Effect of Citrus aurantium L. Essential Oil on Streptococcus mutans Growth, Biofilm Formation and Virulent Genes Expression
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition and Antioxidant Activities of C. aurantium L. Essential Oil
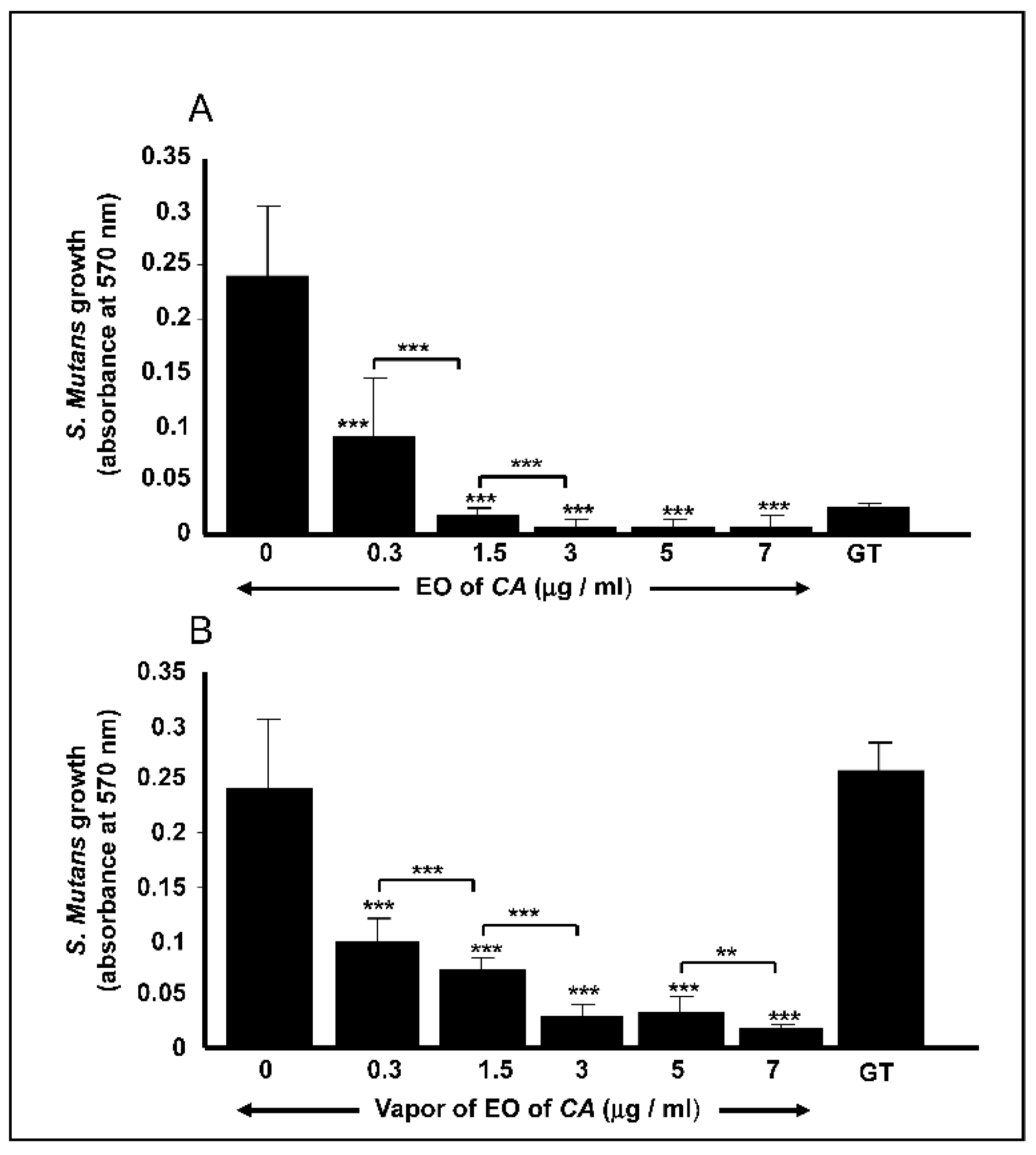
2.2. C. aurantium L. Essential Oil decreased S. mutans Cell Growth
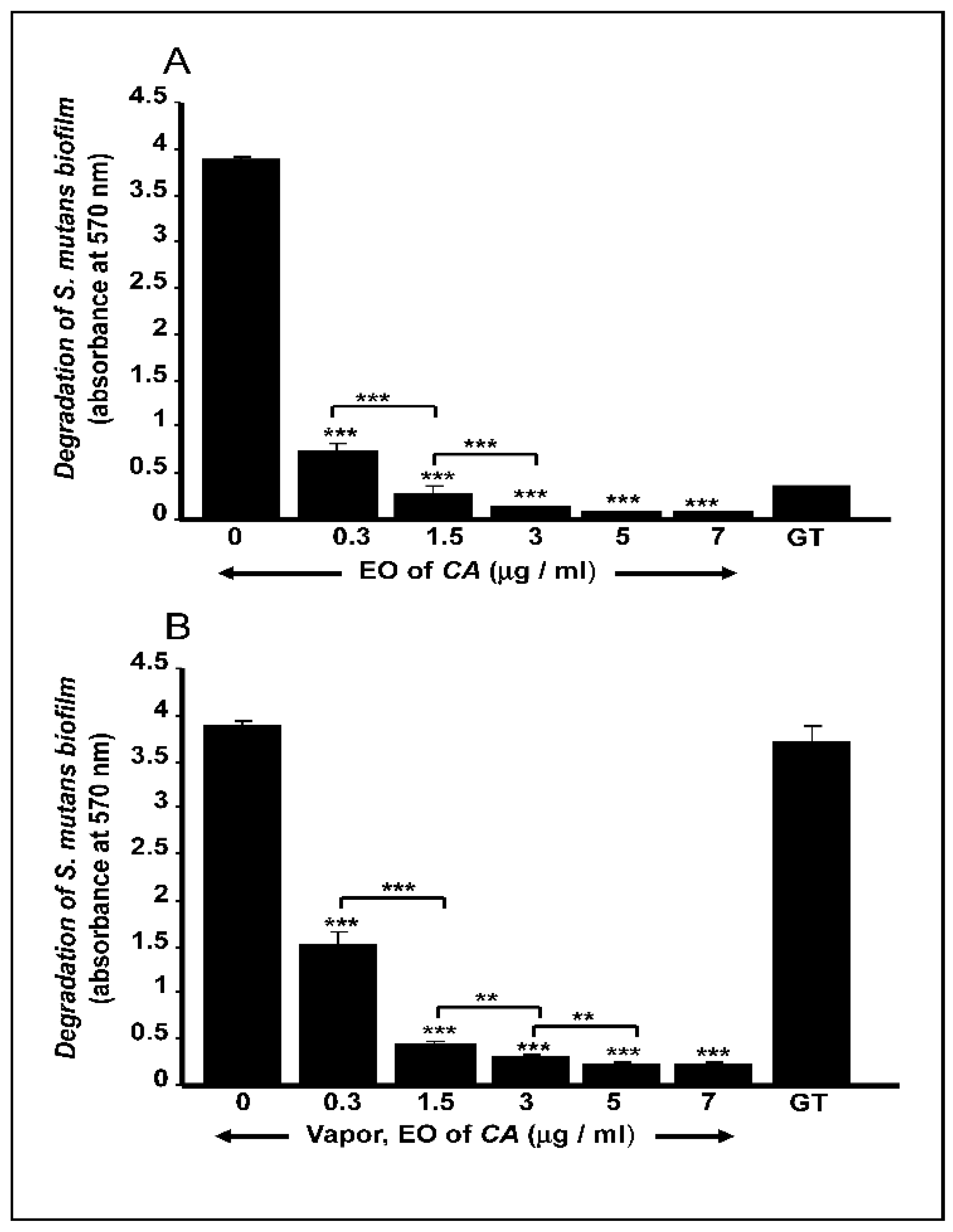
2.3. C. aurantium L. Essential Oil Disrupted Mature S. mutans Biofilms
2.4. C. aurantium L. Essential Oil Decreased the mRNA Expression of Various Virulent Genes by S. mutans
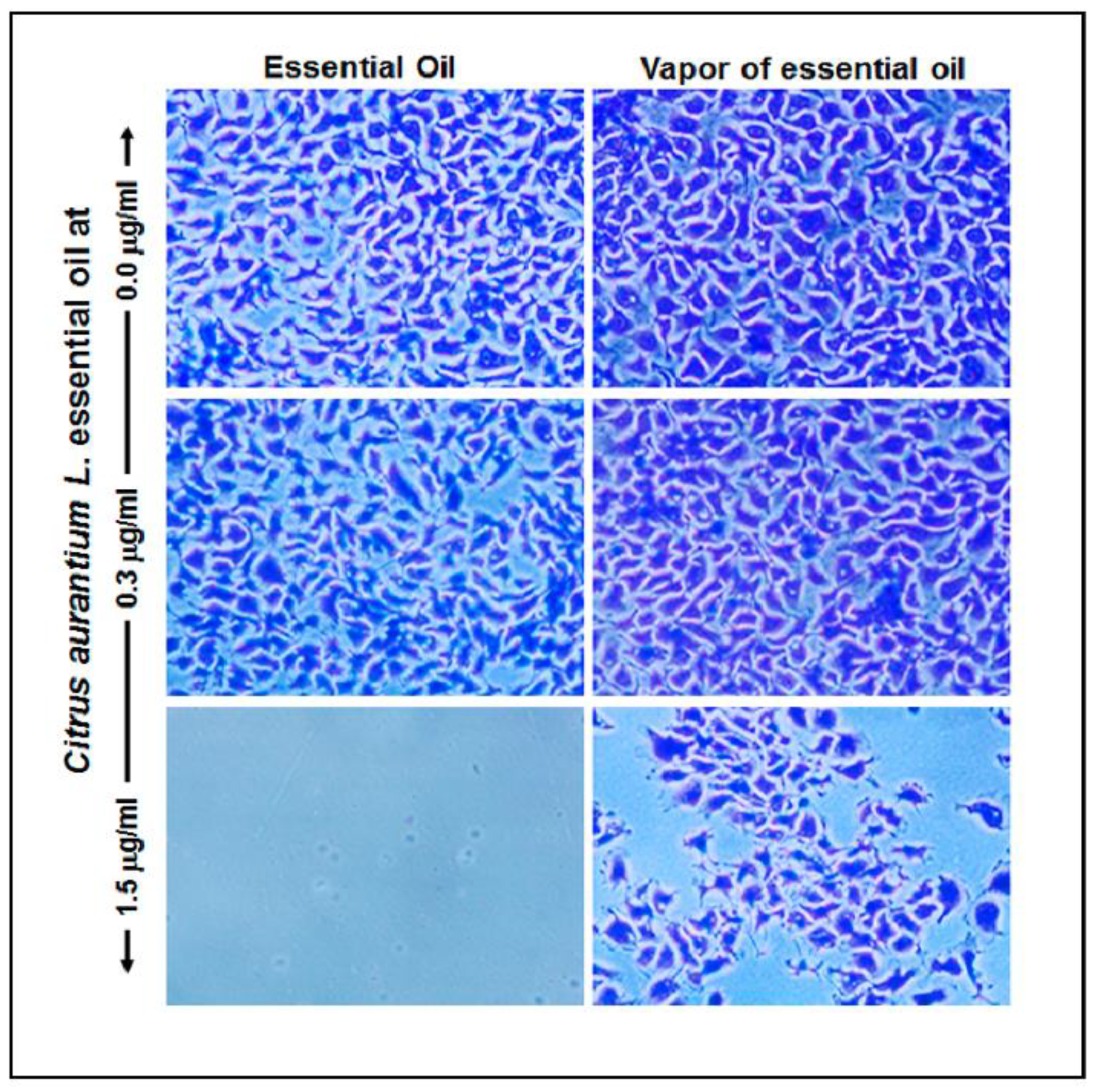
2.5. C. aurantium L. Essential Oil Reduced Gingival Epithelial Cell Attachment
2.6. C. aurantium L. Essential Oil Decreased Gingival Epithelial Cell Viability
3. Discussion
4. Materials and Methods
4.1. Plant Material, Extraction of C. aurantium L. Essential Oil, and Chemical Analyses
4.2. Effect of C. aurantium L. EO on S. mutans Growth
4.3. Effect of C. aurantium L. EO on the Disruption of Mature Biofilm
4.4. Effect of C. aurantium L. EO on the Activation/Repression of Various S. mutans Genes
4.5. Effect of C. aurantium L. EO on Gingival Epithelial Cell Adhesion and Morphology
4.6. Effect of C. aurantium L. EO on Gingival Epithelial Cell Growth and Proliferation
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrentino, G.; Morozova, K.; Horn, C.; Scampicchio, M. Extraction of essential oils from medical plants and their utilization as food antioxidants. Curr. Pharm. Des. 2020, 26, 519–541. [Google Scholar] [CrossRef]
- Bighetti, E.J.; Hiruma-Lima, C.A.; Gracioso, J.S.; Brito, A.R. Anti-inflammatory and antinociceptive effects in rodents of the essential oil of Croton cajucara Benth. J. Pharm. Pharmacol. 1999, 51, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Nasiri Lari, Z.; Hajimonfarednejad, M.; Riasatian, M.; Abolhassanzadeh, Z.; Iraji, A.; Vojoud, M.; Heydari, M.; Shams, M. Efficacy of inhaled Lavandula angustifolia Mill. Essential oil on sleep quality, quality of life and metabolic control in patients with diabetes mellitus type II and insomnia. J. Ethnopharmacol. 2020, 251, 112560. [Google Scholar] [CrossRef]
- Khan, M.; Alkhathlan, H.Z.; Khan, S.T. Antibiotic and Antibiofilm Activities of Salvadora persica L. Essential Oils against Streptococcus mutans: A Detailed Comparative Study with Chlorhexidine Digluconate. Pathogens 2020, 9, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jünger, H.; Jaun-Ventrice, A.; Guldener, K.; Ramseier, C.A.; Reissmann, D.R.; Schimmel, M. Anti-inflammatory potential of an essential oil-containing mouthwash in elderly subjects enrolled in supportive periodontal therapy: A 6-week randomised controlled clinical trial. Clin. Oral Investig. 2020, 24, 3203–3211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, D. Effect of essential oil mouthwashes on plaque and gingivitis. Evid. Based Dent. 2017, 18, 39–40. [Google Scholar] [CrossRef] [PubMed]
- Fanning, S.; Mitchell, A.P. Fungal biofilms. PLoS Pathog. 2012, 8, e1002585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantanella, F.; Valenti, P.; Natalizi, T.; Passeri, D.; Berlutti, F. Analytical techniques to study microbial biofilm on abiotic surfaces: Pros and cons of the main techniques currently in use. Ann Ig. 2013, 25, 31–42. [Google Scholar] [PubMed]
- Li, Y.; Burne, R.A. Regulation of the gtfBC and ftf genes of Streptococcus mutans in biofilms in response to pH and carbohydrate. Microbiology 2001, 147, 2841–2848. [Google Scholar] [CrossRef] [Green Version]
- Rouabhia, M.; Mukherjee, P.K.; Lattif, A.A.; Curt, S.; Chandra, J.; Ghannoum, M.A. Disruption of sphingolipid biosynthetic gene IPT1 reduces Candida albicans adhesion and prevents activation of human gingival epithelial cell innate immune defense. Med. Mycol. 2011, 49, 458–466. [Google Scholar]
- Rouabhia, M.; Semlali, A.; Chandra, J.; Mukherjee, P.; Chmielewski, W.; Ghannoum, M.A. Disruption of the ECM33 gene in Candida albicans prevents biofilm formation, engineered human oral mucosa tissue damage and gingival cell necrosis/apoptosis. Mediat. Inflamm. 2012, 2012, 398207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents. 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samaranayake, L.; Matsubara, V.H. Normal Oral Flora and the Oral Ecosystem. Dent. Clin. North Am. 2017, 61, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Belstrøm, D. The salivary microbiota in health and disease. J. Oral 2020, 12, 1723975. [Google Scholar] [CrossRef] [Green Version]
- Peacock, M.E.; Arce, R.M.; Cutler, C.W. Periodontal and other oral manifestations of immunodeficiency diseases. Oral Dis. 2017, 23, 866–888. [Google Scholar] [CrossRef]
- Kilian, M.; Chapple, I.L.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef]
- Lauritano, D.; Moreo, G.; Della Vella, F.; Di Stasio, D.; Carinci, F.; Lucchese, A.; Petruzzi, M. Oral Health Status and Need for Oral Care in an Aging Population: A Systematic Review. Int. J. Environ. Res. Public Health. 2019, 16, 4558. [Google Scholar] [CrossRef] [Green Version]
- Takenaka, S.; Ohsumi, T.; Noiri, Y. Evidence-based strategy for dental biofilms: Current evidence of mouthwashes on dental biofilm and gingivitis. Jpn. Dent. Sci. Rev. 2019, 55, 33–40. [Google Scholar] [CrossRef]
- Cantarelli, R.; Negrini, T.C.; Muniz, F.W.; Oballe, H.J.; Arthur, R.A.; Rösing, C.K. Antimicrobial potential and gustatory perception of chlorhexidine gluconate mouthwashes with or without alcohol after a single rinse—A randomized controlled crossover clinical trial. Int. J. Dent. Hyg. 2017, 15, 280–286. [Google Scholar] [CrossRef]
- Araujo, M.W.B.; Charles, C.A.; Weinstein, R.B.; McGuire, J.A.; Parikh-Das, A.M.; Du, Q.; Zhang, J.; Berlin, J.A.; Gunsolley, J.C. Meta-analysis of the effect of an essential oil-containing mouthrinse on gingivitis and plaque. J. Am. Dent. Assoc. 2015, 146, 610–622. [Google Scholar] [CrossRef] [Green Version]
- Lynch, M.C.; Cortelli, S.C.; McGuire, J.A.; Zhang, J.; Ricci-Nittel, D.; Mordas, C.J.; Aquino, D.R.; Cortelli, J.R. The effects of essential oil mouthrinses with or without alcohol on plaque and gingivitis: A randomized controlled clinical study. BMC Oral Health 2018, 18, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Değirmenci, H.; Erkurt, H. Relationship between volatile components, antimicrobial and antioxidant properties of the essential oil, hydrosol and extracts of Citrus aurantium L. flowers. J. Infect. Public Health. 2020, 13, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Salma, M.; Abdellah, F.; Houssine, A.; Kawtar, B.; Dalila, B. Comparison of the chemical composition and the bioactivity of the essential oils of three medicinal and aromatic plants from Jacky Garden of Morocco. Int. J. Pharm. Phytochem. Res. 2016, 8, 537–545. [Google Scholar]
- Zhao, H.Y.; Yang, L.; Wei, J.; Huang, M.; Jiang, J.G. Bioactivity evaluations of ingredients extracted from the flowers of Citrus aurantium L. var. amara Engl. Food Chem. 2012, 135, 2175–2181. [Google Scholar] [CrossRef] [PubMed]
- Gironés-Vilaplana, A.; Valentão, P.; Andrade, P.B.; Ferreres, F.; Moreno, D.A.; García-Viguera, C. Beverages of lemon juice and exotic noni and papaya with potential for anticholinergic effects. Food Chem. 2015, 170, 16–21. [Google Scholar] [CrossRef]
- Pedersen, A.M.L.; Darwish, M.; Nicholson, J.; Edwards, M.I.; Gupta, A.K.; Belstrøm, D. Gingival health status in individuals using different types of toothpaste. J. Dent. 2019, 80 (Suppl. 1), S13–S18. [Google Scholar] [CrossRef]
- James, P.; Worthington, H.V.; Parnell, C.; Harding, M.; Lamont, T.; Cheung, A.; Whelton, H.; Riley, P. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst. Rev. 2017, 3, CD008676. [Google Scholar] [CrossRef]
- Guerra, F.; Pasqualotto, D.; Rinaldo, F.; Mazur, M.; Corridore, D.; Nofroni, I.; Ottolenghi, L.; Nardi, G.M. Therapeutic efficacy of chlorhexidine-based mouthwashes and its adverse events: Performance-related evaluation of mouthwashes added with Anti-Discoloration System and cetylpyridinium chloride. Int. J. Dent. Hyg. 2019, 17, 229–236. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [Green Version]
- Suntar, I.; Khan, H.; Patel, S.; Celano, R.; Rastrelli, L. An Overview on Citrus aurantium L.: Its Functions as Food Ingredient and Therapeutic Agent. Oxid. Med. Cell. Longev. 2018, 2018, 7864269. [Google Scholar] [CrossRef] [Green Version]
- Ellouze, I.; Abderrabba, M.; Sabaou, N.; Mathieu, F.; Lebrihi, A.; Bouajila, J. Season’s variation impact on Citrus aurantium leaves essential oil: Chemical composition and biological activities. J. Food Sci. 2012, 77, T173–T180. [Google Scholar] [CrossRef] [PubMed]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta. 2013, 763, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ben Hsouna, A.; Ben Halima, N.; Smaoui, S.; Hamdi, N. Citrus lemon essential oil: Chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017, 16, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maté, J.; Periago, P.M.; Palop, A. Combined effect of a nanoemulsion of d-limonene and nisin on Listeria monocytogenes growth and viability in culture media and foods. Food Sci. Technol. Int. 2016, 22, 146–152. [Google Scholar] [CrossRef]
- Fancello, F.; Petretto, G.L.; Marceddu, S.; Venditti, T.; Pintore, G.; Zara, G.; Mannazzu, I.; Budroni, M.; Zara, S. Antimicrobial activity of gaseous Citrus limon var pompia leaf essential oil against Listeria monocytogenes on ricotta salata cheese. Food Microbiol. 2020, 87, 103386. [Google Scholar] [CrossRef] [PubMed]
- Restuccia, C.; Oliveri Conti, G.; Zuccarello, P.; Parafati, L.; Cristaldi, A.; Ferrante, M. Efficacy of different citrus essential oils to inhibit the growth and B1 aflatoxin biosynthesis of Aspergillus flavus. Environ. Sci. Pollut. Res. Int. 2019, 26, 31263–31272. [Google Scholar] [CrossRef]
- Sahal, G.; Woerdenbag, H.J.; Hinrichs, W.L.J.; Visser, A.; Tepper, P.G.; Quax, W.J.; van der Mei, H.C.; Bilkay, I.S. Antifungal and biofilm inhibitory effect of Cymbopogon citratus (lemongrass) essential oil on biofilm forming by Candida tropicalis isolates; an in vitro study. J. Ethnopharmacol. 2020, 246, 112188. [Google Scholar] [CrossRef]
- Veloz, J.J.; Saavedra, N.; Alvear, M.; Zambrano, T.; Barrientos, L.; Salazar, L.A. Polyphenol-Rich Extract from Propolis Reduces the Expression and Activity of Streptococcus mutans Glucosyltransferases at Subinhibitory Concentrations. Biomed. Res. Int. 2016, 2016, 4302706. [Google Scholar] [CrossRef] [Green Version]
- Duque, C.; Stipp, R.N.; Wang, B.; Smith, D.J.; Höfling, J.F.; Kuramitsu, H.K.; Duncan, M.J.; Mattos-Graner, R.O. Downregulation of GbpB, a component of the VicRK regulon, affects biofilm formation and cell surface characteristics of Streptococcus mutans. Infect. Immun. 2011, 79, 786–796. [Google Scholar] [CrossRef] [Green Version]
- Petersen, F.C.; Scheie, A.A. Genetic transformation in Streptococcus mutans requires a peptide secretion-like apparatus. Oral Microbiol. Immunol. 2000, 15, 329–334. [Google Scholar] [CrossRef]
- Li, Y.H.; Tang, N.; Aspiras, M.B.; Lau, P.C.; Lee, J.H.; Ellen, R.P.; Cvitkovitch, D.G. A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. J. Bacteriol. 2002, 184, 2699–2708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorençoni, M.F.; Figueira, M.M.; Toledo, E.; Silva, M.V.; Pimentel Schmitt, E.F.; Endringer, D.C.; Scherer, R.; Barth, T.; Vilela Bertolucci, S.K.; Fronza, M. Chemical composition and anti-inflammatory activity of essential oil and ethanolic extract of Campomanesia phaea (O. Berg.) Landrum leaves. J. Ethnopharmacol. 2020, 252, 112562. [Google Scholar] [CrossRef] [PubMed]
- Dosoky, N.S.; Satyal, P.; Pokharel, S.; Setzer, W.N. Chemical Composition, Enantiomeric Distribution, and Biological Activities of Rhododendron anthopogon Leaf Essential Oil from Nepal. Nat. Prod. Commun. 2016, 11, 1895–1898. [Google Scholar] [CrossRef] [Green Version]
- Belmadani, A.; Semlali, A.; Rouabhia, M. Dermaseptin-S1 decreases Candida albicans growth, biofilm formation and the expression of hyphal wall protein 1 and aspartic protease genes. J. Appl. Microbiol. 2018, 125, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Rouabhia, M.; Rouabhia, D.; Park, H.J.; Giasson, L.; Zhang, Z. Effect of soft foods on primary human gingival epithelial cell growth and the wound healing process. Food Res. Int. 2017, 100, 433–441. [Google Scholar] [CrossRef] [PubMed]


| Compound | Retention Time (min) | Retention Index | Aire% |
|---|---|---|---|
| α-Pinene | 3.22 | 936 | 0.63 |
| Camphene | 3.59 | 955 | 0.02 |
| β-pinene | 4.30 | 990 | 3.00 |
| Sabinene | 4.59 | 1001 | 5.01 |
| β-Myrcene | 5.17 | 1026 | 2.17 |
| δ-3- carene | 5.54 | 1048 | 0.86 |
| Limonene | 5.62 | 1053 | 50.56 |
| (E)-β—Ocimene | 5.89 | 1063 | 7.82 |
| Z- β—Ocimene | 6.38 | 1075 | 3.54 |
| γ—Terpinene | 6.59 | 1088 | 0.64 |
| Terpinolene | 6.77 | 1102 | 0.48 |
| Citronellal | 7.54 | 1139 | 0.27 |
| Linalool | 7.71 | 1152 | 9.89 |
| Terpinen-4-ol | 8.03 | 1175 | 0.32 |
| Linalylacetate | 8.39 | 1281 | 1.61 |
| α—Terpineol | 8.83 | 1185 | 0.12 |
| Neral | 12.163 | 1268 | 0.08 |
| Geraniol | 13.82 | 1280 | 1.53 |
| Geranial | 14.34 | 1291 | 0.26 |
| Nerylacetate | 19.73 | 1364 | 1.52 |
| Geranylacetate | 20.09 | 1385 | 2.26 |
| Methyl N-methylanthranilate | 22.17 | 1402 | 2.35 |
| Betacaryophyllene | 22.60 | 1416 | 1.21 |
| Total | 96.15% |
| Gene | Primer Sequence (5′ à 3′) | Annealing Temperature (°C) | Amp Size (bp) |
|---|---|---|---|
| 16S rRNA | Forward: CTTACCAGGTCTTGACATCCCG Reverse: ACCCAACATCTCACGACACGAG | 63.3 | 113 |
| gbpB | Forward: AGCAACAGAAGCACAACCATCAG Reverse: CCACCATTACCCCAGTAGTTTCC | 65.6 | 150 |
| gtfB | Forward: ACACTTTCGGGTGGCTTG Reverse: GCTTAGATGTCACTTCGGTTG | 62 | 127 |
| gtfC | Forward: CCAAAATGGTATTATGGCTGTCG Reverse: GAGTCTCTATCAAAGTAACGCAGT | 63 | 135 |
| comC | Forward: GACTTTAAAGAAATTAAGACTG Reverse: AAGCTTGTGTAAAACTTCTGT | 54 | 103 |
| comD | Forward: CTCTGATTGACCATTCTTCTGG Reverse: CATTCTGAGTTTATGCCCCTC | 62 | 150 |
| comE | Forward: CCTGAAAAGGGCAATCACCAG Reverse: GGGGCATAAACTCAGAATGTGTCG | 65 | 148 |
| atpH | Forward: ACCATACATTTCAGGCTG Reverse: TTTTAGCACTTGGGATTG | 56 | 101 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benzaid, C.; Belmadani, A.; Tichati, L.; Djeribi, R.; Rouabhia, M. Effect of Citrus aurantium L. Essential Oil on Streptococcus mutans Growth, Biofilm Formation and Virulent Genes Expression. Antibiotics 2021, 10, 54. https://doi.org/10.3390/antibiotics10010054
Benzaid C, Belmadani A, Tichati L, Djeribi R, Rouabhia M. Effect of Citrus aurantium L. Essential Oil on Streptococcus mutans Growth, Biofilm Formation and Virulent Genes Expression. Antibiotics. 2021; 10(1):54. https://doi.org/10.3390/antibiotics10010054
Chicago/Turabian StyleBenzaid, Chahrazed, Amine Belmadani, Lazhari Tichati, Ryad Djeribi, and Mahmoud Rouabhia. 2021. "Effect of Citrus aurantium L. Essential Oil on Streptococcus mutans Growth, Biofilm Formation and Virulent Genes Expression" Antibiotics 10, no. 1: 54. https://doi.org/10.3390/antibiotics10010054
APA StyleBenzaid, C., Belmadani, A., Tichati, L., Djeribi, R., & Rouabhia, M. (2021). Effect of Citrus aurantium L. Essential Oil on Streptococcus mutans Growth, Biofilm Formation and Virulent Genes Expression. Antibiotics, 10(1), 54. https://doi.org/10.3390/antibiotics10010054





