Enzymatic Low Volume Passive Sweat Based Assays for Multi-Biomarker Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
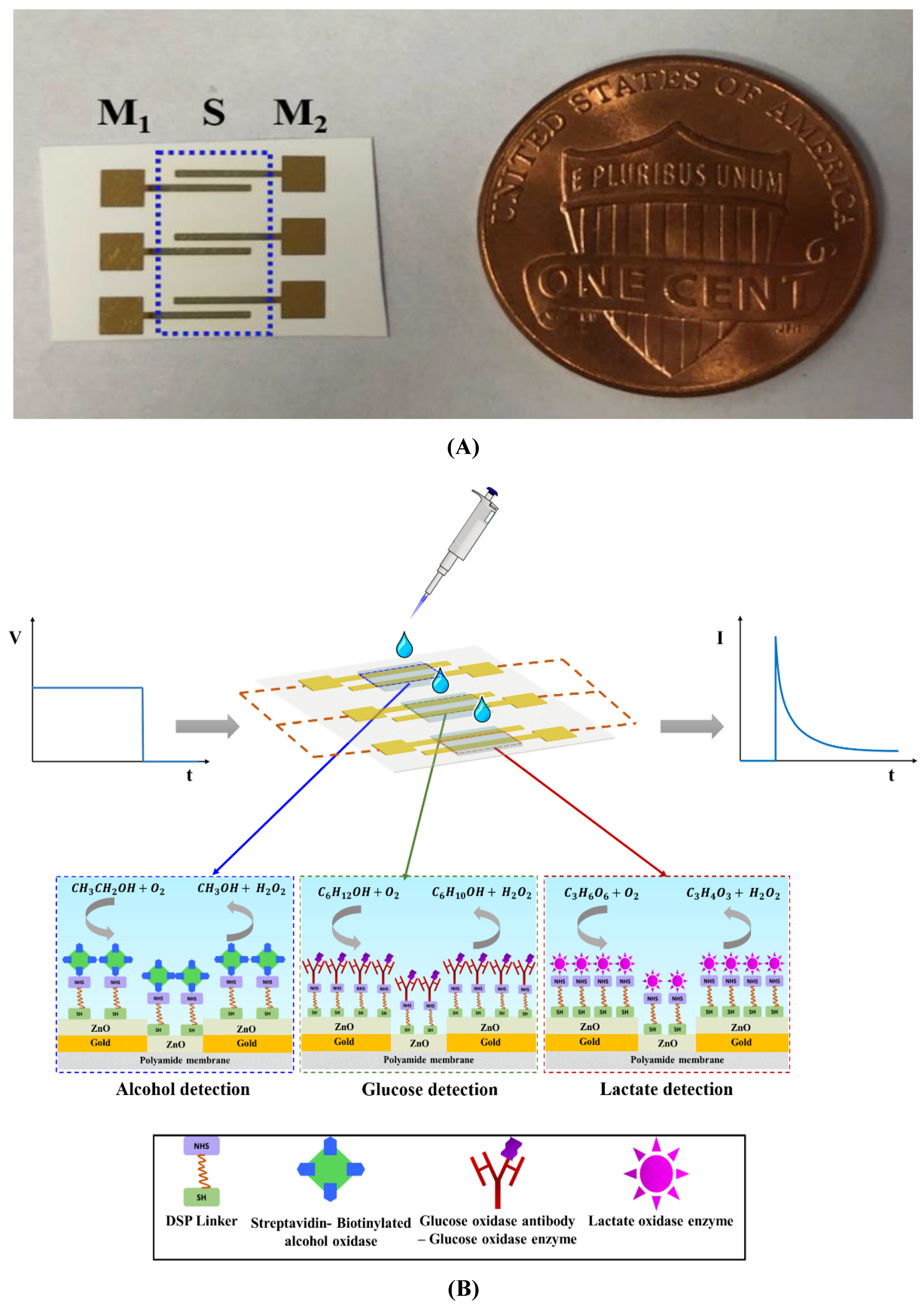
2.2. Sensor Fabrication
2.3. Alcohol Biosensor Calibration in Synthetic Sweat pH 6 and Human Sweat
2.4. Glucose Biosensor Calibration in Synthetic Sweat pH 6 and Human Sweat
2.5 Lactate Biosensor Calibration in Synthetic Sweat pH 6 and Human Sweat
2.6. Specificity Study in Synthetic Sweat pH 6
2.7. Continuous Monitoring in Synthetic Sweat pH 6
3. Results and Discussion
3.1. Non-Faradaic Chronoamperometry as a Technique for Evaluating the Biosensing Performance
3.2. Fluid Wicking Study and Electrical Characterization of the Surface Functionalized Biosensor
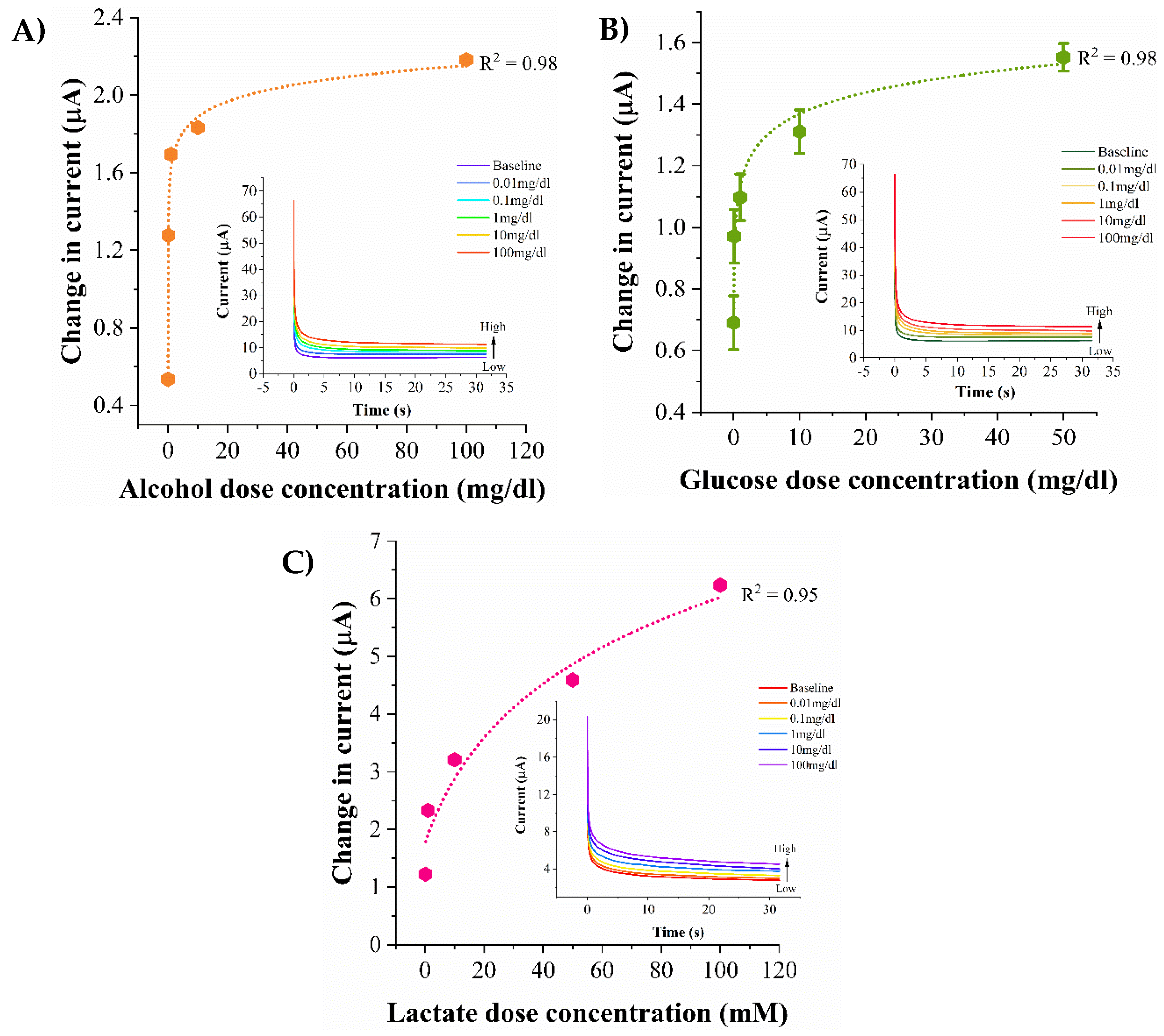
3.3. Biosensor Calibration in pH Variant Synthetic Sweat
3.4. Biosensor Calibration in Human Eccrine Sweat
3.5. Evaluation of Sensor Specificity in Synthetic Sweat pH 6
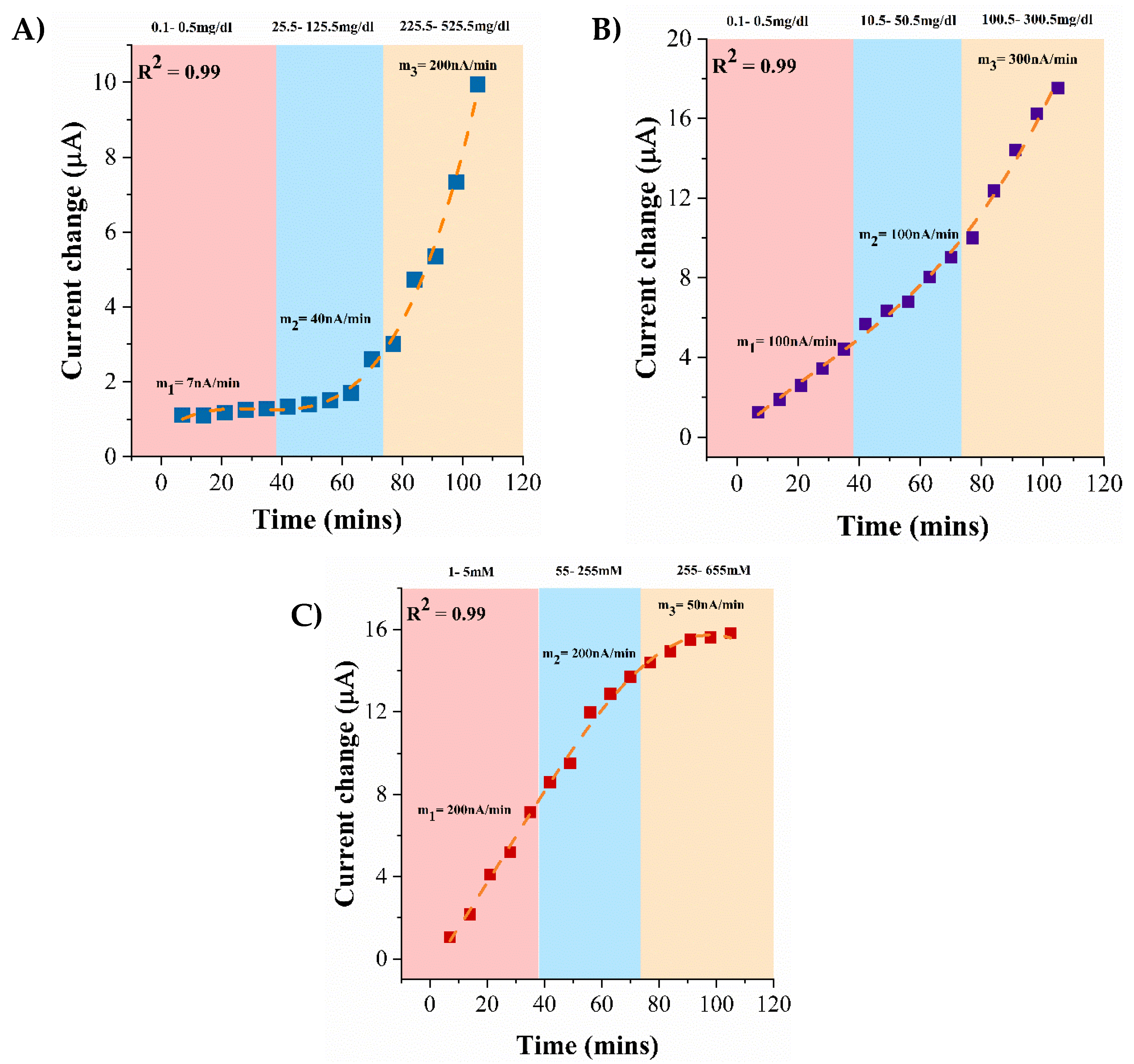
3.6. Continuous Monitoring of Lifestyle Biomarkers in Synthetic Sweat pH 6
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wade, J. Wearable Technology Statistics and Trends 2018. Smart Insights. Available online: https://www.smartinsights.com/digital-marketing-strategy/wearables-statistics-2017/ (accessed on 15 November 2017).
- Piwek, L.; Ellis, D.A.; Andrews, S.; Joinson, A. The Rise of Consumer Health Wearables: Promises and Barriers. PLoS Med. 2016, 13, e1001953. [Google Scholar] [CrossRef] [PubMed]
- Swan, M. Sensor Mania! The Internet of Things, Wearable Computing, Objective Metrics, and the Quantified Self 2.0. J. Sens. Actuator Netw. 2012, 1, 217–253. [Google Scholar] [CrossRef] [Green Version]
- Kovalchick, C.; Sirkar, R.; Regele, O.B.; Kourtis, L.C.; Schiller, M.; Wolpert, H.; Alden, R.G.; Jones, G.B.; Wright, J.M. Can composite digital monitoring biomarkers come of age? A framework for utilization. J. Clin. Transl. Sci. 2017, 1, 373–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, S.; Robinson, A.H. Chemical Composition of Sweat. Physiol. Rev. 1954, 34, 202–220. [Google Scholar] [CrossRef] [PubMed]
- Moyer, J.; Wilson, D.; Finkelshtein, I.; Wong, B.; Potts, R. Correlation between sweat glucose and blood glucose in subjects with diabetes. Diabetes Technol. Ther. 2012, 14, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Gamella, M.; Campuzano, S.; Manso, J.; Rivera, G.G.; Lopez-Colino, F.; Reviejo, A.J.; Pingarron, J.M. A novel non-invasive electrochemical biosensing device for in situ determination of the alcohol content in blood by monitoring ethanol in sweat. Anal. Chim. Acta 2014, 806, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sakharov, D.A.; Shkurnikov, M.U.; Vagin, M.Y.; Yashina, E.I.; Karyakin, A.A.; Tonevitsky, A.G. Relationship between Lactate Concentrations in Active Muscle Sweat and Whole Blood. Bull. Exp. Biol. Med. 2010, 150, 83–85. [Google Scholar] [CrossRef]
- Bhide, A.; Muthukumar, S.; Saini, A.; Prasad, S. Simultaneous lancet-free monitoring of alcohol and glucose from low-volumes of perspired human sweat. Sci. Rep. 2018, 8, 6507. [Google Scholar] [CrossRef]
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical Tattoo Biosensors for Real-Time Noninvasive Lactate Monitoring in Human Perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anastasova, S.; Crewther, B.; Bembnowicz, P.; Curto, V.; Ip, H.M.; Rosa, B.; Yang, G.Z. A wearable multisensing patch for continuous sweat monitoring. Biosens. Bioelectron. 2017, 93, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Devasenathipathy, R.; Chen, S.; Ajmal Ali, M.; Karuppiah, C.; Balakumar, V.; Prakash, P.; Elshikh, M.S.; Al-Hemaid, F. Direct Electrochemistry of Glucose Oxidase at Reduced Graphene Oxide and β-Cyclodextrin Composite Modified Electrode and Application for Glucose Biosensing. Electroanalysis 2015, 27, 2412–2420. [Google Scholar] [CrossRef]
- Kim, J.; Jeerapan, I.; Imani, S.; Cho, T.N.; Bandodkar, A.; Cinti, S.; Mercier, P.P.; Wang, J. Noninvasive Alcohol Monitoring Using a Wearable Tattoo-Based Iontophoretic-Biosensing System. ACS Sens. 2016, 1, 1011–1019. [Google Scholar] [CrossRef]
- Babor, T.; Rehm, J.; Jernigan, D.; Vaeth, P.; Monteiro, M.; Lehman, H. Alcohol, diabetes, and public health in the Americas. Revista Panamericana De Salud Pública 2012, 32, 151. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, N.V.; Swade, T.F.; Emanuele, M.A. Consequences of alcohol use in diabetics. Alcohol Health Res. World 1998, 22, 211–219. [Google Scholar] [PubMed]
- Adelsmayr, G.; Brunner, R.; Holzinger, U. Impact of blood glucose on blood lactate levels in a medical ICU: A retrospective cohort study. Crit. Care 2012, 16, P165. [Google Scholar] [CrossRef]
- Mizock, B.A.; Falk, J.L. Lactic acidosis in critical illness. Crit. Care Med. 1992, 20, 80–93. [Google Scholar] [CrossRef]
- Mathew, M.T.; Ariza, E.; Rocha, L.A.; Fernandes, A.C.; Vaz, F. TiCxOy thin films for decorative applications: Tribocorrosion mechanisms and synergism. Tribol. Int. 2008, 41, 603–615. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Anzai, J.; Osa, T.; Motohashi, R. Amperometric alcohol sensors based on protein multilayers composed of avidin and biotin-labeled alcohol oxidase. Electroanalysis 1996, 8, 813–816. [Google Scholar] [CrossRef]
- Munje, R.D.; Muthukumar, S.; Prasad, S. Lancet-free and label-free diagnostics of glucose in sweat using Zinc Oxide based flexible bioelectronics. Sens. Actuators B Chem. 2017, 238, 482–490. [Google Scholar] [CrossRef]
- Scholz, F. Voltammetric techniques of analysis: The essentials. ChemTexts 2015, 1, 17. [Google Scholar] [CrossRef]
- Munje, R.D.; Muthukumar, S.; Selvam, A.P.; Prasad, S. Flexible nanoporous tunable electrical double layer biosensors for sweat diagnostics. Sci. Rep. 2015, 5, 14586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonner, Z.; Wilder, E.; Heikenfeld, J.; Kasting, G.; Beyette, F.; Swaile, D.; Sherman, F.; Joyce, J.; Hagen, J.; Kelley-Loughnane, N.; et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics 2015, 9, 031301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omar, F.M.; Aziz, H.A.; Stoll, S. Aggregation and disaggregation of ZnO nanoparticles: Influence of pH and adsorption of Suwannee River humic acid. Sci. Total Environ. 2014, 468–469, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, S.; Nikkanen, J.; Huttunen-Saarivirta, E.; Levänen, E. Investigation of long-term chemical stability of structured ZnO films in aqueous solutions of varying conditions. Thin Solid Films 2017, 638, 410–419. [Google Scholar] [CrossRef]
- Armbruster, D.A.; Pry, T. Limit of Blank, Limit of Detection and Limit of Quantitation. Clin. Biochem. Rev. 2008, 29, S52. [Google Scholar]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhide, A.; Cheeran, S.; Muthukumar, S.; Prasad, S. Enzymatic Low Volume Passive Sweat Based Assays for Multi-Biomarker Detection. Biosensors 2019, 9, 13. https://doi.org/10.3390/bios9010013
Bhide A, Cheeran S, Muthukumar S, Prasad S. Enzymatic Low Volume Passive Sweat Based Assays for Multi-Biomarker Detection. Biosensors. 2019; 9(1):13. https://doi.org/10.3390/bios9010013
Chicago/Turabian StyleBhide, Ashlesha, Sarah Cheeran, Sriram Muthukumar, and Shalini Prasad. 2019. "Enzymatic Low Volume Passive Sweat Based Assays for Multi-Biomarker Detection" Biosensors 9, no. 1: 13. https://doi.org/10.3390/bios9010013
APA StyleBhide, A., Cheeran, S., Muthukumar, S., & Prasad, S. (2019). Enzymatic Low Volume Passive Sweat Based Assays for Multi-Biomarker Detection. Biosensors, 9(1), 13. https://doi.org/10.3390/bios9010013





