Antimicrobial Activity and Toxicity of Analogs of Wasp Venom EMP Peptides. Potential Influence of Oxidized Methionine
Abstract
:1. Introduction
2. Results
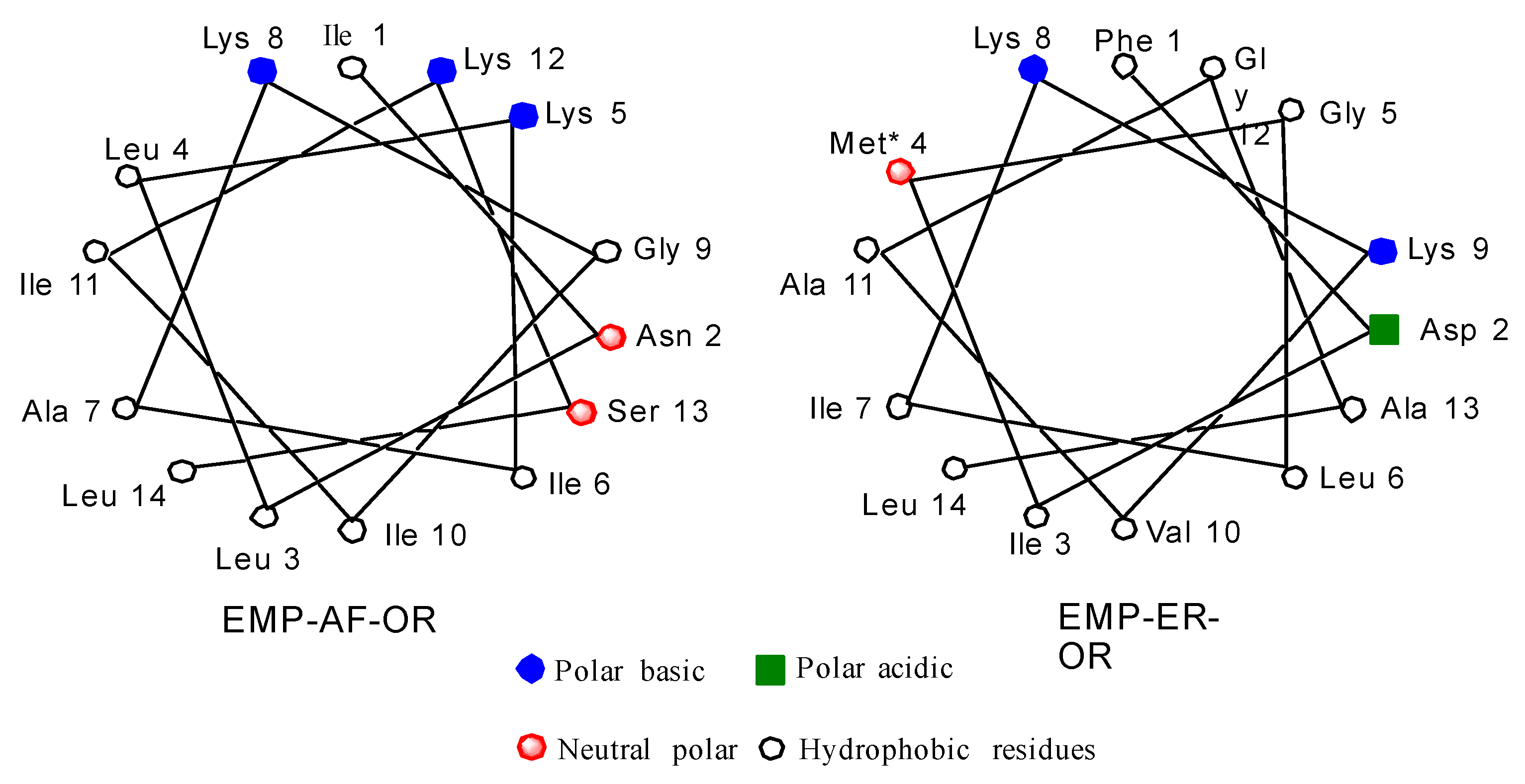
2.1. Peptide Design
2.2. Secondary Structure
2.3. Antibacterial Activities
2.4. Antifungal Activities
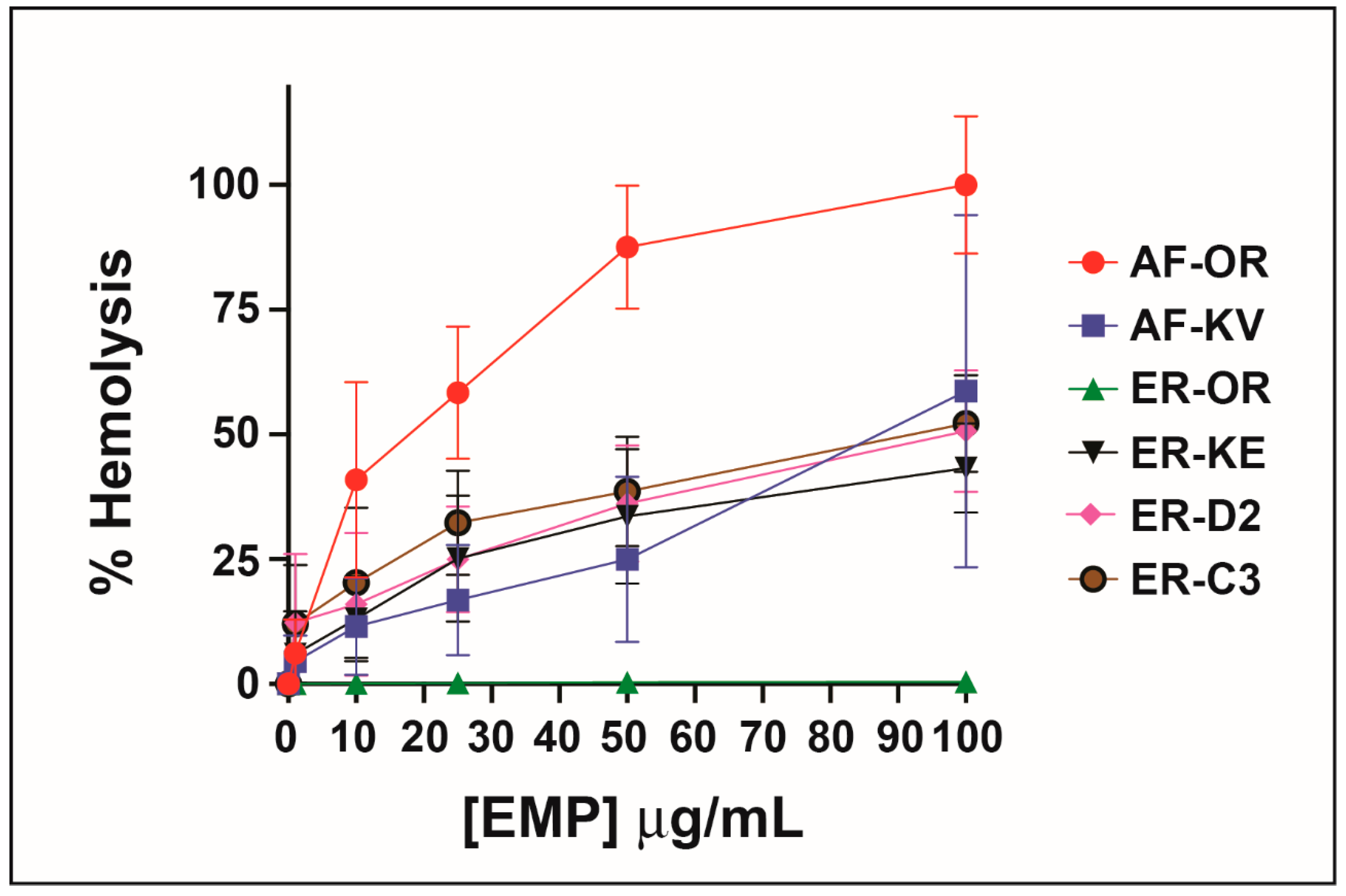
2.5. Hemolytic Activity
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Peptide Synthesis and Purification
4.3. Characterization of Helical Structure
4.4. Bacteria Strains
4.5. Measurement of Antibacterial Activity
4.6. Fungi Strains
4.7. Measurement of Antifungal Activity
4.8. Measurement of Hemolytic Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rentschler, S.; Kaiser, L.; Deigner, H.-P. Emerging Options for the Diagnosis of Bacterial Infections and the Characterization of Antimicrobial Resistance. Int. J. Mol. Sci. 2021, 22, 456. [Google Scholar] [CrossRef]
- Cars, O.C. Resetting the Agenda for Antibiotic Resistance through a Health Systems Perspective. Lancet Glob. Health 2021, 9, e1022–e1027. [Google Scholar] [CrossRef]
- Asenjo, A.; Oteo-Iglesias, J.; Alós, J.I. What’s New in Mechanisms of Antibiotic Resistance in Bacteria of Clinical Origin? Enferm. Infecc. Microbiol. Clin. 2021, 39, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution. Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar] [PubMed] [Green Version]
- Hao, H.; Zheng, X.; Wang, G. Insights into Drug Discovery from Natural Medicines Using Reverse Pharmacokinetics. Trends Pharmacol. Sci. 2014, 35, 168–177. [Google Scholar] [CrossRef]
- Li, X.-J.; Zhang, H.-Y. Synergy in Natural Medicines: Implications for Drug Discovery. Trends Pharmacol. Sci. 2008, 29, 331–332. [Google Scholar] [CrossRef] [PubMed]
- Perumal Samy, R.; Stiles, B.G.; Franco, O.L.; Sethi, G.; Lim, L.H.K. Animal Venoms as Antimicrobial Agents. Biochem. Pharmacol. Amst. Neth. 2017, 134, 127–138. [Google Scholar] [CrossRef]
- Chan, Y.S.; Cheung, R.C.F.; Xia, L.; Wong, J.H.; Ng, T.B.; Chan, W.Y. Snake Venom Toxins: Toxicity and Medicinal Applications. Appl. Microbiol. Biotechnol. 2016, 100, 6165–6181. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, A.B.; Robertson, J.I.S. Captopril in the Treatment of Clinical Hypertension and Cardiac Failure. Lancet 1979, 2, 836–839. [Google Scholar] [CrossRef]
- McGivern, J.G. Ziconotide: A Review of Its Pharmacology and Use in the Treatment of Pain. Neuropsychiatr. Dis. Treat. 2007, 3, 69–85. [Google Scholar] [CrossRef] [Green Version]
- Pal, P.; Roy, S.; Chattopadhyay, S.; Pal, T.K. Medicinal Value of Animal Venom for Treatment of Cancer in Humans—A Review. World Sci. News 2015, 22, 128–144. [Google Scholar]
- Maroti, G.; Kereszt, A.; Kondorosi, E.; Mergaert, P. Natural Roles of Antimicrobial Peptides in Microbes, Plants and Animals. Res. Microbiol. 2011, 162, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Batoni, G.; Maisetta, G.; Esin, S. Therapeutic Potential of Antimicrobial Peptides in Polymicrobial Biofilm-Associated Infections. Int. J. Mol. Sci. 2021, 22, 482. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Dashora, K.; Ameta, K.L.; Singh, N.P.; El-Enshasy, H.A.; Pagano, M.C.; Hesham, A.E.-L.; Sharma, G.D.; Sharma, M.; Bhargava, A. Cysteine-Rich Antimicrobial Peptides from Plants: The Future of Antimicrobial Therapy. Phytother. Res. 2021, 35, 256–277. [Google Scholar] [CrossRef] [PubMed]
- Koehbach, J.; Craik, D.J. The Vast Structural Diversity of Antimicrobial Peptides. Trends Pharmacol. Sci. 2019, 40, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Pirtskhalava, M.; Vishnepolsky, B.; Grigolava, M.; Managadze, G. Physicochemical Features and Peculiarities of Interaction of AMP with the Membrane. Pharmaceuticals 2021, 14, 471. [Google Scholar] [CrossRef]
- Primon-Barros, M.; Macedo, A.J. Animal Venom Peptides: Potential for New Antimicrobial Agents. Curr. Top. Med. Chem. Sharjah United Arab Emir. 2017, 17, 1119–1156. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, K.; Zhu, W.; Ye, X.; Ding, L.; Jiang, H.; Li, F.; Chen, Z.; Luo, X. Two New Cationic alpha-Helical Peptides Identified from the Venom Gland of Liocheles Australasiae Possess Antimicrobial Activity against Methicillin-Resistant Staphylococci. Toxicon 2021, 196, 63–73. [Google Scholar] [CrossRef]
- Konno, K.; Kazuma, K.; Rangel, M.; Stolarz-de-Oliveira, J.; Fontana, R.; Kawano, M.; Fuchino, H.; Hide, I.; Yasuhara, T.; Nakata, Y. New Mastoparan Peptides in the Venom of the Solitary Eumenine Wasp Eumenes Micado. Toxins 2019, 11, 155. [Google Scholar] [CrossRef] [Green Version]
- Prajapati, K.K.; Upadhyay, R.K. Bees and Wasps Venom Toxins, Its Immune-Allergic Responses, Diagnosis and Therapeutics. Int. J. Pharm. Pharm. Sci. 2021, 13, 1–9. [Google Scholar] [CrossRef]
- Dos Santos Cabrera, M.P.; Rangel, M.; Neto, J.R.; Konno, K. Chemical and Biological Characteristics of Antimicrobial alpha-Helical Peptides Found in Solitary Wasp Venoms and Their Interactions with Model Membranes. Toxins 2019, 11, 559. [Google Scholar] [CrossRef] [Green Version]
- El-Wahed, A.A.; Yosri, N.; Sakr, H.H.; Du, M.; Algethami, A.F.M.; Zhao, C.; Abdelazeem, A.H.; Tahir, H.E.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Wasp Venom Biochemical Components and Their Potential in Biological Applications and Nanotechnological Interventions. Toxins 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Singulani, J.D.L.; Galeane, M.C.; Ramos, M.D.; Gomes, P.C.; dos Santos, C.T.; de Souza, B.M.; Palma, M.S.; Fusco Almeida, A.M.; Mendes Giannini, M.J.S. Antifungal Activity, Toxicity, and Membranolytic Action of a Mastoparan Analog Peptide. Front. Cell. Infect. Microbiol. 2019, 9, 419. [Google Scholar] [CrossRef]
- Konno, K.; Kazuma, K.; Nihei, K. Peptide Toxins in Solitary Wasp Venoms. Toxins 2016, 8, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dos Santos Cabrera, M.P.; De Souza, B.M.; Fontana, R.; Konno, K.; Palma, M.S.; de Azevedo, W.F., Jr.; Neto, J.R. Conformation and Lytic Activity of Eumenine Mastoparan: A New Antimicrobial Peptide from Wasp Venom. J. Pept. Res. 2004, 64, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Shinada, T.; Ohfune, Y.; Hisada, M.; Yasuda, A.; Naoki, H.; Nakajima, T. Novel Mastoparan and Protonectin Analogs Isolated from a Solitary Wasp, Orancistrocerus Drewseni Drewseni. Amino Acids 2009, 37, 389–394. [Google Scholar] [CrossRef]
- Sharma, S.; Schiller, M.R. The Carboxy-Terminus, a Key Regulator of Protein Function. Crit. Rev. Biochem. Mol. Biol. 2018, 54, 85–102. [Google Scholar] [CrossRef]
- Konno, K.; Hisada, M.; Naoki, H.; Itagaki, Y.; Kawai, N.; Miwa, A.; Yasuhara, T.; Morimoto, Y.; Nakata, Y. Structure and Biological Activities of Eumenine Mastoparan-AF (EMP-AF), a New Mast Cell Degranulating Peptide in the Venom of the Solitary Wasp (Anterhynchium Flavomarginatum Micado). Toxicon 2000, 38, 1505–1515. [Google Scholar] [CrossRef]
- CLSI Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 10th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; Volume 35.
- Bojsen, R.; Regenberg, B.; Folkesson, A. Saccharomyces Cerevisiae Biofilm Tolerance towards Systemic Antifungals Depends on Growth Phase. BMC Microbiol. 2014, 14, 305. [Google Scholar] [CrossRef] [Green Version]
- Goughenour, K.D.; Balada-Llasat, J.-M.; Rappleye, C.A. Quantitative Microplate-Based Growth Assay for Determination of Antifungal Susceptibility of Histoplasma Capsulatum Yeasts. J. Clin. Microbiol. 2015, 53, 3286–3295. [Google Scholar] [CrossRef] [Green Version]
- Klepser, M.E.; Ernst, E.J.; Lewis, R.E.; Ernst, M.E.; Pfaller, M.A. Influence of Test Conditions on Antifungal Time-Kill Curve Results: Proposal for Standardized Methods. Antimicrob. Agents Chemother. 1998, 42, 1207–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangel, M.; dos Santos Cabrera, M.P.; Kazuma, K.; Ando, K.; Wang, X.; Kato, M.; Nihei, K.I.; Hirata, I.Y.; Cross, T.J.; Garcia, A.N.; et al. Chemical and Biological Characterization of Four New Linear Cationic alpha-Helical Peptides from the Venoms of Two Solitary Eumenine Wasps. Toxicon 2011, 57, 1081–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of Antimicrobial Peptide Action and Resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [Green Version]
- Shai, Y. Mode of Action of Membrane Active Antimicrobial Peptides. Biopolymers 2002, 66, 236–248. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Latge, J.-P.; Munro, C.A. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017, 5, FUNK-2016/25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Rozek, A.; Hancock, R.E.W. Interaction of Cationic Antimicrobial Peptides with Model Membranes. J. Biol. Chem. 2001, 276, 35714–35722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilhelmelli, F.; Vilela, N.; Albuquerque, P.; Derengowski, L.; Silva-Pereira, I.; Kyaw, C. Antibiotic development challenges: The various mechanisms of action of antimicrobial peptides and of bacterial resistance Front. Microbiol. 2013, 4, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dado, G.P.; Gellman, S.H. Redox Control of Secondary Structure in a Designed Peptide. J. Am. Chem. Soc. 1993, 115, 12609–12610. [Google Scholar] [CrossRef]
- Drazic, A.; Winter, J. The Physiological Role of Reversible Methionine Oxidation. Biochim. Biophys. Acta Proteins Proteomics 2014, 1844, 1367–1382. [Google Scholar] [CrossRef]
- Schenck, H.L.; Dado, G.P.; Gellman, S.H. Redox-Triggered Secondary Structure Changes in the Aggregated States of a Designed Methionine-Rich Peptide. J. Am. Chem. Soc. 1996, 118, 12487–12494. [Google Scholar] [CrossRef]
- De la Salud Bea, R.; Ascuitto, M.R.; de Johnson, L.E.L. Synthesis of Analogs of Peptides from Buthus Martensii Scorpion Venom with Potential Antibiotic Activity. Peptides 2015, 68, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Park, E.J.; Yang, S.-T.; Jung, H.J.; Eom, S.H.; Song, W.K.; Kim, Y.; Hahm, K.-S.; Kim, J.I. Structure-Activity Analysis of SMAP-29, a Sheep Leukocytes-Derived Antimicrobial Peptide. Biochem. Biophys. Res. Commun. 2001, 285, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Perego, M.; Spiegelman, G.B.; Hoch, J.A. Structure of the Gene for the Transition State Regulator, AbrB: Regulator Synthesis Is Controlled by the Spo0A Sporulation Gene in Bacillus Subtilis. Mol. Microbiol. 1988, 2, 689–699. [Google Scholar] [CrossRef] [PubMed]
- De la Salud Bea, R.; Petraglia, A.F.; de Johnson, L.E.L. Synthesis, Antimicrobial Activity and Toxicity of Analogs of the Scorpion Venom BmKn Peptides. Toxicon 2015, 101, 79–84. [Google Scholar]
| Peptide | Amino Acid Sequence | Calculated Molecular Mass (Da) | Observed Molecular Mass (Da) | HPLC Retention Time (min) | Charge at pH = 7 |
|---|---|---|---|---|---|
| EMP-AF-OR | I N L L K I A K G I I K S L-NH2 | 1522.96 | 1522.96 | 21.6 | +4 |
| EMP-AF-KV | I N K L V I KV G K I V S L-NH2 | 1522.96 | 1522.96 | 16.8 | +4 |
| EMP-ER-OR | F D I M* G L I K K V A G A L-NH2 | 1474.87 | 1490.75 | 19.7 | +2 |
| EMP-ER-KE | F D I M* G L I E E V A G A L-NH2 | 1487.95 | 1503.90 | 24.5 | −2 |
| EMP-ER-D2K2 | F K I M* G L I K K V A G A L-NH2 | 1476.75 | 1493.80 | 18.4 | +4 |
| EMP-ER-C3 | F D I M* G L I K K V A-NH2 | 1233.58 | 1249.80 | 17.5 | +2 |
| Peptide | Water | 50% TFE | ||
|---|---|---|---|---|
| [θ]222 | % Helix | [θ]222 | % Helix | |
| EMP-AF-OR | −7711.94 | 17.73 | −47,630.55 | 100 |
| EMP-AF-KV | −4719.43 | 7.85 | −13,692.93 | 37.47 |
| EMP-ER-OR | −2759.13 | 1.38 | −13,698.05 | 37.49 |
| EMP-ER-KE | −2256.20 | Random | −12,749.65 | 34.35 |
| EMP-ER-D2K2 | −240.03 | 6.93 | −13,694.72 | 37.47 |
| EMP-ER-C3 | −109.85 | 7.36 | −18,019.75 | 51.74 |
| Peptide | MIC (μg/mL) | |||
|---|---|---|---|---|
| S. aureus | B. subtilis | P. aeruginosa | E. coli | |
| EMP-AF-OR | 25 μg/mL | 10 μg/mL | 200 μg/mL | 25 μg/mL |
| EMP-AF-KV | ND | ND | ND | ND |
| EMP-ER-OR | ND | ND | ND | ND |
| EMP-ER-KE | ND | ND | ND | ND |
| EMP-ER-D2K2 | ND | 200 μg/mL | ND | ND |
| EMP-ER-C3 | ND | ND | ND | ND |
| Peptides | MIC (µg/mL) | ||||
|---|---|---|---|---|---|
| Sc | Sp | An | Ca | Hc | |
| EMP-AF-OR | >256 | >256 | >256 | >256 | 128 |
| EMP-AF-KV | >256 | >256 | >256 | >256 | >256 |
| EMP-ER-OR | >256 | >256 | >256 | 256 | >256 |
| EMP-ER-KE | >256 | >256 | >256 | >256 | >256 |
| EMP-ER-D2K2 | >256 | >256 | >256 | 256 | >256 |
| EMP-ER-C3 | >256 | >256 | >256 | >256 | >256 |
| Amphotericin B | 1.0 | 0.25 | > 16 | 0.5 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Salud Bea, R.; North, L.J.; Horiuchi, S.; Frawley, E.R.; Shen, Q. Antimicrobial Activity and Toxicity of Analogs of Wasp Venom EMP Peptides. Potential Influence of Oxidized Methionine. Antibiotics 2021, 10, 1208. https://doi.org/10.3390/antibiotics10101208
de la Salud Bea R, North LJ, Horiuchi S, Frawley ER, Shen Q. Antimicrobial Activity and Toxicity of Analogs of Wasp Venom EMP Peptides. Potential Influence of Oxidized Methionine. Antibiotics. 2021; 10(10):1208. https://doi.org/10.3390/antibiotics10101208
Chicago/Turabian Stylede la Salud Bea, Roberto, Lily J. North, Sakura Horiuchi, Elaine R. Frawley, and Qian Shen. 2021. "Antimicrobial Activity and Toxicity of Analogs of Wasp Venom EMP Peptides. Potential Influence of Oxidized Methionine" Antibiotics 10, no. 10: 1208. https://doi.org/10.3390/antibiotics10101208






