Casein-Conjugated Gold Nanoparticles for Amperometric Detection of Leishmania infantum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatuses
2.2. Leishmania Parasite Culture
2.3. AuNPs Preparation and Functionalization
2.4. Incubation of the Parasites with casein@AuNPs and Leishmania Quantification
2.5. Electrochemical Assessment of the Effect of Amphotericin B Pretreatment on Leishmania-casein Interaction
3. Results and Discussion
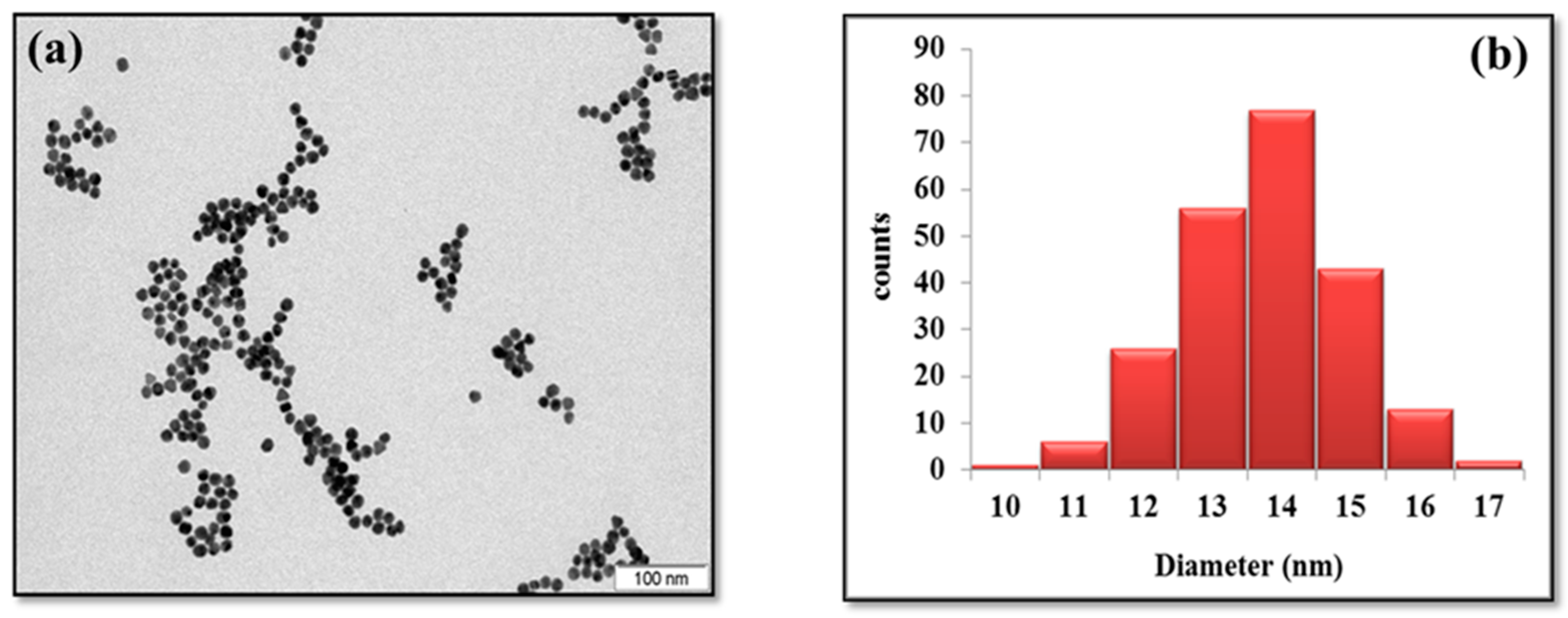
3.1. Characterization of Pure and casein-Capped AuNPs
3.2. Electrocatalytic Activity of AuNPs Towards Hydrogen Ions Reduction
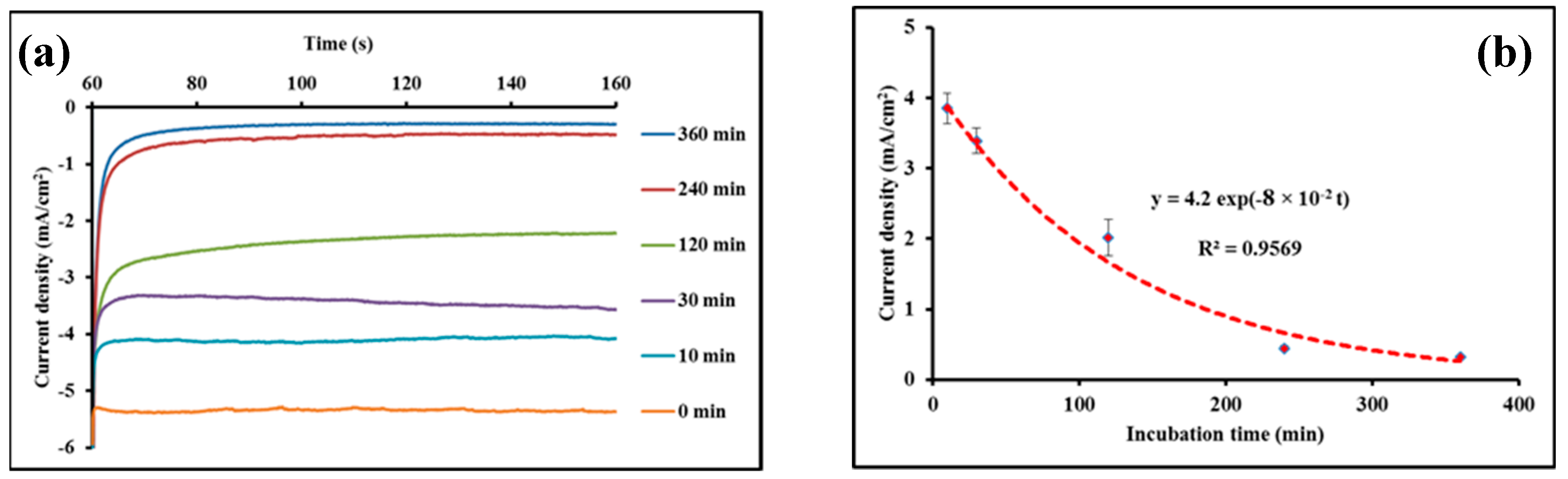
3.3. Detection of Leishmania Parasites
3.4. Effect of the AmB Treatment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alvar, J.; Cañavate, C.; Molina, R.; Moreno, J.; Nieto, J. Canine Leishmaniasis. In Advances in Parasitology; Elsevier: Amsterdam, The Netherlands, 2004; Volume 57, pp. 1–88. ISBN 978-0-12-031757-8. [Google Scholar]
- World Health Organization. Sustaining the Drive to Overcome the Global Impact of Neglected Tropical Diseases: Second Who Report on Neglected Tropical Diseases; World Health Organization: Geneva, Switzerland, 2013; ISBN 978-92-4-156454-0. [Google Scholar]
- Kallel, K.; Pratlong, F.; Haouas, N.; Kaouech, E.; Belhadj, S.; Anane, S.; Dedet, J.P.; Babba, H.; Chaker, E. Isoenzymatic variability of Leishmania infantum in Tunisia concerning 254 human strains. Acta Trop. 2008, 106, 132–136. [Google Scholar] [CrossRef]
- Ndao, M. Diagnosis of Parasitic Diseases: Old and New Approaches. Interdiscip Perspect Infect Dis. 2009, 2009, 1–15. [Google Scholar] [CrossRef] [PubMed]
- de Paiva-Cavalcanti, M.; de Morais, R.C.S.; Pessoa-e-Silva, R.; Trajano-Silva, L.A.M.; da Cunha Gonçalves-de-Albuquerque, S.; de Hollanda Cavalcanti Tavares, D.; Brelaz-de-Castro, M.C.A.; de Freitas e Silva, R.; Pereira, V.R.A. Leishmaniases diagnosis: an update on the use of immunological and molecular tools. Cell Biosci. 2015, 5. [Google Scholar] [CrossRef]
- Travi, B.L.; Cordeiro-da-Silva, A.; Dantas-Torres, F.; Miró, G. Canine visceral leishmaniasis: Diagnosis and management of the reservoir living among us. PLoS Negl. Trop. Dis. 2018, 12, e0006082. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.; Saliba, J.W.; Oliveira, D.; Dias, E.S.; Paz, G.F. A prototype of the direct agglutination test kit (DAT-Canis) for the serological diagnosis of canine visceral leishmaniasis. Vet. Parasitol. 2016, 221, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Reithinger, R.; Dujardin, J.-C.; Louzir, H.; Pirmez, C.; Alexander, B.; Brooker, S. Cutaneous leishmaniasis. Lancet Infect. Dis. 2007, 7, 581–596. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.; Donelson, J.E.; Wilson, M.E. The major surface protease (MSP or GP63) of Leishmania sp. Biosynthesis, regulation of expression, and function. Mol. Biochem. Parasitol. 2003, 132, 1–16. [Google Scholar] [CrossRef]
- Olivier, M.; Atayde, V.D.; Isnard, A.; Hassani, K.; Shio, M.T. Leishmania virulence factors: focus on the metalloprotease GP63. Microbes Infect. 2012, 14, 1377–1389. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.; Rosat, J.-P.; Bouvier, J.; Louis, J.; Bordier, C. Leishmania major: Differential regulation of the surface metalloprotease in amastigote and promastigote stages. Exp. Parasitol. 1992, 75, 196–206. [Google Scholar] [CrossRef]
- Bouvier, J.; Schneider, P.; Etges, R.; Bordier, C. Peptide substrate specificity of the membrane-bound metalloprotease of Leishmania. Biochemistry 1990, 29, 10113–10119. [Google Scholar] [CrossRef]
- Nogueira de Melo, A.C.; d’Avila-Levy, C.M.; Dias, F.A.; Armada, J.L.A.; Silva, H.D.; Lopes, A.H.C.S.; Santos, A.L.S.; Branquinha, M.H.; Vermelho, A.B. Peptidases and gp63-like proteins in Herpetomonas megaseliae: Possible involvement in the adhesion to the invertebrate host. Int. J. Parasitol. 2006, 36, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, R. Interaction between Casein and the Oppositely Charged Surfactant. Biomacromolecules 2007, 8, 2902–2908. [Google Scholar] [CrossRef]
- Huppertz, T.; Smiddy, M.A.; de Kruif, C.G. Biocompatible Micro-Gel Particles from Cross-Linked Casein Micelles. Biomacromolecules 2007, 8, 1300–1305. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, R. The interaction between casein micelles and gold nanoparticles. J. Colloid Interface Sci. 2009, 332, 265–269. [Google Scholar] [CrossRef]
- Yang, T.; Li, Z.; Wang, L.; Guo, C.; Sun, Y. Synthesis, Characterization, and Self-Assembly of Protein Lysozyme Monolayer-Stabilized Gold Nanoparticles. Langmuir 2007, 23, 10533–10538. [Google Scholar] [CrossRef]
- Jiang, X.; Jiang, J.; Jin, Y.; Wang, E.; Dong, S. Effect of Colloidal Gold Size on the Conformational Changes of Adsorbed Cytochrome c: Probing by Circular Dichroism, UV-Visible, and Infrared Spectroscopy. Biomacromolecules 2005, 6, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Castañeda, M.; de la Escosura-Muñiz, A.; González-Ortiz, G.; Martín-Orúe, S.M.; Pérez, J.F.; Merkoçi, A. Casein modified gold nanoparticles for future theranostic applications. Biosens. Bioelectron. 2013, 40, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Jans, H.; Huo, Q. Gold nanoparticle-enabled biological and chemical detection and analysis. Chem. Soc. Rev. 2012, 41, 2849–2866. [Google Scholar] [CrossRef]
- Singh, M.; Harris-Birtill, D.C.C.; Markar, S.R.; Hanna, G.B.; Elson, D.S. Application of gold nanoparticles for gastrointestinal cancer theranostics: A systematic review. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 2083–2098. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Ye, Y.; Liu, S. Gold nanoparticle-based signal amplification for biosensing. Anal. Biochem. 2011, 417, 1–16. [Google Scholar] [CrossRef]
- Baptista, P.; Pereira, E.; Eaton, P.; Doria, G.; Miranda, A.; Gomes, I.; Quaresma, P.; Franco, R. Gold nanoparticles for the development of clinical diagnosis methods. Anal. Bioanal.Chem. 2008, 391, 943–950. [Google Scholar] [CrossRef]
- de la Escosura-Muñiz, A.; Maltez-da Costa, M.; Sánchez-Espinel, C.; Díaz-Freitas, B.; Fernández-Suarez, J.; González-Fernández, Á.; Merkoçi, A. Gold nanoparticle-based electrochemical magnetoimmunosensor for rapid detection of anti-hepatitis B virus antibodies in human serum. Biosens. Bioelectron. 2010, 26, 1710–1714. [Google Scholar] [CrossRef]
- Hassan, A.-R.H.A.-A.; de la Escosura-Muñiz, A.; Merkoçi, A. Highly sensitive and rapid determination of Escherichia coli O157:H7 in minced beef and water using electrocatalytic gold nanoparticle tags. Biosens. Bioelectron. 2015, 67, 511–515. [Google Scholar] [CrossRef]
- de la Escosura-Muñiz, A.; Plichta, Z.; Horák, D.; Merkoçi, A. Alzheimer′s disease biomarkers detection in human samples by efficient capturing through porous magnetic microspheres and labelling with electrocatalytic gold nanoparticles. Biosens. Bioelectron. 2015, 67, 162–169. [Google Scholar] [CrossRef]
- Costa, M.M.; de la Escosura-Muñiz, A.; Merkoçi, A. Electrochemical quantification of gold nanoparticles based on their catalytic properties toward hydrogen formation: Application in magnetoimmunoassays. Electrochem. Commun. 2010, 12, 1501–1504. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Chamorro-Garcia, A.; Merkoçi, A. Electrochemical Impedance Spectroscopy (bio)sensing through hydrogen evolution reaction induced by gold nanoparticles. Biosens. Bioelectron. 2015, 67, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Guerbouj, S.; Djilani, F.; Bettaieb, J.; Lambson, B.; Diouani, M.F.; Ben Salah, A.; Ben Ismail, R.; Guizani, I. Evaluation of a gp63–PCR Based Assay as a Molecular Diagnosis Tool in Canine Leishmaniasis in Tunisia. PLoS ONE 2014, 9, e105419. [Google Scholar] [CrossRef] [PubMed]
- Sayhi, M.; Ouerghi, O.; Belgacem, K.; Arbi, M.; Tepeli, Y.; Ghram, A.; Anik, Ü.; Österlund, L.; Laouini, D.; Diouani, M.F. Electrochemical detection of influenza virus H9N2 based on both immunomagnetic extraction and gold catalysis using an immobilization-free screen printed carbon microelectrode. Biosens. Bioelectron. 2018, 107, 170–177. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Spec. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chemical Society Reviews 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Brülle, T.; Ju, W.; Niedermayr, P.; Denisenko, A.; Paschos, O.; Schneider, O.; Stimming, U. Size-Dependent Electrocatalytic Activity of Gold Nanoparticles on HOPG and Highly Boron-Doped Diamond Surfaces. Molecules 2011, 16, 10059–10077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich Method for Gold Nanoparticle Synthesis Revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiang, Y.; Ding, D.; Guo, R. Structural effects of amphiphilic protein/gold nanoparticle hybrid based nanozyme on peroxidase-like activity and silver-mediated inhibition. RSC Adv. 2016, 6, 112435–112444. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Longo, A.; Martorana, A.; Prestianni, A.; Venezia, A.M. XPS study of supported gold catalysts: the role of Au0 and Au+δ species as active sites. Surf. Interface Anal. 2006, 38, 215–218. [Google Scholar] [CrossRef]
- Maye, M.M.; Lou, Y.; Zhong, C.-J. Core−Shell Gold Nanoparticle Assembly as Novel Electrocatalyst of CO Oxidation. Langmuir 2000, 16, 7520–7523. [Google Scholar] [CrossRef]
- Haruta, M.; Daté, M. Advances in the catalysis of Au nanoparticles. Appl. Catal. A 2001, 222, 427–437. [Google Scholar] [CrossRef]
- Alivisatos, A.P. Semiconductor Clusters, Nanocrystals, and Quantum Dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef] [Green Version]
- Sen, I.K.; Maity, K.; Islam, S.S. Green synthesis of gold nanoparticles using a glucan of an edible mushroom and study of catalytic activity. Carbohydr. Polym. 2013, 91, 518–528. [Google Scholar] [CrossRef]
- de la Escosura-Muñiz, A.; Sánchez-Espinel, C.; Díaz-Freitas, B.; González-Fernández, Á.; Maltez-da Costa, M.; Merkoçi, A. Rapid Identification and Quantification of Tumor Cells Using an Electrocatalytic Method Based on Gold Nanoparticles. Anal. Chem. 2009, 81, 10268–10274. [Google Scholar] [CrossRef]
- Andreadou, M.; Liandris, E.; Gazouli, M.; Mataragka, A.; Tachtsidis, I.; Goutas, N.; Vlachodimitropoulos, D.; Ikonomopoulos, J. Detection of Leishmania -specific DNA and surface antigens using a combination of functionalized magnetic beads and cadmium selenite quantum dots. J. Microbiol. Methods 2016, 123, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, P.; Rojas, H.; Medina, M.; Arrivillaga, J.; Francisco, Y.; Dager, F.; Piscitelli, V.; Caetano, M.; Fernández, A.; Castillo, J. Study of Functionalized Gold Nanoparticles with Anti-gp63 IgG Antibody for the Detection of Glycoprotein gp63 in Membrane Surface of Leishmania Genus Parasites. Am. J. Analyt. Chem. 2013, 04, 100–108. [Google Scholar] [CrossRef] [Green Version]
- de la Escosura-Muñiz, A.; Ambrosi, A.; Merkoçi, A. Electrochemical analysis with nanoparticle-based biosystems. TrAC Trends Anal. Chem. 2008, 27, 568–584. [Google Scholar] [CrossRef]
- Silverman, J.M.; Clos, J.; de’Oliveira, C.C.; Shirvani, O.; Fang, Y.; Wang, C.; Foster, L.J.; Reiner, N.E. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J. Cell Sci. 2010, 123, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Mantel, P.-Y.; Marti, M. The role of extracellular vesicles in P lasmodium and other protozoan parasites: Extracellular vesicles in protozoan parasites. Cell. Microbiol. 2014, 16, 344–354. [Google Scholar] [CrossRef]
- Marcilla, A.; Martin-Jaular, L.; Trelis, M.; de Menezes-Neto, A.; Osuna, A.; Bernal, D.; Fernandez-Becerra, C.; Almeida, I.C.; del Portillo, H.A. Extracellular vesicles in parasitic diseases. J. Extracell. Vesicles 2014, 3, 25040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britton, C.; Winter, A.D.; Marks, N.D.; Gu, H.; McNeilly, T.N.; Gillan, V.; Devaney, E. Application of small RNA technology for improved control of parasitic helminths. Vet. Parasitol. 2015, 212, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Sun, X.; Zhao, J.; Yang, Y.; Cai, X.; Xu, J.; Cao, P. Exosomes: A Novel Strategy for Treatment and Prevention of Diseases. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Wasan, K.M.; Wasan, E.K.; Gershkovich, P.; Zhu, X.; Tidwell, R.R.; Werbovetz, K.A.; Clement, J.G.; Thornton, S.J. Highly Effective Oral Amphotericin B Formulation against Murine Visceral Leishmaniasis. J. Infect. Dis. 2009, 200, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Sundar, S.; Singh, A. Recent developments and future prospects in the treatment of visceral leishmaniasis. Ther. Adv. Infect. Dis. 2016, 3, 98–109. [Google Scholar] [CrossRef]
- Kamiński, D.M. Recent progress in the study of the interactions of amphotericin B with cholesterol and ergosterol in lipid environments. Eur. Biophys. J. 2014, 43, 453–467. [Google Scholar] [CrossRef] [Green Version]
- Paila, Y.D.; Saha, B.; Chattopadhyay, A. Amphotericin B inhibits entry of Leishmania donovani into primary macrophages. Biochem. Biophys. Res. Commun. 2010, 399, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Vermeersch, M.; da Luz, R.I.; Tote, K.; Timmermans, J.-P.; Cos, P.; Maes, L. In Vitro Susceptibilities of Leishmania donovani Promastigote and Amastigote Stages to Antileishmanial Reference Drugs: Practical Relevance of Stage-Specific Differences. Antimicrob. Agents Chemother. 2009, 53, 3855–3859. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diouani, M.F.; Ouerghi, O.; Belgacem, K.; Sayhi, M.; Ionescu, R.; Laouini, D. Casein-Conjugated Gold Nanoparticles for Amperometric Detection of Leishmania infantum. Biosensors 2019, 9, 68. https://doi.org/10.3390/bios9020068
Diouani MF, Ouerghi O, Belgacem K, Sayhi M, Ionescu R, Laouini D. Casein-Conjugated Gold Nanoparticles for Amperometric Detection of Leishmania infantum. Biosensors. 2019; 9(2):68. https://doi.org/10.3390/bios9020068
Chicago/Turabian StyleDiouani, Mohamed Fethi, Oussama Ouerghi, Kamel Belgacem, Maher Sayhi, Radu Ionescu, and Dhafer Laouini. 2019. "Casein-Conjugated Gold Nanoparticles for Amperometric Detection of Leishmania infantum" Biosensors 9, no. 2: 68. https://doi.org/10.3390/bios9020068
APA StyleDiouani, M. F., Ouerghi, O., Belgacem, K., Sayhi, M., Ionescu, R., & Laouini, D. (2019). Casein-Conjugated Gold Nanoparticles for Amperometric Detection of Leishmania infantum. Biosensors, 9(2), 68. https://doi.org/10.3390/bios9020068




