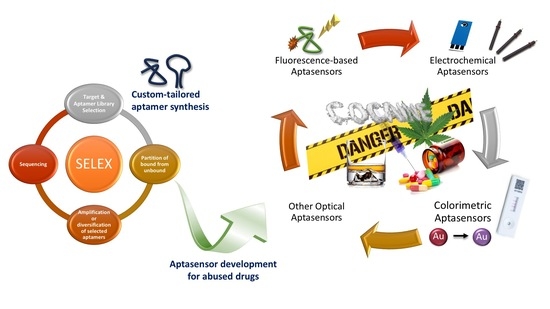
A Bottom-Up Approach for Developing Aptasensors for Abused Drugs: Biosensors in Forensics
Abstract
:1. Introduction
2. Custom-Tailored Aptamer Development
2.1. Principles of Aptamer Selection
2.2. Experimental Design of SELEX
2.3. Selection and Characterization of Aptamers Against Abused Drugs
3. Aptasensor Development
3.1. Advantages of Aptamers in Biosensor Development
3.2. Immobilization Techniques of Aptamers for Aptasensors
3.3. Optical Aptasensors
3.3.1. Colorimetric Aptasensors for Abused Drugs
3.3.2. Fluorescence-based Aptasensors for Abused Drugs
3.3.3. Other Optical Aptasensors for Abused Drugs
3.4. Electrochemical Aptasensors
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thompson, T.; Black, S. Forensic Human Identification: An Introduction; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Clauwaert, K.M.; Van Bocxlaer, J.F.; Lambert, W.E.; De Leenheer, A.P. Segmental analysis for cocaine and metabolites by HPLC in hair of suspected drug overdose cases. Forensic Sci. Int. 2000, 110, 157–166. [Google Scholar] [CrossRef]
- Anastos, N.; Barnett, N.W.; Lewis, S.W. Capillary electrophoresis for forensic drug analysis: A review. Talanta 2005, 67, 269–279. [Google Scholar] [CrossRef]
- Stout, P.R.; Horn, C.K.; Klette, K.L. Solid-phase extraction and GC-MS analysis of THC-COOH method optimized for a high-throughput forensic drug-testing laboratory. J. Anal. Toxicol. 2001, 25, 550–554. [Google Scholar] [CrossRef]
- Manchikanti, L.; Malla, Y.; Wargo, B.W.; Fellows, B. Comparative evaluation of the accuracy of benzodiazepine testing in chronic pain patients utilizing immunoassay with liquid chromatography tandem mass spectrometry (LC/MS/MS) of urine drug testing. Pain Physician 2011, 14, 259–270. [Google Scholar] [PubMed]
- Smith, F. Handbook of Forensic Drug Analysis; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Healy, D.A.; Hayes, C.J.; Leonard, P.; McKenna, L.; O’Kennedy, R. Biosensor developments: Application to prostate-specific antigen detection. Trends Biotechnol. 2007, 25, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Mabey, D.; Peeling, R.W.; Ustianowski, A.; Perkins, M.D. Diagnostics for the developing world. Nat. Rev. Microbiol. 2004, 2, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Gubala, V.; Harris, L.F.; Ricco, A.J.; Tan, M.X.; Williams, D.E. Point of care diagnostics: Status and future. Anal. Chem. 2011, 84, 487–515. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M. Portable biosensing devices for point-of-care diagnostics: Recent developments and applications. TrAC Trends Anal. Chem. 2017, 91, 26–41. [Google Scholar] [CrossRef]
- Bozokalfa, G.; Akbulut, H.; Demir, B.; Guler, E.; Gumus, Z.P.; Odaci Demirkol, D.; Aldemir, E.; Yamada, S.; Endo, T.; Coskunol, H.; et al. Polypeptide functional surface for the aptamer immobilization: Electrochemical cocaine biosensing. Anal. Chem. 2016, 88, 4161–4167. [Google Scholar] [CrossRef]
- Guler, E.; Yilmaz Sengel, T.; Gumus, Z.P.; Arslan, M.; Coskunol, H.; Timur, S.; Yagci, Y. Mobile phone sensing of cocaine in a lateral flow assay combined with a biomimetic material. Anal. Chem. 2017, 89, 9629–9632. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; Agüí, L.; Villalonga, R.; Pingarrón, J.M. Biosensors in forensic analysis: A review. Anal. Chim. Acta 2014, 823, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hermann, T.; Patel, D.J. Adaptive recognition by nucleic acid aptamers. Science 2000, 287, 820. [Google Scholar] [CrossRef] [PubMed]
- Song, K.-M.; Lee, S.; Ban, C. Aptamers and their biological applications. Sensors 2012, 12, 612–631. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.G.; Stahl, F.; Scheper, T. Aptamers as affinity ligands for downstream processing. Eng. Life Sci. 2012, 12, 496–506. [Google Scholar] [CrossRef]
- Que-Gewirth, N.; Sullenger, B. Gene therapy progress and prospects: RNA aptamers. Gene Ther. 2007, 14, 283. [Google Scholar] [CrossRef] [PubMed]
- Dollins, C.M.; Nair, S.; Sullenger, B.A. Aptamers in immunotherapy. Hum. Gene Ther. 2008, 19, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Liang, Z.; Zhou, N. Design strategies for aptamer-based biosensors. Sensors 2010, 10, 4541–4557. [Google Scholar] [CrossRef]
- Cho, E.J.; Lee, J.-W.; Ellington, A.D. Applications of aptamers as sensors. Ann. Rev. Anal. Chem. 2009, 2, 241–264. [Google Scholar] [CrossRef]
- Bruno, J.G. Predicting the uncertain future of aptamer-based diagnostics and therapeutics. Molecules 2015, 20, 6866–6887. [Google Scholar] [CrossRef]
- Gooch, J.; Daniel, B.; Parkin, M.; Frascione, N. Developing aptasensors for forensic analysis. TrAC Trends Anal. Chem. 2017, 94, 150–160. [Google Scholar] [CrossRef]
- Bremer, P.T.; Janda, K.D. Conjugate vaccine immunotherapy for substance use disorder. Pharmacol. Rev. 2017, 69, 298–315. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Siddiqua, A.; Chinnappan, R.; Zourob, M. Electrochemical SELEX technique for the selection of DNA aptamers against the small molecule 11-deoxycortisol. ACS Appl. Biomater. 2019, 2, 2624–2632. [Google Scholar] [CrossRef]
- Mairal Lerga, T.; Jauset-Rubio, M.; Skouridou, V.; Bashammakh, A.S.; El-Shahawi, M.S.; Alyoubi, A.O.; O’Sullivan, C.K. High affinity aptamer for the detection of the biogenic amine histamine. Anal. Chem. 2019, 91, 7104–7111. [Google Scholar] [CrossRef] [PubMed]
- Bayat, P.; Nosrati, R.; Alibolandi, M.; Rafatpanah, H.; Abnous, K.; Khedri, M.; Ramezani, M. SELEX methods on the road to protein targeting with nucleic acid aptamers. Biochimie 2018, 154, 132–155. [Google Scholar] [CrossRef]
- Li, Y.; Lee, J.-S. Recent developments in affinity-based selection of aptamers for binding disease-related protein targets. Chem. Pap. 2019, 1–17. [Google Scholar] [CrossRef]
- Kaur, H. Recent developments in cell-SELEX technology for aptamer selection. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2323–2329. [Google Scholar] [CrossRef]
- Philippou, S.; Mastroyiannopoulos, N.P.; Makrides, N.; Lederer, C.W.; Kleanthous, M.; Phylactou, L.A. Selection and identification of skeletal-muscle-targeted RNA aptamers. Mol. Ther. Nucl. Acids 2018, 10, 199–214. [Google Scholar] [CrossRef]
- Zhong, W.; Pu, Y.; Tan, W.; Liu, J.; Liao, J.; Liu, B.; Chen, K.; Yu, B.; Hu, Y.; Deng, Y.; et al. Identification and application of an aptamer targeting papillary thyroid carcinoma using tissue-SELEX. Anal. Chem. 2019, 91, 8289–8297. [Google Scholar] [CrossRef]
- Pfeiffer, F.; Mayer, G. Selection and biosensor application of aptamers for small molecules. Front. Chem. 2016, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Ruscito, A.; DeRosa, M.C. Small-Molecule binding aptamers: Selection strategies, characterization, and applications. Front. Chem. 2016, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Stoltenburg, R.; Reinemann, C.; Strehlitz, B. FluMag-SELEX as an advantageous method for DNA aptamer selection. Anal. Bioanal. Chem. 2005, 383, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, C.; Li, H. Selection of DNA aptamers and establishment of an effective aptasensor for highly sensitive detection of cefquinome residues in milk. Analyst 2018, 143, 3202–3208. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Lu, X.; Hu, X.; Zhang, Y.; Zeng, L.; Chen, L.; Sun, M. In vitro selection of DNA aptamers binding pesticide fluoroacetamide. Biosci. Biotechnol. Biochem. 2016, 80, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Yan, J.; Xiong, H.; Liu, Y.; Peng, D.; Liu, Z. Investigations on the interface of nucleic acid aptamers and binding targets. Analyst 2018, 143, 5317–5338. [Google Scholar] [CrossRef] [PubMed]
- Stoltenburg, R.; Nikolaus, N.; Strehlitz, B. Capture-SELEX: Selection of DNA aptamers for aminoglycoside antibiotics. J. Anal. Methods Chem. 2012, 2012, 14. [Google Scholar] [CrossRef]
- Spiga, F.M.; Maietta, P.; Guiducci, C. More DNA–aptamers for small drugs: A capture–SELEX coupled with surface plasmon resonance and high-throughput sequencing. ACS Comb. Sci. 2015, 17, 326–333. [Google Scholar] [CrossRef]
- Boussebayle, A.; Groher, F.; Suess, B. RNA-based capture-SELEX for the selection of small molecule-binding aptamers. Methods 2019, 161, 10–15. [Google Scholar] [CrossRef]
- Park, J.-W.; Tatavarty, R.; Kim, D.W.; Jung, H.-T.; Gu, M.B. Immobilization-free screening of aptamers assisted by graphene oxide. Chem. Commun. 2012, 48, 2071–2073. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Kwon, Y.S.; Kim, J.H.; Gu, M.B. Multiple GO-SELEX for efficient screening of flexible aptamers. Chem. Commun. 2014, 50, 10513–10516. [Google Scholar] [CrossRef] [PubMed]
- Komarova, N.; Andrianova, M.; Glukhov, S.; Kuznetsov, A. Selection, characterization, and application of ssDNA aptamer against furaneol. Molecules 2018, 23, 3159. [Google Scholar] [CrossRef] [PubMed]
- Paniel, N.; Istamboulie, G.; Triki, A.; Lozano, C.; Barthelmebs, L.; Noguer, T. Selection of DNA aptamers against penicillin G using Capture-SELEX for the development of an impedimetric sensor. Talanta 2017, 162, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Duan, N.; Wu, S.; Hao, L.; Xia, Y.; Ma, X.; Wang, Z. Graphene oxide-assisted non-immobilized SELEX of okdaic acid aptamer and the analytical application of aptasensor. Sci. Rep. 2016, 6, 21665. [Google Scholar] [CrossRef] [PubMed]
- Ozyurt, C.; Canbay, Z.C.; Dinckaya, E.; Evran, S. A highly sensitive DNA aptamer-based fluorescence assay for sarcosine detection down to picomolar levels. Int. J. Biol. Macromol. 2019, 129, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, C.; Larcher, L.M.; Barrero, R.A.; Veedu, R.N. Three decades of nucleic acid aptamer technologies: Lessons learned, progress and opportunities on aptamer development. Biotechnol. Adv. 2019, 37, 28–50. [Google Scholar] [CrossRef]
- Vorobyeva, M.A.; Davydova, A.S.; Vorobjev, P.E.; Venyaminova, A.G. Key aspects of nucleic acid library design for in vitro selection. Int. J. Mol. Sci. 2018, 19, 470. [Google Scholar] [CrossRef]
- Sun, Z.; Nguyen, T.; McAuliffe, K.; You, M. Intracellular imaging with genetically encoded RNA-based molecular sensors. Nanomaterials 2019, 9, 233. [Google Scholar] [CrossRef]
- Rothlisberger, P.; Hollenstein, M. Aptamer chemistry. Adv. Drug. Deliv. Rev. 2018, 134, 3–21. [Google Scholar] [CrossRef]
- Pfeiffer, F.; Rosenthal, M.; Siegl, J.; Ewers, J.; Mayer, G. Customised nucleic acid libraries for enhanced aptamer selection and performance. Curr. Opin. Biotechnol. 2017, 48, 111–118. [Google Scholar] [CrossRef]
- Kraemer, S.; Vaught, J.D.; Bock, C.; Gold, L.; Katilius, E.; Keeney, T.R.; Kim, N.; Saccomano, N.A.; Wilcox, S.K.; Zichi, D.; et al. From SOMAmer-based biomarker discovery to diagnostic and clinical applications: A SOMAmer-based, streamlined multiplex proteomic assay. PLoS ONE 2011, 6, e26332. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, F.; Tolle, F.; Rosenthal, M.; Brandle, G.M.; Ewers, J.; Mayer, G. Identification and characterization of nucleobase-modified aptamers by click-SELEX. Nat. Protoc. 2018, 13, 1153–1180. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; McKeague, M.; Pitre, S.; Dumontier, M.; Green, J.; Golshani, A.; Derosa, M.C.; Dehne, F. Computational approaches toward the design of pools for the in vitro selection of complex aptamers. RNA 2010, 16, 2252–2262. [Google Scholar] [CrossRef] [PubMed]
- Kinghorn, A.B.; Fraser, L.A.; Liang, S.; Shiu, S.C.-C.; Tanner, J.A. Aptamer bioinformatics. Int. J. Mol. Sci. 2017, 18, 2516. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xia, X.; Luo, Z.; Liang, H.; Shakhnovich, E. Searching the sequence space for potent aptamers using SELEX in Silico. J. Chem. Theory Comput. 2015, 11, 5939–5946. [Google Scholar] [CrossRef] [PubMed]
- Wondergem, J.A.J.; Schiessel, H.; Tompitak, M. Performing SELEX experiments in silico. J. Chem. Phys. 2017, 147, 174101. [Google Scholar] [CrossRef]
- Fa, Y.; Guan, M.; Zhao, H.; Li, F.; Liu, H. Affinity analysis between trypsin and aptamers using surface plasmon resonance competition experiments in a steady state. Anal. Methods 2019, 11, 3061–3065. [Google Scholar] [CrossRef]
- Lotz, T.S.; Halbritter, T.; Kaiser, C.; Rudolph, M.M.; Kraus, L.; Groher, F.; Steinwand, S.; Wachtveitl, J.; Heckel, A.; Suess, B. A light-responsive RNA aptamer for an azobenzene derivative. Nucl. Acids Res. 2018, 47, 2029–2040. [Google Scholar] [CrossRef]
- Samokhvalov, A.V.; Safenkova, I.V.; Eremin, S.A.; Zherdev, A.V.; Dzantiev, B.B. Measurement of (Aptamer–Small Target) KD using the competition between fluorescently labeled and unlabeled targets and the detection of fluorescence anisotropy. Anal. Chem. 2018, 90, 9189–9198. [Google Scholar] [CrossRef]
- Jaeger, J.; Groher, F.; Stamm, J.; Spiehl, D.; Braun, J.; Dörsam, E.; Suess, B. Characterization and inkjet printing of an RNA aptamer for paper-based biosensing of ciprofloxacin. Biosensors 2019, 9, 7. [Google Scholar] [CrossRef]
- Cruz-Aguado, J.A.; Penner, G. Determination of ochratoxin A with a DNA aptamer. J. Agricult. Food Chem. 2008, 56, 10456–10461. [Google Scholar] [CrossRef] [PubMed]
- Kammer, M.N.; Olmsted, I.R.; Kussrow, A.K.; Morris, M.J.; Jackson, G.W.; Bornhop, D.J. Characterizing aptamer small molecule interactions with backscattering interferometry. Analyst 2014, 139, 5879–5884. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Qiao, P.; Jing, L.; Song, Y.; Zhang, J.; Chen, Q.; Han, Q. Screening and application of a new aptamer for the rapid detection of sudan dye III. Eur. J. Lipid Sci. Technol. 2018, 120, 1700112. [Google Scholar] [CrossRef]
- Moreno, M.; Fernández-Algar, M.; Fernández-Chamorro, J.; Ramajo, J.; Martínez-Salas, E.; Briones, C. A combined ELONA-(RT)qPCR approach for characterizing DNA and RNA aptamers selected against PCBP-2. Molecules 2019, 24, 1213. [Google Scholar] [CrossRef]
- Gao, S.; Zheng, X.; Jiao, B.; Wang, L. Post-SELEX optimization of aptamers. Anal. Bioanal. Chem. 2016, 408, 4567–4573. [Google Scholar] [CrossRef] [PubMed]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucl. Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef] [PubMed]
- Reuter, J.S.; Mathews, D.H. RNAstructure: Software for RNA secondary structure prediction and analysis. BMC Bioinf. 2010, 11, 129. [Google Scholar] [CrossRef]
- Akitomi, J.; Kato, S.; Yoshida, Y.; Horii, K.; Furuichi, M.; Waga, I. ValFold: Program for the aptamer truncation process. Bioinformatics 2011, 7, 38–40. [Google Scholar] [CrossRef]
- Gao, S.; Hu, W.; Zheng, X.; Cai, S.; Wu, J. Functionalized aptamer with an antiparallel G-quadruplex: Structural remodeling, recognition mechanism, and diagnostic applications targeting CTGF. Biosens. Bioelectron. 2019, 142, 111475. [Google Scholar] [CrossRef]
- Wolter, A.C.; Pianu, A.; Kremser, J.; Strebitzer, E.; Schnieders, R.; Furtig, B.; Kreutz, C.; Duchardt-Ferner, E.; Wohnert, J. NMR resonance assignments for the GTP-binding RNA aptamer 9-12 in complex with GTP. Biomol. NMR Assign 2019, 13, 281–286. [Google Scholar] [CrossRef]
- Khan, N.H.; Bui, A.A.; Xiao, Y.; Sutton, R.B.; Shaw, R.W.; Wylie, B.J.; Latham, M.P. A DNA aptamer reveals an allosteric site for inhibition in metallo-beta-lactamases. PLoS ONE 2019, 14, e0214440. [Google Scholar] [CrossRef] [PubMed]
- Krüger, A.; Zimbres, F.M.; Kronenberger, T.; Wrenger, C. Molecular modeling applied to nucleic acid-based molecule development. Biomolecules 2018, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, L.; Lu, W.; Zhai, Q.; Fan, D.; Liu, X.; Zhao, J.; Zhang, H.; Chen, W. Selection, identification and application of DNA aptamers for the detection of Bifidobacterium breve. RSC Adv. 2017, 7, 11672–11679. [Google Scholar] [CrossRef]
- Alhadrami, H.A.; Chinnappan, R.; Eissa, S.; Rahamn, A.A.; Zourob, M. High affinity truncated DNA aptamers for the development of fluorescence based progesterone biosensors. Anal. Biochem. 2017, 525, 78–84. [Google Scholar] [CrossRef]
- Dhiman, A.; Anand, A.; Malhotra, A.; Khan, E.; Santra, V.; Kumar, A.; Sharma, T.K. Rational truncation of aptamer for cross-species application to detect krait envenomation. Sci. Rep. 2018, 8, 17795. [Google Scholar] [CrossRef]
- Virgilio, A.; Amato, T.; Petraccone, L.; Esposito, F.; Grandi, N.; Tramontano, E.; Romero, R.; Haider, S.; Gomez-Monterrey, I.; Novellino, E.; et al. Improvement of the activity of the anti-HIV-1 integrase aptamer T30175 by introducing a modified thymidine into the loops. Sci. Rep. 2018, 8, 7447. [Google Scholar] [CrossRef]
- Li, L.; Yang, X.; Li, K.; Zhang, G.; Ma, Y.; Cai, B.; Li, S.; Ding, H.; Deng, J.; Nan, X.; et al. d-/l-Isothymidine incorporation in the core sequence of aptamer BC15 enhanced its binding affinity to the hnRNP A1 protein. Org. Biomol. Chem. 2018, 16, 7488–7497. [Google Scholar] [CrossRef]
- Zheng, X.; Hu, B.; Gao, S.X.; Liu, D.J.; Sun, M.J.; Jiao, B.H.; Wang, L.H. A saxitoxin-binding aptamer with higher affinity and inhibitory activity optimized by rational site-directed mutagenesis and truncation. Toxicon 2015, 101, 41–47. [Google Scholar] [CrossRef]
- Stojanovic, M.N.; de Prada, P.; Landry, D.W. Aptamer-Based folding fluorescent sensor for cocaine. J. Am. Chem. Soc. 2001, 123, 4928–4931. [Google Scholar] [CrossRef]
- Neves, M.A.D.; Reinstein, O.; Johnson, P.E. Defining a stem length-dependent binding mechanism for the cocaine-binding aptamer: A combined NMR and calorimetry study. Biochemistry 2010, 49, 8478–8487. [Google Scholar] [CrossRef]
- Neves, M.A.D.; Reinstein, O.; Saad, M.; Johnson, P.E. Defining the secondary structural requirements of a cocaine-binding aptamer by a thermodynamic and mutation study. Biophys. Chem. 2010, 153, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Reinstein, O.; Yoo, M.; Han, C.; Palmo, T.; Beckham, S.A.; Wilce, M.C.J.; Johnson, P.E. Quinine binding by the cocaine-binding aptamer: Thermodynamic and hydrodynamic analysis of high-affinity binding of an off-target ligand. Biochemistry 2013, 52, 8652–8662. [Google Scholar] [CrossRef] [PubMed]
- Slavkovic, S.; Altunisik, M.; Reinstein, O.; Johnson, P.E. Structure–affinity relationship of the cocaine-binding aptamer with quinine derivatives. Bioorg. Med. Chem. 2015, 23, 2593–2597. [Google Scholar] [CrossRef] [PubMed]
- Roncancio, D.; Yu, H.; Xu, X.; Wu, S.; Liu, R.; Debord, J.; Lou, X.; Xiao, Y. A label-free aptamer-fluorophore assembly for rapid and specific detection of cocaine in biofluids. Anal. Chem. 2014, 86, 11100–11106. [Google Scholar] [CrossRef] [PubMed]
- Sachan, A.; Ilgu, M.; Kempema, A.; Kraus, G.A.; Nilsen-Hamilton, M. Specificity and ligand affinities of the cocaine aptamer: Impact of structural features and physiological NaCl. Anal. Chem. 2016, 88, 7715–7723. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.A.D.; Slavkovic, S.; Churcher, Z.R.; Johnson, P.E. Salt-mediated two-site ligand binding by the cocaine-binding aptamer. Nucl. Acids Res. 2017, 45, 1041–1048. [Google Scholar] [CrossRef]
- Grytz, C.M.; Marko, A.; Cekan, P.; Sigurdsson, S.T.; Prisner, T.F. Flexibility and conformation of the cocaine aptamer studied by PELDOR. Phys. Chem. Chem. Phys. 2016, 18, 2993–3002. [Google Scholar] [CrossRef]
- Win, M.N.; Klein, J.S.; Smolke, C.D. Codeine-binding RNA aptamers and rapid determination of their binding constants using a direct coupling surface plasmon resonance assay. Nucl. Acids Res. 2006, 34, 5670–5682. [Google Scholar] [CrossRef]
- Huang, L.; Yang, X.; Qi, C.; Niu, X.; Zhao, C.; Zhao, X.; Shangguan, D.; Yang, Y. A label-free electrochemical biosensor based on a DNA aptamer against codeine. Anal. Chim. Acta 2013, 787, 203–210. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Hamzeiy, H.; Barar, J.; Barzegari, A.; Omidi, Y. Systematic evolution of ligands by exponential enrichment selection of specific aptamer for sensing of methamphetamine. Sens. Lett. 2013, 11, 566–570. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, Y.; Yang, J. Graphene oxide-assisted non-immobilized SELEX of chiral drug ephedrine aptamers and the analytical binding mechanism. Biochem. Biophys. Res. Commun. 2019, 514, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Willner, I.; Zayats, M. Electronic aptamer-based sensors. Angew. Chem. Int. Ed. 2007, 46, 6408–6418. [Google Scholar] [CrossRef] [PubMed]
- Tombelli, S.; Minunni, M.; Mascini, M. Analytical applications of aptamers. Biosens. Bioelectron. 2005, 20, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Berglund, L.; Bjorling, E.; Oksvold, P.; Fagerberg, L.; Asplund, A.; Szigyarto, C.A.; Persson, A.; Ottosson, J.; Wernerus, H.; Nilsson, P.; et al. A genecentric human protein atlas for expression profiles based on antibodies. Mol. Cell Proteom. 2008, 7, 2019–2027. [Google Scholar] [CrossRef]
- Mayer, G. The chemical biology of aptamers. Angew. Chem. Int. Ed. Engl. 2009, 48, 2672–2689. [Google Scholar] [CrossRef]
- Ruigrok, V.J.; Levisson, M.; Eppink, M.H.; Smidt, H.; van der Oost, J. Alternative affinity tools: More attractive than antibodies? Biochem. J. 2011, 436, 1–13. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Electrochemical aptasensors. Electroanalysis 2009, 21, 1237–1250. [Google Scholar] [CrossRef]
- O’Sullivan, C.K. Aptasensors—The future of biosensing? Anal. Bioanal. Chem. 2002, 372, 44–48. [Google Scholar] [CrossRef]
- Cheng, A.K.H.; Sen, D.; Yu, H.-Z. Design and testing of aptamer-based electrochemical biosensors for proteins and small molecules. Bioelectrochemistry 2009, 77, 1–12. [Google Scholar] [CrossRef]
- Zuo, X.; Xiao, Y.; Plaxco, K.W. High specificity, electrochemical sandwich assays based on single aptamer sequences and suitable for the direct detection of small-molecule targets in blood and other complex matrices. J. Am. Chem. Soc. 2009, 131, 6944–6945. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Huang, H.; Xuan, J.; Zhang, J.; Zhu, J.-J. Quantum dots electrochemical aptasensor based on three-dimensionally ordered macroporous gold film for the detection of ATP. Biosens. Bioelectron. 2010, 26, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhang, X.; Yang, W.; Jia, H.; Li, Y. Fluorescence detection of adenosine triphosphate through an aptamer—Molecular beacon multiple probe. Anal. Biochem. 2012, 424, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Guler, E.; Bozokalfa, G.; Demir, B.; Gumus, Z.P.; Guler, B.; Aldemir, E.; Timur, S.; Coskunol, H. An aptamer folding-based sensory platform decorated with nanoparticles for simple cocaine testing. Drug Test. Anal. 2017, 9, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Polonschii, C.; David, S.; Tombelli, S.; Mascini, M.; Gheorghiu, M. A novel low-cost and easy to develop functionalization platform. Case study: Aptamer-based detection of thrombin by surface plasmon resonance. Talanta 2010, 80, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, J.; Cao, X.; Wang, L.; Li, D.; Song, S.; Ye, B.; Fan, C. Adenosine detection by using gold nanoparticles and designed aptamer sequences. Analyst 2009, 134, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, S.; Obubuafo, A.; Soper, S.A.; Spivak, D.A. Surface immobilization methods for aptamer diagnostic applications. Anal. Bioanal. Chem. 2008, 390, 1009–1021. [Google Scholar] [CrossRef]
- Hunt, H.K.; Armani, A.M. Label-free biological and chemical sensors. Nanoscale 2010, 2, 1544–1559. [Google Scholar] [CrossRef]
- Wittmann, C. Immobilisation of DNA on Chips: Immobilization of DNA on Microarrays; Springer Sci. & Business Media: Berlin, Germany, 2006. [Google Scholar]
- Rhouati, A.; Catanante, G.; Nunes, G.; Hayat, A.; Marty, J.-L. Label-Free aptasensors for the detection of mycotoxins. Sensors 2016, 16, 2178. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, M.-H.; Wang, J.-P.; Ye, Z.-Z. Application of biosensor surface immobilization methods for aptamer. Chin. J. Anal. Chem. 2011, 39, 432–438. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar] [CrossRef] [PubMed]
- Liss, M.; Petersen, B.; Wolf, H.; Prohaska, E. An aptamer-based quartz crystal protein biosensor. Anal. Chem. 2002, 74, 4488–4495. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.C.; Kawde, A.-N.; Wang, J. Aptamer biosensor for label-free impedance spectroscopy detection of proteins based on recognition-induced switching of the surface charge. Chem. Commun. 2005, 4267–4269. [Google Scholar] [CrossRef] [PubMed]
- McCracken, K.E.; Yoon, J.-Y. Recent approaches for optical smartphone sensing in resource-limited settings: A brief review. Anal. Methods 2016, 8, 6591–6601. [Google Scholar] [CrossRef]
- Feng, C.; Dai, S.; Wang, L. Optical aptasensors for quantitative detection of small biomolecules: A review. Biosens. Bioelectron. 2014, 59, 64–74. [Google Scholar] [CrossRef]
- Wang, L.; Musile, G.; McCord, B.R. An aptamer-based paper microfluidic device for the colorimetric determination of cocaine. Electrophoresis 2018, 39, 470–475. [Google Scholar] [CrossRef]
- Liu, J.; Mazumdar, D.; Lu, Y. A simple and sensitive “dipstick” test in serum based on lateral flow separation of aptamer-linked nanostructures. Angew. Chem. Int. Ed. 2006, 45, 7955–7959. [Google Scholar] [CrossRef]
- Du, Y.; Li, B.; Guo, S.; Zhou, Z.; Zhou, M.; Wang, E.; Dong, S. G-Quadruplex-based DNAzyme for colorimetric detection of cocaine: Using magnetic nanoparticles as the separation and amplification element. Analyst 2011, 136, 493–497. [Google Scholar] [CrossRef]
- Mao, K.; Yang, Z.; Du, P.; Xu, Z.; Wang, Z.; Li, X. G-quadruplex–hemin DNAzyme molecular beacon probe for the detection of methamphetamine. RSC Adv. 2016, 6, 62754–62759. [Google Scholar] [CrossRef]
- Shi, Q.; Shi, Y.; Pan, Y.; Yue, Z.; Zhang, H.; Yi, C. Colorimetric and bare eye determination of urinary methylamphetamine based on the use of aptamers and the salt-induced aggregation of unmodified gold nanoparticles. Microchim. Acta 2015, 182, 505–511. [Google Scholar] [CrossRef]
- Mao, K.; Yang, Z.; Li, J.; Zhou, X.; Li, X.; Hu, J. A novel colorimetric biosensor based on non-aggregated Au@Ag core–shell nanoparticles for methamphetamine and cocaine detection. Talanta 2017, 175, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Yarbakht, M.; Nikkhah, M. Unmodified gold nanoparticles as a colorimetric probe for visual methamphetamine detection. J. Exp. Nanosci. 2016, 11, 593–601. [Google Scholar] [CrossRef]
- Nie, J.; Deng, Y.; Deng, Q.-P.; Zhang, D.-W.; Zhou, Y.-L.; Zhang, X.-X. A self-assemble aptamer fragment/target complex based high-throughput colorimetric aptasensor using enzyme linked aptamer assay. Talanta 2013, 106, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Qadami, F.; Molaeirad, A.; Alijanianzadeh, M.; Azizi, A.; Kamali, N. Localized surface plasmon resonance (LSPR)-based nanobiosensor for methamphetamin measurement. Plasmonics 2018, 13, 2091–2098. [Google Scholar] [CrossRef]
- Emrani, A.S.; Danesh, N.M.; Ramezani, M.; Taghdisi, S.M.; Abnous, K. A novel fluorescent aptasensor based on hairpin structure of complementary strand of aptamer and nanoparticles as a signal amplification approach for ultrasensitive detection of cocaine. Biosens. Bioelectron. 2016, 79, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yan, L.; Wang, C.; Lin, H.; Wang, C.; Chen, X.; Yang, C.J. A general excimer signaling approach for aptamer sensors. Biosens. Bioelectron. 2010, 25, 2232–2237. [Google Scholar] [CrossRef]
- Tang, Y.; Long, F.; Gu, C.; Wang, C.; Han, S.; He, M. Reusable split-aptamer-based biosensor for rapid detection of cocaine in serum by using an all-fiber evanescent wave optical biosensing platform. Anal. Chim. Acta 2016, 933, 182–188. [Google Scholar] [CrossRef]
- He, J.-L.; Wu, Z.-S.; Zhou, H.; Wang, H.-Q.; Jiang, J.-H.; Shen, G.-L.; Yu, R.-Q. Fluorescence aptameric sensor for strand displacement amplification detection of cocaine. Anal. Chem. 2010, 82, 1358–1364. [Google Scholar] [CrossRef]
- Saberi, Z.; Rezaei, B.; Faroukhpour, H.; Ensafi, A.A. A fluorometric aptasensor for methamphetamine based on fluorescence resonance energy transfer using cobalt oxyhydroxide nanosheets and carbon dots. Microchim. Acta 2018, 185, 303. [Google Scholar] [CrossRef]
- Sun, B.; Qi, H.; Ma, F.; Gao, Q.; Zhang, C.; Miao, W. Double covalent coupling method for the fabrication of highly sensitive and reusable electrogenerated chemiluminescence sensors. Anal. Chem. 2010, 82, 5046–5052. [Google Scholar] [CrossRef]
- Yan, X.; Cao, Z.; Lau, C.; Lu, J. DNA aptamer folding on magnetic beads for sequential detection of adenosine and cocaine by substrate-resolved chemiluminescence technology. Analyst 2010, 135, 2400–2407. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ji, X.; Liu, B. Chemiluminescence aptasensor for cocaine based on double-functionalized gold nanoprobes and functionalized magnetic microbeads. Anal. Bioanal. Chem. 2011, 401, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Golub, E.; Pelossof, G.; Freeman, R.; Zhang, H.; Willner, I. Electrochemical, photoelectrochemical, and surface plasmon resonance detection of cocaine using supramolecular aptamer complexes and metallic or semiconductor nanoparticles. Anal. Chem. 2009, 81, 9291–9298. [Google Scholar] [CrossRef] [PubMed]
- Chansuvarn, W.; Tuntulani, T.; Imyim, A. Colorimetric detection of mercury(II) based on gold nanoparticles, fluorescent gold nanoclusters and other gold-based nanomaterials. TrAC Trends Anal. Chem. 2015, 65, 83–96. [Google Scholar] [CrossRef]
- Aldewachi, H.; Chalati, T.; Woodroofe, M.N.; Bricklebank, N.; Sharrack, B.; Gardiner, P. Gold nanoparticle-based colorimetric biosensors. Nanoscale 2018, 10, 18–33. [Google Scholar] [CrossRef]
- Aydindogan, E.; Guler Celik, E.; Timur, S. Paper-based analytical methods for smartphone sensing with functional nanoparticles: Bridges from smart surfaces to global health. Anal. Chem. 2018, 90, 12325–12333. [Google Scholar] [CrossRef]
- Nath, N.; Chilkoti, A. Label free colorimetric biosensing using nanoparticles. J. Fluoresc. 2004, 14, 377–389. [Google Scholar] [CrossRef]
- Vilela, D.; Gonzalez, M.C.; Escarpa, A. Sensing colorimetric approaches based on gold and silver nanoparticles aggregation: Chemical creativity behind the assay: A review. Anal. Chim. Acta 2012, 751, 24–43. [Google Scholar] [CrossRef]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Lin, M.Y.; Lindsay, H.M.; Weitz, D.A.; Ball, R.C.; Klein, R.; Meakin, P. Universality in colloid aggregation. Nature 1989, 339, 360–362. [Google Scholar] [CrossRef]
- Sun, M.; Liu, F.; Zhu, Y.; Wang, W.; Hu, J.; Liu, J.; Dai, Z.; Wang, K.; Wei, Y.; Bai, J.; et al. Salt-induced aggregation of gold nanoparticles for photoacoustic imaging and photothermal therapy of cancer. Nanoscale 2016, 8, 4452–4457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Q. Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 2016, 75, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Michelini, E.; Zangheri, M.; Di Fusco, M.; Calabria, D.; Simoni, P. Smartphone-based biosensors: A critical review and perspectives. TrAC Trends Anal. Chem. 2016, 79, 317–325. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Smith, J.E.; Griffin, D.K.; Leny, J.K.; Hagen, J.A.; Chavez, J.L.; Kelley-Loughnane, N. Colorimetric detection with aptamer-gold nanoparticle conjugates coupled to an android-based color analysis application for use in the field. Talanta 2014, 121, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Pan, D.; Song, S.; Boey, F.Y.C.; Zhang, H.; Fan, C. Visual cocaine detection with gold nanoparticles and rationally engineered aptamer structures. Small 2008, 4, 1196–1200. [Google Scholar] [CrossRef]
- Reynolds, R.A.; Mirkin, C.A.; Letsinger, R.L. Homogeneous, nanoparticle-based quantitative colorimetric detection of oligonucleotides. J. Am. Chem. Soc. 2000, 122, 3795–3796. [Google Scholar] [CrossRef]
- Wong, R.; Tse, H. Lateral Flow Immunoassay; Springer Sci. & Business Media: Berlin, Germany, 2008. [Google Scholar]
- Liang, R.-L.; Xu, X.-P.; Liu, T.-C.; Zhou, J.-W.; Wang, X.-G.; Ren, Z.-Q.; Hao, F.; Wu, Y.-S. Rapid and sensitive lateral flow immunoassay method for determining alpha fetoprotein in serum using europium (III) chelate microparticles-based lateral flow test strips. Anal. Chim. Acta 2015, 891, 277–283. [Google Scholar] [CrossRef]
- Parolo, C.; de la Escosura-Muñiz, A.; Merkoçi, A. Enhanced lateral flow immunoassay using gold nanoparticles loaded with enzymes. Biosens. Bioelectron. 2013, 40, 412–416. [Google Scholar] [CrossRef]
- Zangheri, M.; Cevenini, L.; Anfossi, L.; Baggiani, C.; Simoni, P.; Di Nardo, F.; Roda, A. A simple and compact smartphone accessory for quantitative chemiluminescence-based lateral flow immunoassay for salivary cortisol detection. Biosens. Bioelectron. 2015, 64, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, C.; Wang, J.; Zhang, Y. Development of colloidal gold-based flow-through and lateral-flow immunoassays for the rapid detection of the insecticide carbaryl. Anal. Chim. Acta 2005, 546, 161–166. [Google Scholar] [CrossRef]
- Chen, A.; Yang, S. Replacing antibodies with aptamers in lateral flow immunoassay. Biosens. Bioelectron. 2015, 71, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Dong, M.; Zhang, C.; Wang, Y.; Xie, M.; Chen, Y. Magnetic lateral flow strip for the detection of cocaine in urine by naked eyes and smart phone camera. Sensors 2017, 17, 1286. [Google Scholar] [CrossRef]
- Luby, B.M.; Charron, D.M.; MacLaughlin, C.M.; Zheng, G. Activatable fluorescence: From small molecule to nanoparticle. Adv. Drug Deliv. Rev. 2017, 113, 97–121. [Google Scholar] [CrossRef]
- Zhang, H.; Uselman, R.R.; Yee, D. Exogenous near-infrared fluorophores and their applications in cancer diagnosis: Biological and clinical perspectives. Exp. Opin. Med. Diagn. 2011, 5, 241–251. [Google Scholar] [CrossRef]
- Ke, S.; Wen, X.; Gurfinkel, M.; Charnsangavej, C.; Wallace, S.; Sevick-Muraca, E.M.; Li, C. Near-infrared optical imaging of epidermal growth factor receptor in breast cancer xenografts. Cancer Res. 2003, 63, 7870–7875. [Google Scholar]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef]
- Bunz, U.H.F.; Rotello, V.M. Gold nanoparticle–fluorophore complexes: Sensitive and discerning “noses” for biosystems sensing. Angew. Chem. Int. Ed. 2010, 49, 3268–3279. [Google Scholar] [CrossRef]
- Wang, Q.X.; Xue, S.F.; Chen, Z.H.; Ma, S.H.; Zhang, S.; Shi, G.; Zhang, M. Dual lanthanide-doped complexes: The development of a time-resolved ratiometric fluorescent probe for anthrax biomarker and a paper-based visual sensor. Biosens. Bioelectron. 2017, 94, 388–393. [Google Scholar] [CrossRef]
- Lee, L.G.; Nordman, E.S.; Johnson, M.D.; Oldham, M.F. A low-cost, high-performance system for fluorescence lateral flow assays. Biosensors 2013, 3, 360–373. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Lin, M.; Gong, Y.; Wang, S.; Li, A.; Ji, L.; Zhao, H.; Ling, K.; Wen, T.; Huang, Y.; et al. Household fluorescent lateral flow strip platform for sensitive and quantitative prognosis of heart failure using dual-color upconversion nanoparticles. ACS Nano 2017, 11, 6261–6270. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Pasini, P.; Mirasoli, M.; Michelini, E.; Guardigli, M. Biotechnological applications of bioluminescence and chemiluminescence. Trends Biotechnol. 2004, 22, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bard, A.J. Electrostatic electrochemistry at insulators. Nat. Mater. 2008, 7, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J. Electrogenerated Chemiluminescence; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Miao, W. Electrogenerated chemiluminescence and its biorelated applications. Chem. Rev. 2008, 108, 2506–2553. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: Review. Sens. Actuators B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Haes, A.J.; Zou, S.; Schatz, G.C.; Van Duyne, R.P. Nanoscale optical biosensor: Short range distance dependence of the localized surface plasmon resonance of noble metal nanoparticles. J. Phys. Chem. B 2004, 108, 6961–6968. [Google Scholar] [CrossRef]
- De Araujo, W.R.; Cardoso, T.M.G.; da Rocha, R.G.; Santana, M.H.P.; Muñoz, R.A.A.; Richter, E.M.; Paixão, T.R.L.C.; Coltro, W.K.T. Portable analytical platforms for forensic chemistry: A review. Anal. Chim. Acta 2018, 1034, 1–21. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical biosensors: Towards point-of-care cancer diagnostics. Biosens. Bioelectron. 2006, 21, 1887–1892. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical biosensors—Sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Bahadır, E.B.; Sezgintürk, M.K. Electrochemical biosensors for hormone analyses. Biosens. Bioelectron. 2015, 68, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Roushani, M.; Shahdost-fard, F. A novel ultrasensitive aptasensor based on silver nanoparticles measured via enhanced voltammetric response of electrochemical reduction of riboflavin as redox probe for cocaine detection. Sens. Actuators B Chem. 2015, 207, 764–771. [Google Scholar] [CrossRef]
- Taghdisi, S.M.; Danesh, N.M.; Emrani, A.S.; Ramezani, M.; Abnous, K. A novel electrochemical aptasensor based on single-walled carbon nanotubes, gold electrode and complimentary strand of aptamer for ultrasensitive detection of cocaine. Biosens. Bioelectron. 2015, 73, 245–250. [Google Scholar] [CrossRef]
- Roushani, M.; Shahdost-fard, F. An aptasensor for voltammetric and impedimetric determination of cocaine based on a glassy carbon electrode modified with platinum nanoparticles and using rutin as a redox probe. Microchim. Acta 2016, 183, 185–193. [Google Scholar] [CrossRef]
- Shahdost-fard, F.; Roushani, M. Conformation switching of an aptamer based on cocaine enhancement on a surface of modified GCE. Talanta 2016, 154, 7–14. [Google Scholar] [CrossRef]
- Tavakkoli, N.; Soltani, N.; Mohammadi, F. A nanoporous gold-based electrochemical aptasensor for sensitive detection of cocaine. RSC Adv. 2019, 9, 14296–14301. [Google Scholar] [CrossRef]
- Su, F.; Zhang, S.; Ji, H.; Zhao, H.; Tian, J.-Y.; Liu, C.-S.; Zhang, Z.; Fang, S.; Zhu, X.; Du, M. Two-Dimensional zirconium-based metal–organic framework nanosheet composites embedded with au nanoclusters: A highly sensitive electrochemical aptasensor toward detecting cocaine. ACS Sens. 2017, 2, 998–1005. [Google Scholar] [CrossRef]
- Hua, M.; Tao, M.; Wang, P.; Zhang, Y.; Wu, Z.; Chang, Y.; Yang, Y. Label-free electrochemical cocaine aptasensor based on a target-inducing aptamer switching conformation. Anal. Sci. 2010, 26, 1265–1270. [Google Scholar] [CrossRef]
- Roushani, M.; Shahdost-fard, F. A highly selective and sensitive cocaine aptasensor based on covalent attachment of the aptamer-functionalized AuNPs onto nanocomposite as the support platform. Anal. Chim. Acta 2015, 853, 214–221. [Google Scholar] [CrossRef]
- Du, Y.; Chen, C.; Yin, J.; Li, B.; Zhou, M.; Dong, S.; Wang, E. Solid-state probe based electrochemical aptasensor for cocaine: A potentially convenient, sensitive, repeatable, and integrated sensing platform for drugs. Anal. Chem. 2010, 82, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Li, J.; Cheng, W.; Yan, Y.; Tang, R.; Li, Y.; Ju, H.; Ding, S. Electrochemical aptasensor for highly sensitive determination of cocaine using a supramolecular aptamer and rolling circle amplification. Microchim. Acta 2015, 182, 361–367. [Google Scholar] [CrossRef]
- Yilmaz, T.; Guler, E.; Gumus, Z.P.; Akbulut, H.; Aldemir, E.; Coskunol, H.; Colak, D.G.; Cianga, I.; Yamada, S.; Timur, S. Synthesis and application of a novel poly-L-phenylalanine electroactive macromonomer as matrix for the biosensing of ‘Abused Drug’model. Polymer Chem. 2016, 7, 7304–7315. [Google Scholar] [CrossRef]
- Kohzadi, R.; Molaeirad, A.; Alijanianzadeh, M.; Kamali, N.; Mohtashamifar, M. Designing a label free aptasensor for detection of methamphetamine. Biomacromol. J. 2016, 2, 28–33. [Google Scholar]
- Azadbakht, A.; Abbasi, A.R. Engineering an aptamer-based recognition sensor for electrochemical opium alkaloid biosensing. J. Mater. Sci. Mater. Electron. 2019, 30, 3432–3442. [Google Scholar] [CrossRef]
- Niu, X.; Huang, L.; Zhao, J.; Yin, M.; Luo, D.; Yang, Y. An ultrasensitive aptamer biosensor for the detection of codeine based on a Au nanoparticle/polyamidoamine dendrimer-modified screen-printed carbon electrode. Anal. Methods 2016, 8, 1091–1095. [Google Scholar] [CrossRef]
- Xiong, W.; Wu, S.F.; Liao, F.S.; Hong, N.; Fan, H.; Wei, G.B. A ZnS-Nanoparticle-Label-Based Electrochemical Codeine Sensor. Proc. Appl. Mech. Mater. 2017, 872, 173–177. [Google Scholar] [CrossRef]
- Dong, H.; Chen, H.; Jiang, J.; Zhang, H.; Cai, C.; Shen, Q. Highly sensitive electrochemical detection of tumor exosomes based on aptamer recognition-induced multi-dna release and cyclic enzymatic amplification. Anal. Chem. 2018, 90, 4507–4513. [Google Scholar] [CrossRef]
- Zhang, D.-W.; Zhang, F.-T.; Cui, Y.-R.; Deng, Q.-P.; Krause, S.; Zhou, Y.-L.; Zhang, X.-X. A label-free aptasensor for the sensitive and specific detection of cocaine using supramolecular aptamer fragments/target complex by electrochemical impedance spectroscopy. Talanta 2012, 92, 65–71. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Johari-Ahar, M.; Hamzeiy, H.; Barar, J.; Mashinchian, O.; Omidi, Y. Electrochemical impedance spectroscopic sensing of methamphetamine by a specific aptamer. Bioimpacts 2012, 2, 91–95. [Google Scholar] [CrossRef]
- Luppa, P.B.; Sokoll, L.J.; Chan, D.W. Immunosensors—Principles and applications to clinical chemistry. Clin. Chim. Acta 2001, 314, 1–26. [Google Scholar] [CrossRef]
- Hoyos-Arbeláez, J.; Vázquez, M.; Contreras-Calderón, J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: A review. Food Chem. 2017, 221, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- ErtuğruL, H.D.; Uygun, Z.O. Impedimetric biosensors for label-free and enzymless detection. In State of the Arts in Biosensors; Intech Rjeka: Rijeka, Croatia, 2013; pp. 179–196. [Google Scholar]
- Yuan, X.-Z.R.; Song, C.; Wang, H.; Zhang, J. Electrochemical Impedance Spectroscopy in PEM Fuel Cells: Fundamentals and Applications; Springer Sci. & Business Media: Berlin, Germany, 2009. [Google Scholar]
- Prodromidis, M.I. Impedimetric immunosensors—A review. Electrochim. Acta 2010, 55, 4227–4233. [Google Scholar] [CrossRef]
- Wang, J.; Anik Kirgöz, Ü.; Mo, J.-W.; Lu, J.; Nasser Kawde, A.; Muck, A. Glassy carbon paste electrodes. Electrochem. Commun. 2001, 3, 203–208. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, N.; Yu, H.; Niu, Y.; Sun, C. Covalent attachment of glucose oxidase to an Au electrode modified with gold nanoparticles for use as glucose biosensor. Bioelectrochemistry 2005, 67, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guan, Z.; Lv, Z.; Jiang, X.; Yang, S.; Chen, A. Improving sensitivity of gold nanoparticle based fluorescence quenching and colorimetric aptasensor by using water resuspended gold nanoparticle. Biosens. Bioelectron. 2014, 52, 265–270. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, J.; Li, Y.; Gao, H.; Guo, J.; Shen, F.; Sun, C. A novel colorimetric aptasensor using cysteamine-stabilized gold nanoparticles as probe for rapid and specific detection of tetracycline in raw milk. Food Control 2015, 54, 7–15. [Google Scholar] [CrossRef]
- Wägli, P.; Chang, Y.-C.; Homsy, A.; Hvozdara, L.; Herzig, H.P.; de Rooij, N.F. Microfluidic droplet-based liquid–liquid extraction and on-chip IR spectroscopy detection of cocaine in human saliva. Anal. Chem. 2013, 85, 7558–7565. [Google Scholar] [CrossRef]
- Sheng, Q.; Liu, R.; Zhang, S.; Zheng, J. Ultrasensitive electrochemical cocaine biosensor based on reversible DNA nanostructure. Biosens. Bioelectron. 2014, 51, 191–194. [Google Scholar] [CrossRef]
- López, P.; Bermejo, A.M.; Tabernero, M.J.; Fernández, P.; Álvarez, I. Determination of cocaine and heroin with their respective metabolites in human hair using gas chromatography-mass spectrometry. Anal. Lett. 2006, 39, 2307–2316. [Google Scholar] [CrossRef]








| Strengths (S) | Weaknesses (W) | Opportunities (O) | Threats (T) |
|---|---|---|---|
| Low-cost and widely applicable | Requirement of advanced devices | POC diagnosis of abused drug | In-laboratory, controlled environment testing |
| Sensitive and rapid | Requirement of expert scientists | On-site applications | Rapidly changing technologies |
| Simple and user-friendly | Lack of real-life sample studies | Real-time measurements | Difficulty in standardization |
| Adaptation possibilities for the newly designed drugs | Decision on choosing target analytes | Adjusting selectivity towards either a target analyte or a target group | Rapidly changing chemical formulations of designed drugs |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celikbas, E.; Balaban, S.; Evran, S.; Coskunol, H.; Timur, S. A Bottom-Up Approach for Developing Aptasensors for Abused Drugs: Biosensors in Forensics. Biosensors 2019, 9, 118. https://doi.org/10.3390/bios9040118
Celikbas E, Balaban S, Evran S, Coskunol H, Timur S. A Bottom-Up Approach for Developing Aptasensors for Abused Drugs: Biosensors in Forensics. Biosensors. 2019; 9(4):118. https://doi.org/10.3390/bios9040118
Chicago/Turabian StyleCelikbas, Eda, Simge Balaban, Serap Evran, Hakan Coskunol, and Suna Timur. 2019. "A Bottom-Up Approach for Developing Aptasensors for Abused Drugs: Biosensors in Forensics" Biosensors 9, no. 4: 118. https://doi.org/10.3390/bios9040118
APA StyleCelikbas, E., Balaban, S., Evran, S., Coskunol, H., & Timur, S. (2019). A Bottom-Up Approach for Developing Aptasensors for Abused Drugs: Biosensors in Forensics. Biosensors, 9(4), 118. https://doi.org/10.3390/bios9040118