Future Trends for In Situ Monitoring of Polycyclic Aromatic Hydrocarbons in Water Sources: The Role of Immunosensing Techniques
Abstract
:1. Introduction
2. Existing Challenges with Conventional PAH Analysis Methods
3. Emerging Technologies
4. Immunoassays and Immunoassay Kits
5. Immunosensors
6. Surface Functionalization Techniques
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Baklanov, A.; Hänninen, O.; Slørdal, L.H.; Kukkonen, J.; Bjergene, N.; Fay, B. Integrated systems for forecasting urban meteorology, air pollution and population exposure. Atmos. Chem. Phys. 2007, 7, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Latimer, J.; Zheng, J. The Sources, Transport, and Fate of PAH in the Marine Environment. In PAHs: An Ecotoxicological Perspective; Douben, P.E.T., Ed.; John Wiley and Sons Ltd.: New York, NY, USA, 2003. [Google Scholar]
- Menzie, A.C.; Potocki, B.B.; Santodonato, J. Exposure to carcinogenic PAHs in the environment. Environ. Sci. Technol. 1992, 26, 1278–1284. [Google Scholar] [CrossRef]
- Arey, J.; Atkinson, R. Photochemical reactions of PAH in the atmosphere. In PAHs: An Ecotoxicological Perspective; Douben, P.E.T., Ed.; John Wiley and Sons Ltd.: New York, NY, USA, 2003; pp. 47–63. [Google Scholar]
- Di Toro, D.M.; McGrath, J.A.; Hansen, D.J. Technical basis for narcotic chemicals and polycyclic aromatic hydrocarbon criteria. I. Water and tissue. Environ. Toxicol. Chem. 2000, 19, 1951–1970. [Google Scholar] [CrossRef]
- Kim, K.; Jahan, S.A.; Kabir, E.; Brown, C.J. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef]
- Armstrong, B.G.; Hutchinson, E.; Unwin, J.; Fletcher, T. Lung cancer risk after exposure to polycyclic aromatic hydrocarbons: A review and meta-analysis. Environ. Health Perspect. 2004, 112, 970–978. [Google Scholar] [CrossRef]
- Canadian Council of Ministers of the Environment. Canadian Soil Quality Guidelines for Potentially Carcinogenic and Other PAHs: Scientific Criteria Document; CCME: Winnipeg, MB, Canada, 2010.
- Suess, M.J. The environmental load and cycle of polycyclic aromatic hydrocarbons. Sci. Total Environ. 1976, 6, 239–250. [Google Scholar] [CrossRef]
- European Communities. The quality of water intended for human consumption. Off. J. Eur. Communities 2016, 98, 83. [Google Scholar]
- Polycyclic aromatic hydrocarbons (PAHs) biologic exposure indices (BEI). Available online: https://www.acgih.org/docs/default-source/presentations/2005/aihce2005_presentation.pdf?sfvrsn=6afadf0d_2 (accessed on 12 April 2018).
- Unwin, J.; Cocker, J.; Scobbie, E.H. An assessment of occupational exposure to polycyclic aromatic hydrocarbons in the UK. Ann. Occup. Hyg. 2006, 50, 395–403. [Google Scholar]
- International Programme on Chemical Safety. Polycyclic Aromatic Hydrocarbons, Selected Non-Heterocyclic. 2010. Available online: http://www.inchem.org/documents/ehc/ehc/ehc202htm (accessed on 10 December 2019).
- Bach, B.P.; Kelley, J.M.; Tate, C.R.; McCrory, C.D. Screening for lung cancer: A review of the current literature. Chest 2003, 123, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Diggs, D.L.; Huderson, A.C.; Harris, K.L.; Myers, J.N.; Banks, L.D.; Rekhadevi, P.V. Polycyclic aromatic hydrocarbons and digestive tract cancers: A perspective. J. Environ. Sci. Health. Part C 2011, 29, 324–357. [Google Scholar] [CrossRef] [Green Version]
- Olsson, A.C.; Fevotte, J.; Fletcher, T.; Cassidy, A.; Mannetje, A.; Brennan, P. Occupational exposure to polycyclic aromatic hydrocarbons and lung cancer risk: A multicenter study in Europe. Occup. Environ. Med. 2010, 67, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Jafarabadi, A.R.; Bakhtiari, A.R.; Yaghoobi, Z.; Yap, C.K.; Maisano, M.; Cappello, T. Distributions and compositional patterns of polycyclic aromatic hydrocarbons (PAHs) and their derivatives in three edible fishes from Kharg coral Island, Persian Gulf, Iran. Chemosphere 2019, 215, 835–845. [Google Scholar] [CrossRef] [PubMed]
- US Environmental Protection Agency. Polycyclic Aromatic Hydrocarbons (PAHs)—EPA Fact Sheet; National Center for Environmental Assessment, Office of Research and Development: Washington, DC, USA, 2008.
- Burchiel, S.W.; Luster, M.I. Signaling by environmental polycyclic aromatic hydrocarbons in human lymphocytes. Clin. Immunol. 2001, 98, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.T.; Achten, C. Time to say goodbye to the 16 EPA PAHs? Toward an up-to-date use of PACs for environmental purposes. Christine Polycycl. Aromat. Compd. 2015, 35, 330–354. [Google Scholar] [CrossRef] [Green Version]
- Rajasärkkä, J.; Pernica, M.; Kuta, J.; Lašňák, J.; Šimek, Z.; Bláha, L. Drinking water contaminants from epoxy resin-coated pipes: A field study. Water Res. 2016, 103, 133–140. [Google Scholar] [CrossRef] [Green Version]
- The European Water Framework Directive. Introduction to EU WFD 2013/39/EU. In Thermo Scientific Environmental Solutions Reference Guide; Thermo Scientific: Brussels, Belgium, 2013; Available online: https://assets.thermofisher.com/TFS-Assets/CMD/brochures/XX-71741-EU-Water-Framework-Directive-Reference-Guide-XX71741-EN.pdf (accessed on 3 February 2017).
- Nowacka, A.; Włodarczyk-Makuła, M. Monitoring of polycyclic aromatic hydrocarbons in water during preparation processes. Polycycl. Aromat. Compd. 2013, 33, 430–450. [Google Scholar] [CrossRef]
- Maliszewska-Kordybach, B. Sources, concentrations, fate and effects of polycyclic aromatic hydrocarbons (PAHs) in the environment. Part A: PAHs in air. Pol. J. Environ. Stud. 1999, 8, 131–136. [Google Scholar]
- Akyuz, M.; Cabuk, H. Gas—particle partitioning and seasonal variation of polycyclic aromatic hydrocarbons in the atmosphere of Zonguldak, Turkey. Sci. Total Environ. 2010, 408, 5550–5558. [Google Scholar] [CrossRef]
- Determination of Parent and Alkylated Polycyclic Aromatic Hydrocarbons (PAHs) in Biota and Sediment. Available online: https://pdfs.semanticscholar.org/c83a/9554342d38c4eeab6004b44b9b285278714d.pdf (accessed on 3 December 2019).
- Masih, J.; Singhvi, R.; Kumar, K.; Jain, V.K. Seasonal variation and sources of polycyclic aromatic hydrocarbons (PAHs) in indoor and outdoor air in a semi-arid tract of northern India. Aerosol Air Qual Res. 2012, 12, 515–525. [Google Scholar] [CrossRef] [Green Version]
- Wild, S.R.; Jones, K.C. Polynuclear aromatic hydrocarbons in the United Kingdom environment: A preliminary source inventory and budget. Environ. Pollut. 1995, 88, 91–108. [Google Scholar] [CrossRef]
- Sarria-Villa, R.; Ocampo-Duque, W.; Páez, M.; Schuhmacher, M. Presence of PAHs in water and sediments of the Colombian Cauca River during heavy rain episodes, and implications for risk assessment. Sci. Total Environ. 2016, 540, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Singare, P.U. Studies on polycyclic aromatic hydrocarbons in surface sediments of Mithi River near Mumbai, India: Assessment of sources, toxicity risk and biological impact. Mar. Pollut. Bull. 2015, 101, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fernández, B.; Viñas, L.; Franco, M.Á.; Bargiela, J. PAHs in the Ría de Arousa (NW Spain): A consideration of PAHs sources and abundance. Mar. Pollut. Bull. 2015, 95, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Song, D.; Chen, F. Towards the fabrication of a label-free amperometric immunosensor using SWNTs for direct detection of paraoxon. Talanta 2013, 104, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, J.; Yu, G.; Hong, H. Occurrence of PAHs, PCBs and organochlorine pesticides in the Tonghui River of Beijing, China. Environ. Pollut. 2004, 130, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Yunker, M.B.; Macdonald, R.W.; Vingarzan, R.; Mitchell, R.H.; Goyette, D.; Sylvestre, S. PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 2000, 33, 489–515. [Google Scholar] [CrossRef]
- Neff, J.; Stout, S.; Gunster, D. Ecological Risk Assessment of Polycyclic Aromatic Hydrocarbons in Sediments: Identifying Sources and Ecological Hazard. Integr. Environ. Assess. Manag. 2004, 1, 22–23. [Google Scholar] [CrossRef]
- Pies, C.; Hoffmann, B.; Petrowsky, J.; Yang, Y.; Ternes, T.A.; Hofmann, T. Characterization and source identification of polycyclic aromatic hydrocarbons (PAHs) in river bank soils. Chemosphere 2008, 72, 1594–1601. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Wan, C.; Yue, D.; Ye, Y.; Wang, X. Source diagnostics of polycyclic aromatic hydrocarbons in urban road runoff, dust, rain and canopy throughfall. Environ. Pollut. 2008, 153, 594–601. [Google Scholar] [CrossRef]
- Ding, S.; Xu, Y.; Wang, Y.; Zhang, X.; Zhao, L.; Ruan, J.; Wu, W. Spatial and Temporal Variability of Polycyclic Aromatic Hydrocarbons in Sediments from Yellow River-Dominated Margin. Sci. World J. 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- El Nemr, A.; Moneer, A.; Ragab, S.; El Sikaily, A. Distribution and sources of n-alkanes and polycyclic aromatic hydrocarbons in shellfish of the Egyptian Red Sea coast. Egypt. J. Aquat. Res. 2016, 42, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Callén, M.; de la Cruz, M.; López, J.; Mastral, A. PAH in airborne particulate matter: Carcinogenic character of PM10 samples and assessment of the energy generation impact. Fuel Process. Technol. 2011, 92, 176–182. [Google Scholar] [CrossRef]
- Tipmanee, D.; Deelaman, W.; Pongpiachan, S.; Schwarzer, K.; Sompongchaiyakul, P. Using Polycyclic Aromatic Hydrocarbons (PAHs) as a chemical proxy to indicate Tsunami 2004 backwash in Khao Lak coastal area, Thailand. Nat. Hazards Earth Syst. Sci. 2012, 12, 1441–1451. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, X.; Zhang, Z. Co-biodegradation of pyrene and other PAHs by the bacterium Acinetobacter johnsonii. Ecotoxicol. Environ. Saf. 2018, 163, 465–470. [Google Scholar] [CrossRef]
- Dong, C.D.; Chen, C.F.; Chen, C.W. Determination of polycyclic aromatic hydrocarbons in industrial harbor sediments by GC-MS. Int. J. Environ. Res. Public Health 2012, 9, 2175–2188. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, R. A structure-activity relationship for the estimation of rate constants for the gas-phase reactions of OH radicals with organic compounds. Int. J. Chem. Kinet. 1987, 19, 799–828. [Google Scholar] [CrossRef]
- Elcoroaristizabal, S.; de Juan, A.; García, J.; Durana, N.; Alonso, C.L. Comparison of second-order multivariate methods for screening and determination of PAHs by total fluorescence spectroscopy. Chem. Intel. Lab. Syst. 2014, 132, 63–74. [Google Scholar] [CrossRef]
- Molaei, S.; Saleh, A.; Ghoulipour VSeidi, S. Centrifuge-less Emulsification Microextraction Using Effervescent CO2 Tablet for On-site Extraction of PAHs in Water Samples Prior to GC–MS Detection. Chromatographia 2016, 79, 629–640. [Google Scholar] [CrossRef]
- Wang, W.; Ma, R.; Wu, Q.; Wang, C.; Wang, Z. Magnetic microsphere-confined graphene for the extraction of polycyclic aromatic hydrocarbons from environmental water samples coupled with high performance liquid chromatography–fluorescence analysis. J. Chromatogr. A 2013, 1293, 20–27. [Google Scholar] [CrossRef]
- Gilgenast, E.; Boczkaj, G.; Przyjazny, A.; Kamiński, A. Sample preparation procedure for the determination of polycyclic aromatic hydrocarbons in petroleum vacuum residue and bitumen. Anal. Bioanal. Chem. 2011, 401, 1059–1069. [Google Scholar] [CrossRef] [Green Version]
- Lux, G.; Langer, A.; Pschenitza, M.; Karsunke, X.; Strasser, R.; Niessner, R.; Knopp, D.; Rant, U. Detection of the carcinogenic water pollutant benzo [a] pyrene with an electro-switchable biosurface. Anal. Chem. 2015, 87, 4538–4545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Q.X.; Guo, Z.H.; Lin, J.H. Practical Application of Aptamer-Based Biosensors in Detection of Low Molecular Weight Pollutants in Water Sources. Molecules 2018, 23, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manoli, E.; Kouras, A.; Samara, C. Profile analysis of ambient and source emitted particle-bound polycyclic aromatic hydrocarbons from three sites in northern Greece. Chemosphere 2004, 56, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Mostert, M.; Ayoko, G.A.; Kokot, S. Application of chemometrics to analysis of soil pollutants. Trends Anal. Chem. 2010, 29, 430–445. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.; Wade, T.; Sericano, J. Concentrations and source characterization of polycyclic aromatic hydrocarbons in pine needles from Korea, Mexico, and United States. Atmos. Environ. 2003, 37, 2259–2267. [Google Scholar] [CrossRef]
- Kielhorn, J.; Boehncke, A. Polynuclear Aromatic Hydrocarbons. In Guidelines for Drinking-Water Quality, 2nd ed.; Health Criteria and other Supporting Information; World Health Organization: Geneva, Switzerland, 1998; pp. 123–152. [Google Scholar]
- Vo-Dinh, T.; Fetzer, J.; Campiglia, A. Monitoring and characterization of polyaromatic compounds in the environment. Talanta 1998, 47, 943–969. [Google Scholar] [CrossRef]
- Hund, K.; Traunspurger, W. Ecotox-evaluation strategy for soil bioremediation exemplified for a PAH-contaminated site. Chemosphere 1994, 29, 371–390. [Google Scholar] [CrossRef]
- Hamza-Chaffai, A. Usefulness of bioindicators and biomarkers in pollution biomonitoring. Int. J. Biotechnol. Wellness Ind. 2014, 3, 19–26. [Google Scholar] [CrossRef]
- Del Carlo, M.; Di Marcello, M.; Perugini, M.; Ponzielli, V.; Sergi, M.; Mascini, M.; Compagnone, D. Electrochemical DNA biosensor for polycyclic aromatic hydrocarbon detection. Microchim. Acta 2008, 163, 163–169. [Google Scholar] [CrossRef]
- Gu, M.B.; Chang, S.T. Soil biosensor for the detection of PAH toxicity using an immobilized recombinant bacterium and a biosurfactant. Biosens. Bioelectron. 2001, 16, 667–674. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, B.; Tian, S.; Tang, H.; Liu, Z.; Li, C.; Li, G. A whole-cell bioreporter approach for the genotoxicity assessment of bioavailability of toxic compounds in contaminated soil in China. Environ. Pollut. 2014, 195, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Anderson, J.W.; Tukey, R.H. Using the metabolism of PAHs in a human cell line to characterize environmental samples. Environ. Toxicol. Pharm. 2000, 8, 119–126. [Google Scholar] [CrossRef]
- Fent, K. Fish cell lines as versatile tools in ecotoxicology: Assessment of cytotoxicity, cytochrome P4501A induction potential and estrogenic activity of chemicals and environmental samples. Toxicol. Vitr. 2001, 15, 477–488. [Google Scholar] [CrossRef]
- Ahmad, A.; Moore, E. Comparison of Cell-Based Biosensors with Traditional Analytical Techniques for Cytotoxicity Monitoring and Screening of Polycyclic Aromatic Hydrocarbons in the Environment. Anal. Lett. 2009, 42, 1–28. [Google Scholar] [CrossRef]
- Anderson, J.W.; Zeng, E.; Jones, J.M. Correlation between the response of a human cell line (P450RGS) and the distribution of sediment PAHs and PCBs on the Palos Verdes Shelf, California. Environ. Toxicol. Chem. 1999, 18, 1506–1510. [Google Scholar] [CrossRef]
- Lawal, A.T. Polycyclic aromatic hydrocarbons. A review. Cogent Environ. Sci. 2017, 3, 1339841. [Google Scholar] [CrossRef]
- Srogi, K. Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: A review. Environ. Chem. Lett. 2007, 5, 169–195. [Google Scholar] [CrossRef] [Green Version]
- Engvall, E.; Jonsson, K.; Perlmann, P. Enzyme-linked immunosorbent assay. II. Quantitative assay of protein antigen, immunoglobulin G, by means of enzyme-labelled antigen and antibody-coated tubes. Biochim. Biophys. Acta 1971, 251, 427–434. [Google Scholar] [CrossRef]
- Bansal, V.; Kumar, P.; Kwon, E.E.; Kim, K.H. Review of the quantification techniques for polycyclic aromatic hydrocarbons (PAHs) in food products. Crit. Rev. Food Sci. Nutr. 2017, 57, 3297–3312. [Google Scholar] [CrossRef]
- Fillmann, G.; Bicego, M.C.; Zamboni, A.; Fileman, T.W.; Depledge, M.H.; Readman, J.W. Readma Validation of immunoassay methods to determine hydrocarbon contamination a in estuarine Sediments. J. Braz. Chem. Soc. 2016, 18, 774–781. [Google Scholar] [CrossRef]
- National Environmental Methods Index (NEMI). Available online: https://acwi.gov/methods/pubs/nemi_pubs/nemi_fs2.pdf (accessed on 3 December 2019).
- National Environmental Methods Index (NEMI). Rapid Assay PAH Test Kit A00156/A00157; Strategic diagnostics Inc.: Newark, DE, USA, 2018. [Google Scholar]
- National Environmental Methods Index (NEMI). EPA Method 70620; Strategic diagnostics Inc.: Newark, DE, USA, 2018. [Google Scholar]
- Hennion, M.C.; Barcelo, D. Strengths and limitations of immunoassays for effective and efficient use for pesticide analysis in water samples: A review. Anal. Chim. Acta 1998, 362, 3–34. [Google Scholar] [CrossRef]
- Pollap, A.; Kochana, J. Electrochemical Immunosensors for Antibiotic Detection. Biosensor 2019, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Rhouati, A.; Catanante, G.; Nunes, G.; Hayat, A.; Marty, J.L. Label-Free Aptasensors for the Detection of Mycotoxins. Sensors 2016, 16, 2178. [Google Scholar] [CrossRef]
- Fähnrich, K.; Pravda, M.; Guilbault, G. Immunochemical detection of polycyclic aromatic hydrocarbons (PAHs). Anal. Lett. 2002, 35, 1269–1300. [Google Scholar] [CrossRef]
- Ramírez, N.B.; Salgado, A.M.; Valdman, B. The evolution and developments of immunosensors for health and environmental monitoring: Problems and perspectives. Braz. J. Chem. Eng. 2009, 26, 227–249. [Google Scholar] [CrossRef]
- Trilling, A.K.; Beekwilder, J.; Zuilhof, H. Antibody orientation on biosensor surfaces: A mini review. Analyst 2013, 138, 1619–1627. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.H.; Su, Q.; Xu, J.H.; Zhang, Y.; Chen, S.T. Detecting of Benzo [a] pyrene Using a Label-free Amperometric Immunosensor. Int. J. Electrochem. Sci. 2014, 9, 3736–3745. [Google Scholar]
- Sassolas, A.; Prieto-Simón, B.; Marty, J.L. Biosensors for pesticide detection: New trends. Am. J. Anal. Chem. 2012, 3, 210. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Moore, E. Electrochemical immunosensor modified with self-assembled monolayer of 11-mercaptoundecanoic acid on gold electrodes for detection of benzo [a] pyrene in water. Analyst 2012, 137, 5839–5844. [Google Scholar] [CrossRef]
- Zheng, X.; Tian, D.; Duan, S.; Wei, M.; Liu, S.; Zhou, C.; Wu, G. Polypyrrole composite film for highly sensitive and selective electrochemical determination sensors. Electrochim. Acta 2014, 130, 187–193. [Google Scholar] [CrossRef]
- Nanomaterials for advancing the health immunosensor. Available online: https://www.intechopen.com/books/biosensors-micro-and-nanoscale-applications/nanomaterials-for-advancing-the-health-immunosensor (accessed on 10 December 2019).
- Barathi, P.; Senthil Kumar, A. Electrochemical conversion of unreactive pyrene to highly redox-active 1, 2-quinone derivatives on a carbon nanotube-modified gold electrode surface and its selective hydrogen peroxide sensing. Langmuir 2013, 29, 10617–10623. [Google Scholar] [CrossRef]
- Lin, M.; Liu, Y.; Sun, Z.; Zhang, S.; Yang, Z.; Ni, C. Electrochemical immunoassay of benzo [a] pyrene based on dual amplification strategy of electron-accelerated Fe3O4/polyaniline platform and multi-enzyme-functionalized carbon sphere label. Anal. Chim. Acta 2012, 722, 100–106. [Google Scholar] [CrossRef]
- Akanda, M.R.; Aziz, M.A.; Jo, K.; Tamilavan, V.; Hyun, M.H.; Kim, S.; Yang, H. Optimization of phosphatase-and redox cycling-based immunosensors and its application to ultrasensitive detection of troponin I. Anal. Chem. 2011, 83, 3926–3933. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.Y.; Wen, S.D.; Yang, X.Q.; Guo, L.H. Development of redox-labeled electrochemical immunoassay for polycyclic aromatic hydrocarbons with controlled surface modification and catalytic voltammetric detection. Biosens. Bioelectron. 2009, 24, 2909–2914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cong, Y.; Huang, Y.; Du, X. Nanomaterials-Based Electrochemical Immunosensors. Micromachines 2019, 10, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yost, A.L.; Shahsavari, S.; Bradwell, G.M.; Polak, R.; Fachin, F.; Cohen, R.E.; Wardle, B.L. Layer-by-layer functionalized nanotube arrays: A versatile microfluidic platform for biodetection. Microsyst. Nanoeng. 2015, 1, 15037. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.L.; Li, Z.H.; Liu, P.; Song, B.B.; Cai, Q.Y.; Grimes, C.A. Aminocalix [4] arene monolayers as magnetoelastic sensor sensing elements for selective detection of benzo [a] pyrene. Anal. Methods 2016, 8, 912–918. [Google Scholar] [CrossRef]
- Penezić, A.; Gašparović, B.; Stipaničev, D.; Nelson, A. In situ electrochemical method for detecting freely dissolved polycyclic aromatic hydrocarbons in water. Environ. Chem. 2014, 11, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Bunkoed, O.; Kanatharana, P. Extraction of polycyclic aromatic hydrocarbons with a magnetic sorbent composed of alginate, magnetite nanoparticles and multiwalled carbon nanotubes. Microchim. Acta 2015, 182, 1519–1526. [Google Scholar] [CrossRef]
- Mehdinia, A.; Khodaee, N.; Jabbari, A. Fabrication of graphene/Fe3O4@ polythiophene nanocomposite and its application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Anal. Chim. Acta 2015, 868, 1–9. [Google Scholar] [CrossRef]
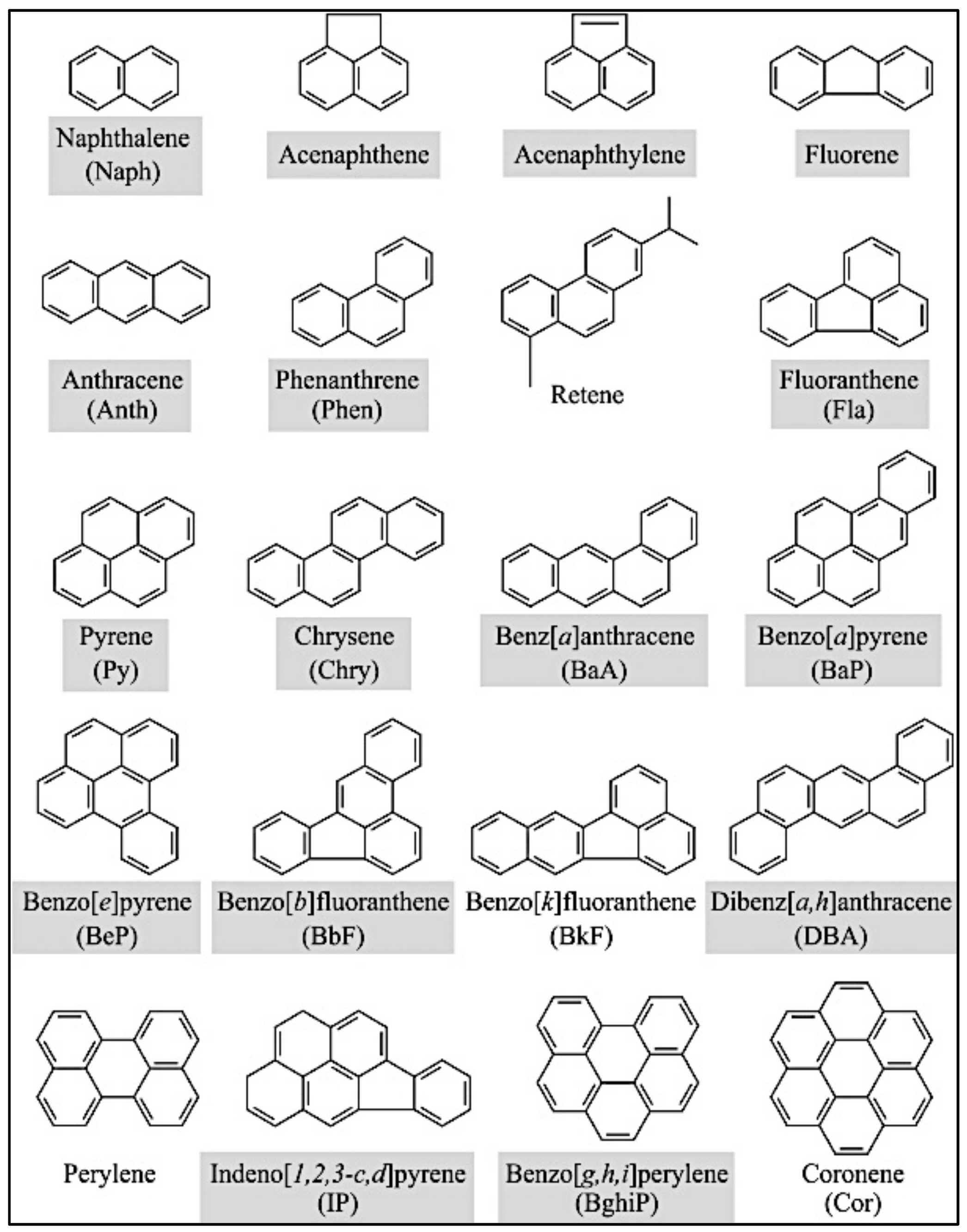

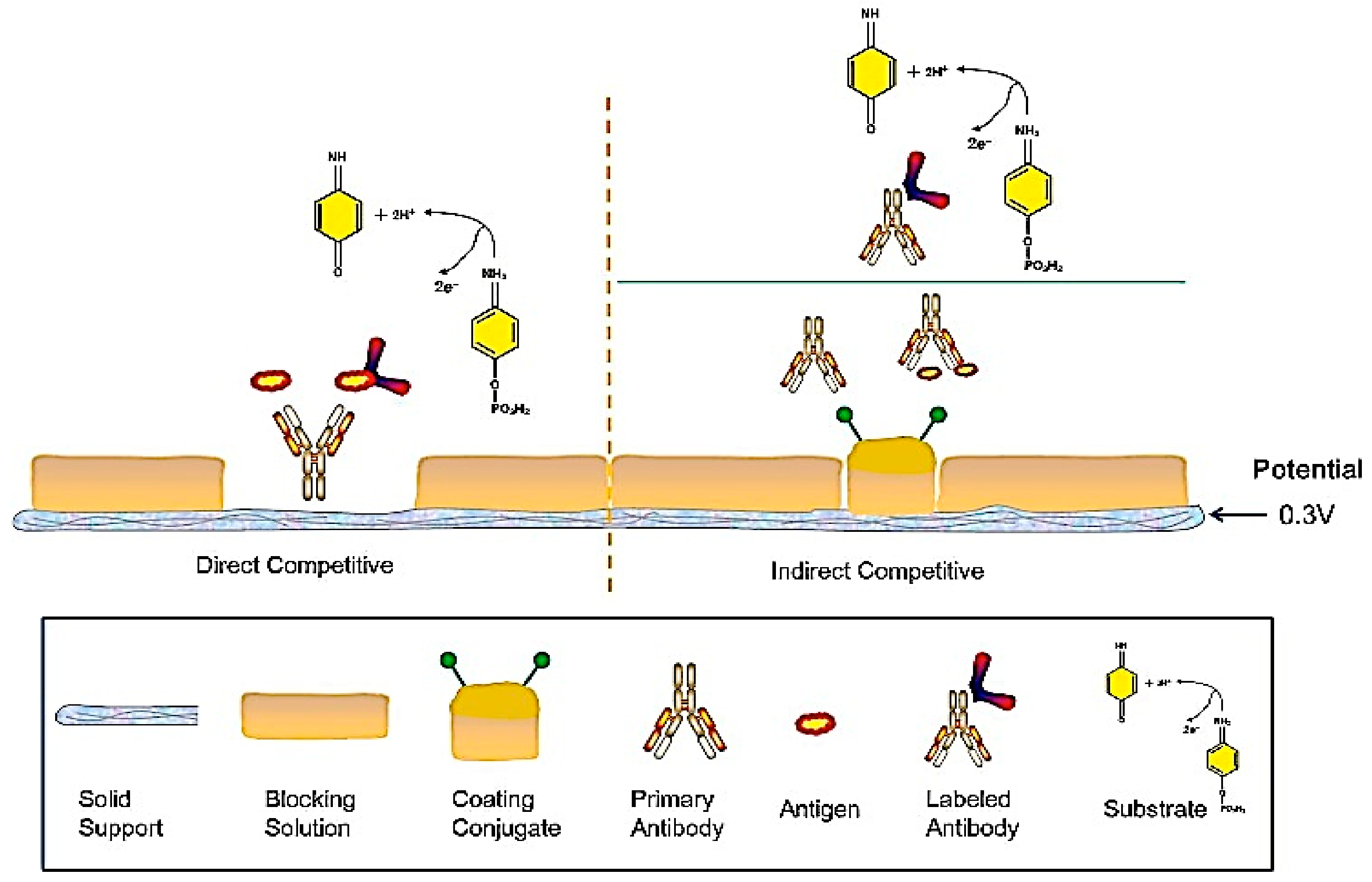



| Short | Long-Standing Contact |
|---|---|
| Impaired lung function and a coronary heart condition by inhalation; intake of water contaminated with PAHs has resulted in diarrhea, vomiting, and nausea conditions; when the human skin is exposed to PAHs, it results in irritation, swelling, and allergic reactions of the skin [11,12,13] | Detrimental effects on the reproductive and development systems; kidney and liver infection; cataracts inducement; jaundice; malfunction of red blood cells (RBCs) [14,15,16] |
| Compound | 2013/39/EU | |
|---|---|---|
| Annual Average—Environmental Quality Standard Inland Surface Water (µg/L) | Environmental Quality Standard/Required Limit (µg/L) | |
| Anthracene | 0.1 | 0.034 |
| Benzo(a)pyrene | 0.00017 | 0.000057 |
| Benzo(b)fluoranthene | 0.00017 | 0.000057 |
| Benzo(k)fluoranthene | 0.00017 | 0.000057 |
| Benzo(ghi)perylene | 0.00017 | 0.000057 |
| Indeno(123cd)pyrene | 0.00017 | 0.000057 |
| Fluoranthene | 0.0063 | 0.0021 |
| Naphtalene | 2 | 0.67 |
| Pentachlorobenzene | 0.007 | 0.0023 |
| PAH Ratio | Value Range | PAHs Source | Country | Ref |
|---|---|---|---|---|
| IcdP/(IcdP + BghiP) | <0.2 | petrogenic | Fraser River basin, Canada | [34] |
| Flpy/(Flpy + C24Ph) | >0.2–0.5 | Pyrolytic | Eagle Harbor, USA | [35] |
| Ant/(Ant + Phe) | <0.1 | petrogenic | Mosel and Saar Rivers in German | [36] |
| ΣLMW/HMW | >0.75 | Pyrolytic | Beijing, China | [37] |
| Fla/(Fla + Pyr) | <0.1 | petrogenic | Yellow River, China. | [38] |
| Ant/(Ant + Phe) vs. Flur/(Flur + Pyr) | >0.1 | Pyrolytic | Egyptian Red Sea coast | [39] |
| ΣMePhe/Phe | <1 | Pyrolytic | Zaragoza city, Spain | [40] |
| Ind/Ind + B(g,h,i)P | >1 | petrogenic | Khao Lak coastal area, Thailand | [41] |
| Immunosensors | Definitions |
|---|---|
| Electrochemical | An antibody can be used as a receptor and can be grouped depending on the detection method [66,79], such as electrochemical impedance spectroscopy, potentiometric, conductometric, or amperometric [80,81,82,83]. |
| Optical | The antibody–antigen complex triggers changes in the optical characteristics of the substrate, which can be detected by the use of different techniques, such as total internal reflection fluorescence (TIRF) and polarization–modulation infrared reflection–absorption spectroscopy (PM-IRRAS) [76]. Additional methods of detection include chemiluminescence, fluorescence [77], and Raman spectroscopy [73]. |
| Mechanical | The basis in of this type of transduction is the response of a surface upon variations in the stress and loading applied to it. Velocity and position can be used in detecting measurement performance [74,84]. In addition, piezoelectric materials (where mechanical stress generates an accumulation of electric charge) such as quartz crystals have been used to immobilize antibodies and antigens [76,81]. |
| PAHs | Detection Limit (μg/L) |
|---|---|
| Phenanthrene | 0.33 |
| Pyrene | 0.35 |
| Anthracene | 0.15 |
| Fluoranthene | 0.32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felemban, S.; Vazquez, P.; Moore, E. Future Trends for In Situ Monitoring of Polycyclic Aromatic Hydrocarbons in Water Sources: The Role of Immunosensing Techniques. Biosensors 2019, 9, 142. https://doi.org/10.3390/bios9040142
Felemban S, Vazquez P, Moore E. Future Trends for In Situ Monitoring of Polycyclic Aromatic Hydrocarbons in Water Sources: The Role of Immunosensing Techniques. Biosensors. 2019; 9(4):142. https://doi.org/10.3390/bios9040142
Chicago/Turabian StyleFelemban, Shifa, Patricia Vazquez, and Eric Moore. 2019. "Future Trends for In Situ Monitoring of Polycyclic Aromatic Hydrocarbons in Water Sources: The Role of Immunosensing Techniques" Biosensors 9, no. 4: 142. https://doi.org/10.3390/bios9040142




