensitive bioimaging with coherent Raman scattering. Nat. Photonics 2015, 9, 295–305. [Google Scholar] [CrossRef]
- Cheng, J.-X.; Xie, X.S. Vibrational spectroscopic imaging of living systems: An emerging platform for biology and medicine. Science 2015, 350, aaa8870. [Google Scholar] [CrossRef] [PubMed]
- Matousek, P.; Stone, N. Development of deep subsurface Raman spectroscopy for medical diagnosis and disease monitoring. Chem. Soc. Rev. 2016, 45, 1794–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghita, A.; Matousek, P.; Stone, N. Exploring the effect of laser excitation wavelength on signal recovery with deep tissue transmission Raman spectroscopy. Analyst 2016, 141, 5738–5746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matousek, P. Spatially offset Raman spectroscopy for non-invasive analysis of turbid samples. TrAC Trends Anal. Chem. 2018. [Google Scholar] [CrossRef]
- Ong, Y.H.; Lim, M.; Liu, Q. Comparison of principal component analysis and biochemical component analysis in Raman spectroscopy for the discrimination of apoptosis and necrosis in K562 leukemia cells. Opt. Express 2012, 20, 22158–22171. [Google Scholar] [CrossRef] [PubMed]
- MacRitchie, N.; Grassia, G.; Noonan, J.; Garside, P.; Graham, D.; Maffia, P. Molecular imaging of atherosclerosis: Spotlight on Raman spectroscopy and surface-enhanced Raman scattering. Heart 2018, 104, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Kostamovaara, J.; Tenhunen, J.; Kögler, M.; Nissinen, I.; Nissinen, J.; Keränen, P. Fluorescence suppression in Raman spectroscopy using a time-gated CMOS SPAD. Opt. Express 2013, 21, 31632–31645. [Google Scholar] [CrossRef] [PubMed]
- Knorr, F.; Smith, Z.J.; Wachsmann-Hogiu, S. Development of a time-gated system for Raman spectroscopy of biological samples. Opt. Express 2010, 18, 20049–20058. [Google Scholar] [CrossRef] [PubMed]
- Kallaway, C.; Almond, L.M.; Barr, H.; Wood, J.; Hutchings, J.; Kendall, C.; Stone, N. Advances in the clinical application of Raman spectroscopy for cancer diagnostics. Photodiagn. Photodyn. Ther. 2013, 10, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Siddhanta, S.; Narayana, C. Surface enhanced Raman spectroscopy of proteins: Implications in drug designing. Nanomater. Nanotechnol. 2012, 2. [Google Scholar] [CrossRef]
- Feng, S.; Lin, D.; Lin, J.; Li, B.; Huang, Z.; Chen, G.; Zhang, W.; Wang, L.; Pan, J.; Chen, R. Blood plasma surface-enhanced Raman spectroscopy for non-invasive optical detection of cervical cancer. Analyst 2013, 138, 3967–3974. [Google Scholar] [CrossRef] [PubMed]
- González-Solís, J.L.; Martínez-Espinosa, J.C.; Torres-González, L.A.; Aguilar-Lemarroy, A.; Jave-Suárez, L.F.; Palomares-Anda, P. Cervical cancer detection based on serum sample Raman spectroscopy. Lasers Med Sci. 2014, 29, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Lyng, F.M.; Traynor, D.; Ramos, I.R.; Bonnier, F.; Byrne, H.J. Raman spectroscopy for screening and diagnosis of cervical cancer. Anal. Bioanal. Chem. 2015, 407, 8279–8289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lui, H.; Zhao, J.; McLean, D.I.; Zeng, H. Real-time Raman spectroscopy for in vivo skin cancer diagnosis. Cancer Res. 2012. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.A.; Kirshnamoorthi, H.; Ellis, D.L.; van Leeuwen, T.G.; Mahadevan-Jansen, A. A clinical instrument for combined Raman spectroscopy-optical coherence tomography of skin cancers. Lasers Surg. Med. 2011, 43, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lui, H.; Kalia, S.; Zeng, H. Real-time Raman spectroscopy for automatic in vivo skin cancer detection: An independent validation. Anal. Bioanal. Chem. 2015, 407, 8373–8379. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Wang, J.; Zheng, W.; Ho, K.Y.; Teh, M.; Yeoh, K.G.; Huang, Z. Rapid fiber-optic Raman spectroscopy for real-time in vivo detection of gastric intestinal metaplasia during clinical gastroscopy. Cancer Prev. Res. 2016, 9, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Tay, L.-L.; Tremblay, R.G.; Hulse, J.; Zurakowski, B.; Thompson, M.; Bani-Yaghoub, M. Detection of acute brain injury by Raman spectral signature. Analyst 2011, 136, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- El-Said, W.A.; Fouad, D.M.; El-Safty, S.A. Ultrasensitive label-free detection of cardiac biomarker myoglobin based on surface-enhanced Raman spectroscopy. Sens. Actuators B Chem. 2016, 228, 401–409. [Google Scholar] [CrossRef]
- Kumar, V.; Brent, J.R.; Shorie, M.; Kaur, H.; Chadha, G.; Thomas, A.G.; Lewis, E.A.; Rooney, A.P.; Nguyen, L.; Zhong, X.L. Nanostructured aptamer-functionalized black phosphorus sensing platform for label-free detection of myoglobin, a cardiovascular disease biomarker. ACS Appl. Mater. Interfaces 2016, 8, 22860–22868. [Google Scholar] [CrossRef] [PubMed]
- Donofrio, M.T.; Moon-Grady, A.J.; Hornberger, L.K.; Copel, J.A.; Sklansky, M.S.; Abuhamad, A.; Cuneo, B.F.; Huhta, J.C.; Jonas, R.A.; Krishnan, A. Diagnosis and treatment of fetal cardiac disease: A scientific statement from the American Heart Association. Circulation 2014, 129, 2183–2242. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.F.; Collins, R.A.; Anderson, A.J.; Hess, M.; Farley, I.M.; Hagemann, D.A.; Harkins, H.J.; Zwicke, D. Value of serial myoglobin levels in the early diagnosis of patients admitted for acute myocardial infarction. Ann. Emerg. Med. 1994, 24, 704–708. [Google Scholar] [CrossRef]
- Tang, J.; Guo, H.; Zhao, M.; Liu, W.; Chou, X.; Zhang, B.; Xue, C.; Zhang, W.; Liu, J. Ag nanoparticles cladded with parylene for high-stability microfluidic surface-enhanced Raman scattering (SERS) biochemical sensing. Sens. Actuators B Chem. 2017, 242, 1171–1176. [Google Scholar] [CrossRef]
- Myers, R.W.; Guan, H.-P.; Ehrhart, J.; Petrov, A.; Prahalada, S.; Tozzo, E.; Yang, X.; Kurtz, M.M.; Trujillo, M.; Gonzalez Trotter, D.; et al. Systemic pan-AMPK activator MK-8722 improves glucose homeostasis but induces cardiac hypertrophy. Science 2017, 357, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, I.; Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2016, 97, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.K.; Bernardo, B.C.; Ooi, J.Y.Y.; Weeks, K.L.; McMullen, J.R. Pathophysiology of cardiac hypertrophy and heart failure: Signaling pathways and novel therapeutic targets. Arch. Toxicol. 2015, 89, 1401–1438. [Google Scholar] [CrossRef] [PubMed]
- Saliminasab, M.; Bahrampour, A.; Zandi, M.H. Human cardiac troponin I sensor based on silver nanoparticle doped microsphere resonator. J. Opt. 2012, 14, 122301. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, L.; Liu, B.; Ni, H.; Sun, L.; Su, E.; Chen, H.; Gu, Z.; Zhao, X. Quantitative and ultrasensitive detection of multiplex cardiac biomarkers in lateral flow assay with core-shell SERS nanotags. Biosens. Bioelectron. 2018, 106, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Kallepitis, C.; Bergholt, M.S.; Mazo, M.M.; Leonardo, V.; Skaalure, S.C.; Maynard, S.A.; Stevens, M.M. Quantitative volumetric Raman imaging of three dimensional cell cultures. Nat. Commun. 2017, 8, 14843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, R.; Wright, K.L.; Ashton, L. Raman spectroscopy: An evolving technique for live cell studies. Analyst 2016, 141, 3590–3600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almohammedi, A.; Kapetanaki, S.; Wood, B.; Raven, E.L.; Storey, N.; Hudson, A.J. Spectroscopic analysis of myoglobin and cytochrome c dynamics in isolated cardiomyocytes during hypoxia and reoxygenation. J. R. Soc. Interface 2015, 12, 20141339. [Google Scholar] [CrossRef] [PubMed]
- Pascut, F.C.; Kalra, S.; George, V.; Welch, N.; Denning, C.; Notingher, I. Non-invasive label-free monitoring the cardiac differentiation of human embryonic stem cells in-vitro by Raman spectroscopy. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3517–3524. [Google Scholar] [CrossRef] [PubMed]
- Brazhe, N.A.; Treiman, M.; Brazhe, A.R.; Maksimov, G.V.; Sosnovtseva, O.V. Mapping of redox state of mitochondrial cytochromes in live cardiomyocytes using Raman microspectroscopy. PLoS ONE 2012, 7, e41990. [Google Scholar] [CrossRef] [PubMed]
- Ohira, S.; Tanaka, H.; Harada, Y.; Minamikawa, T.; Kumamoto, Y.; Matoba, S.; Yaku, H.; Takamatsu, T. Label-free detection of myocardial ischaemia in the perfused rat heart by spontaneous Raman spectroscopy. Sci. Rep. 2017, 7, 42401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, T.; Minamikawa, T.; Harada, Y.; Yamaoka, Y.; Tanaka, H.; Yaku, H.; Takamatsu, T. Label-free evaluation of myocardial infarct in surgically excised ventricular myocardium by Raman spectroscopy. arXiv 2018, arXiv:1806.05333. [Google Scholar] [CrossRef] [PubMed]
- Nishiki-Muranishi, N.; Harada, Y.; Minamikawa, T.; Yamaoka, Y.; Dai, P.; Yaku, H.; Takamatsu, T. Label-free evaluation of myocardial infarction and its repair by spontaneous Raman spectroscopy. Anal. Chem. 2014, 86, 6903–6910. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Irmak, S.; Lu, Y.; Pipinos, I.; Casale, G.; Subbiah, J. Spontaneous and coherent anti-Stokes Raman spectroscopy of human gastrocnemius muscle biopsies in CH-stretching region for discrimination of peripheral artery disease. Biomed. Opt. Express 2015, 6, 2766–2777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salenius, J.P.; Brennan, J.F.; Miller, A.; Wang, Y.; Aretz, T.; Sacks, B.; Dasari, R.R.; Feld, M.S. Biochemical composition of human peripheral arteries examined with nearinfrared Raman spectroscopy. J. Vasc. Surg. 1998, 27, 710–719. [Google Scholar] [CrossRef] [Green Version]
- Cluff, K.; Kelly, A.M.; Koutakis, P.; He, X.N.; Huang, X.; Lu, Y.F.; Pipinos, I.I.; Casale, G.P.; Subbiah, J. Surface-enhanced Raman spectral biomarkers correlate with Ankle Brachial Index and characterize leg muscle biochemical composition of patients with peripheral arterial disease. Physiol. Rep. 2014, 2, e12148. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, A.; Bonifacio, A.; Della Mora, A.; Livi, U.; Marchini, M.; Ortolani, F. Carotenoids co-localize with hydroxyapatite, cholesterol, and other lipids in calcified stenotic aortic valves. Ex vivo Raman maps compared to histological patterns. Eur. J. Histochem. EJH 2015, 59, 2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilarczyk, M.; Czamara, K.; Baranska, M.; Natorska, J.; Kapusta, P.; Undas, A.; Kaczor, A. Calcification of aortic human valves studied in situ by Raman microimaging: Following mineralization from small grains to big deposits. J. Raman Spectrosc. 2013, 44, 1222–1229. [Google Scholar] [CrossRef]
- You, A.Y.; Bergholt, M.S.; St-Pierre, J.-P.; Kit-Anan, W.; Pence, I.J.; Chester, A.H.; Yacoub, M.H.; Bertazzo, S.; Stevens, M.M. Raman spectroscopy imaging reveals interplay between atherosclerosis and medial calcification in the human aorta. Sci. Adv. 2017, 3, e1701156. [Google Scholar] [CrossRef] [PubMed]
- Brazhe, N.A.; Treiman, M.; Faricelli, B.; Vestergaard, J.H.; Sosnovtseva, O. In situ Raman study of redox state changes of mitochondrial cytochromes in a perfused rat heart. PLoS ONE 2013, 8, e70488. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Harada, Y.; Yamaoka, Y.; Fujita, K.; Yaku, H.; Takamatsu, T. Label-free biochemical imaging of heart tissue with high-speed spontaneous Raman microscopy. Biochem. Biophys. Res. Commun. 2009, 382, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Fritzsche, W.; Popp, J. Optical Nano-and Microsystems for Bioanalytics; Springer Science & Business Media: Berlin, Germany, 2012; Volume 10. [Google Scholar]
- Siebert, F.; Hildebrandt, P. Theory of infrared absorption and Raman spectroscopy. Vib. Spectrosc. Life Sci. 2008, 11–16. [Google Scholar]
- Mohammed, A. Theoretical Studies of Raman Scattering. Ph.D. Thesis, KTH, Stockholm, Sweden, 2011. [Google Scholar]
- Lewis, I.R.; Edwards, H. Handbook of Raman Spectroscopy: From the Research Laboratory to the Process Line; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Szymanski, H.A. Raman Spectroscopy: Theory and Practice; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Musunuru, K.; Domian, I.J.; Chien, K.R. Stem cell models of cardiac development and disease. Annu. Rev. Cell Dev. Boil. 2010, 26, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Ghita, A.; Pascut, F.C.; Sottile, V.; Denning, C.; Notingher, I. Applications of Raman micro-spectroscopy to stem cell technology: Label-free molecular discrimination and monitoring cell differentiation. EPJ Tech. Instrum. 2015, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Rudehill, A.; Sundqvist, K.; Sylven, C. QT and QT-peak interval measurements. A methodological study in patients with subarachnoid haemorrhage compared to a reference group. Clin. Physiol. 1986, 6, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Mahato, K.; Prasad, A.; Maurya, P.; Chandra, P. Nanobiosensors: Next generation point-of-care biomedical devices for personalized diagnosis. J. Anal. Bioanal. Tech. 2016, 7, e125. [Google Scholar]
- Frohlich, M.; Grayson, W.L.; Wan, L.Q.; Marolt, D.; Drobnic, M.; Vunjak-Novakovic, G. Tissue engineered bone grafts: Biological requirements, tissue culture and clinical relevance. Curr. Stem Cell Res. Ther. 2008, 3, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto-Ida, M.; Akao, M.; Takeda, T.; Kato, M.; Kita, T. Real-time 2-photon imaging of mitochondrial function in perfused rat hearts subjected to ischemia/reperfusion. Circulation 2006, 114, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y.; Shimizu, S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 2007, 12, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Lesnefsky, E.J.; Gudz, T.I.; Migita, C.T.; Ikeda-Saito, M.; Hassan, M.O.; Turkaly, P.J.; Hoppel, C.L. Ischemic injury to mitochondrial electron transport in the aging heart: Damage to the iron–sulfur protein subunit of electron transport complex III. Arch. Biochem. Biophys. 2001, 385, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Vivaldi, M.; Kloner, R.; Schoen, F. Triphenyltetrazolium staining of irreversible ischemic injury following coronary artery occlusion in rats. Am. J. Pathol. 1985, 121, 522. [Google Scholar] [PubMed]
- Almohammedi, A.; Kapetanaki, S.M.; Hudson, A.J.; Storey, N.M. Monitoring changes in the redox state of myoglobin in cardiomyocytes by Raman spectroscopy enables the protective effect of NO donors to be evaluated. Anal. Chem. 2015, 87, 10605–10612. [Google Scholar] [CrossRef] [PubMed]
- St-Arnaud, K.; Aubertin, K.; Strupler, M.; Madore, W.-J.; Grosset, A.-A.; Petrecca, K.; Trudel, D.; Leblond, F. Development and characterization of a handheld hyperspectral Raman imaging probe system for molecular characterization of tissue on mesoscopic scales. Med. Phys. 2018, 45, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Frontiera, R.R.; Henry, A.-I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Premasiri, W.R.; Lee, J.C.; Sauer-Budge, A.; Théberge, R.; Costello, C.E.; Ziegler, L.D. The biochemical origins of the surface-enhanced Raman spectra of bacteria: A metabolomics profiling by SERS. Anal. Bioanal. Chem. 2016, 408, 4631–4647. [Google Scholar] [CrossRef] [PubMed]
- Freudiger, C.W.; Yang, W.; Holtom, G.R.; Peyghambarian, N.; Xie, X.S.; Kieu, K.Q. Stimulated Raman scattering microscopy with a robust fibre laser source. Nat. Photonics 2014, 8, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duboisset, J.; Berto, P.; Gasecka, P.; Bioud, F.-Z.; Ferrand, P.; Rigneault, H.; Brasselet, S. Molecular Orientational Order Probed by Coherent Anti-Stokes Raman Scattering (CARS) and Stimulated Raman Scattering (SRS) Microscopy: A Spectral Comparative Study. J. Phys. Chem. B 2015, 119, 3242–3249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhar, N.; Sil, S.; Verma, T.; Umapathy, S. Challenges in application of Raman spectroscopy to biology and materials. RSC Adv. 2018, 8, 25888–25908. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Sil, S.; Umapathy, S. Raman spectroscopy explores molecular structural signatures of hidden materials in depth: Universal Multiple Angle Raman Spectroscopy. Sci. Rep. 2014, 4, 5308. [Google Scholar] [CrossRef] [PubMed]
- Chrimes, A.F.; Khoshmanesh, K.; Stoddart, P.R.; Mitchell, A.; Kalantar-zadeh, K. Microfluidics and Raman microscopy: Current applications and future challenges. Chem. Soc. Rev. 2013, 42, 5880–5906. [Google Scholar] [CrossRef] [PubMed]



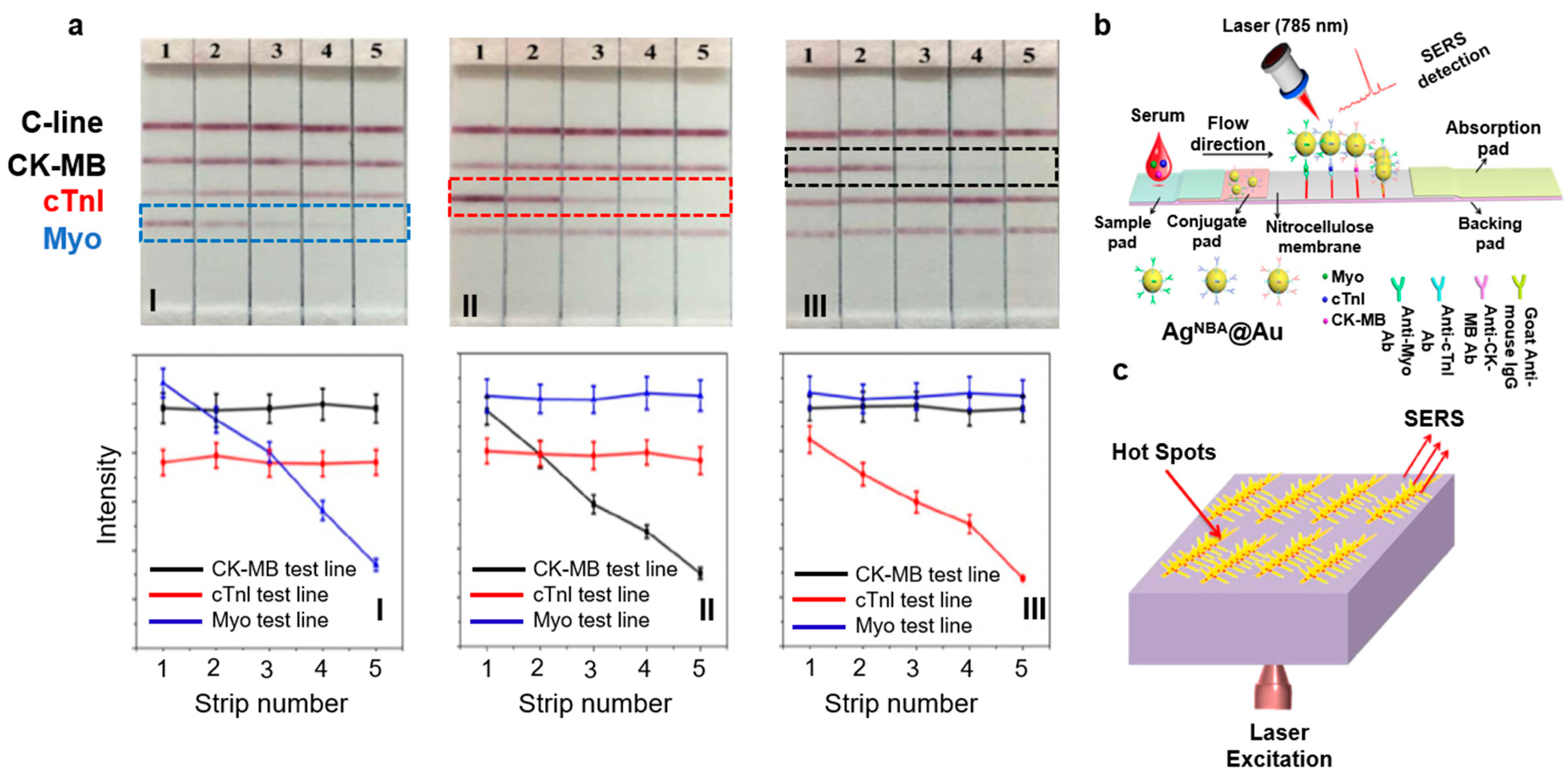




| Category | Types | Findings | Reference |
|---|---|---|---|
| Biomarkers | cTnI | Detection of cTnI molecules after 3–4 h of stroke with ~1.3 ng/mL concentration. cTnI is adsorbed onto AgNPs to generate LSPR enhanced Raman signals. | [31] |
| Myoglobin, cTnI, and CK-MB | LFA on paper microfluidics by immobilizing NPs encapsulated with Raman dyes. LOD for myoglobin was 50 ng/mL, cTnI and CK-MB were 10 ng/mL. | [32] | |
| Myoglobin | SERS-based myoglobin sensor based on Ag NPT/ITO substrate. LOD was 10 ng/mL. | [23] | |
| Cardiac cells |
| Confocal Raman spectroscopy was used to study cell cytology. CMhiPSCs displayed cardiomyocyte-like colonies. rCMadult displayed elongated rod-like shapes and sarcomeres. | [33] |
| Cardiomyocytes from rat | Raman spectrometer coupled with a charge-coupled device (CCD) of the camera was used to visualize, image, map, and collect the Raman spectra of the cells. | [34] | |
| Raman microspectroscopy (RMS) was used to evaluate NO release at the single-cell level. | [35] | ||
| hESCs differentiated into cardiomyocytes | Raman microspectroscopy was used to study the fate of cardiomyocytes and acquire spectra from the beating embryoid bodies. | [36] | |
| Cardiomyocytes | Raman microspectroscopy was used to identify redox mitochondrial states and create a map to distinguish between rod- and round-shaped cardiomyocytes. | [37] | |
| Tissues | Subepicardial myocardial tissue | Raman microscopy was used for label-free evaluation of mitochondrial membrane and reduced cytochromes in early myocardial ischemic phase. | [38] |
| Ischemic myocardial tissue | Label-free Raman spectroscopy was used to study infarcted and noninfarcted regions from five patients who suffered a stroke. | [39] | |
| Myocardium infarcted tissue | Spontaneous Raman spectroscopy was used to identify the five sequential stages of myocardial infarcted tissue. | [40] | |
| In vivo | Atherosclerosis | SERRS was used to study aortic sinus tissues by tagging with intercellular adhesion molecule-1 (ICAM1) protein attached to gold nanoparticles. | [10] |
| Ex vivo | Atherosclerosis | Spontaneous and coherent anti-Stokes Raman scattering (CARS) was used to study healthy and diseased tissues from biopsies of human gastrocnemius peripheral arterial disease (PAD) and control groups. | [41] |
| Near-infrared Raman spectroscopy was used to evaluate lipid (cholesterol) and calcium salt content in human peripheral arteries. | [42] | ||
| Raman spectroscopy was used to acquire spectra from skeletal muscle of PAD versus control. | [43] | ||
| Raman spectroscopy was used to study stenotic aortic valves to monitor mineral deposits, and cholesterol and lipid levels. | [44,45] | ||
| SERS was used to identify plaques in blocked arteries. | [10] | ||
| Raman spectroscopy was used to study cardiovascular calcification. | [46] | ||
| Whole heart | Raman spectroscopy was used to study the reduction state of mitochondrial cytochromes and myoglobin oxygenation at infarct sites of whole rat hearts. | [47] | |
| Raman confocal microscope integrated with a slit-scanning apparatus was used to acquire spectra from whole rat hearts. | [48] |
| Marker | <6 h | 6–12 h | 12–24 h | 24–48 h | >48 h |
|---|---|---|---|---|---|
| Myoglobin | + + + | + | - | - | - |
| Troponin I | + | + + | + + + | + + + | + + + |
| Troponin T | + | + + | + + + | + + + | + + + |
| CK-MB | + | + + | + + + | - | - |
| MB-isoforms | + + | + + + | + | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaichi, A.; Prasad, A.; Gartia, M.R. Raman Spectroscopy and Microscopy Applications in Cardiovascular Diseases: From Molecules to Organs. Biosensors 2018, 8, 107. https://doi.org/10.3390/bios8040107
Chaichi A, Prasad A, Gartia MR. Raman Spectroscopy and Microscopy Applications in Cardiovascular Diseases: From Molecules to Organs. Biosensors. 2018; 8(4):107. https://doi.org/10.3390/bios8040107
Chicago/Turabian StyleChaichi, Ardalan, Alisha Prasad, and Manas Ranjan Gartia. 2018. "Raman Spectroscopy and Microscopy Applications in Cardiovascular Diseases: From Molecules to Organs" Biosensors 8, no. 4: 107. https://doi.org/10.3390/bios8040107
APA StyleChaichi, A., Prasad, A., & Gartia, M. R. (2018). Raman Spectroscopy and Microscopy Applications in Cardiovascular Diseases: From Molecules to Organs. Biosensors, 8(4), 107. https://doi.org/10.3390/bios8040107







