An Antibacterial Peptide with High Resistance to Trypsin Obtained by Substituting d-Amino Acids for Trypsin Cleavage Sites
Abstract
:1. Introduction
2. Results
2.1. The Structure-Activity Relationship (SAR) of OM19R
2.2. Peptide Design and Antibacterial Activity
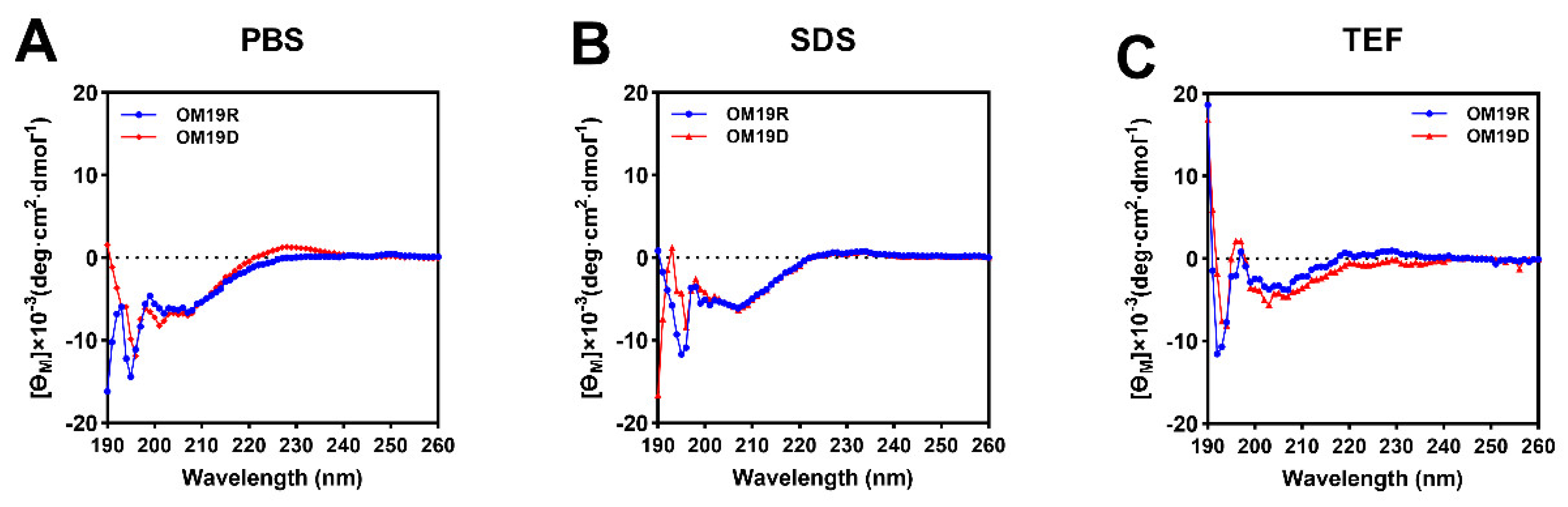
2.3. Circular Dichroism (CD) Spectroscopy
2.4. Antibacterial Activity
2.5. Stability toward Tryptic Degradation
2.6. Condition Sensitivity Assays
2.7. In Vitro Safety Evaluation
2.8. Antimicrobial Mechanism Study
2.8.1. Growth Curves of Bacteria and Time-Kill Curve
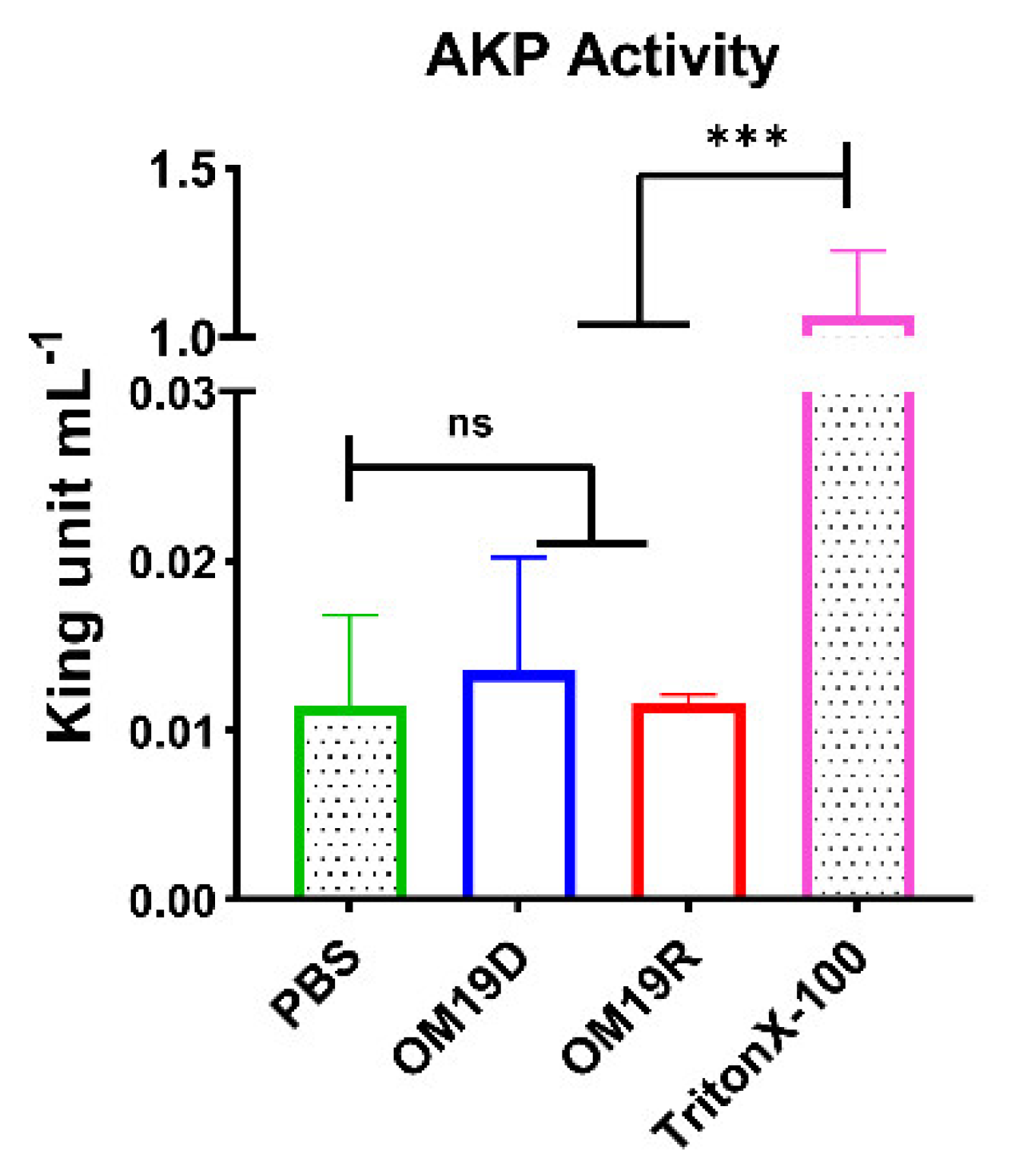
2.8.2. AKP Activity Determination
2.8.3. Outer Membrane and Inner Membrane Permeability Assay
2.8.4. Proton Motive Force
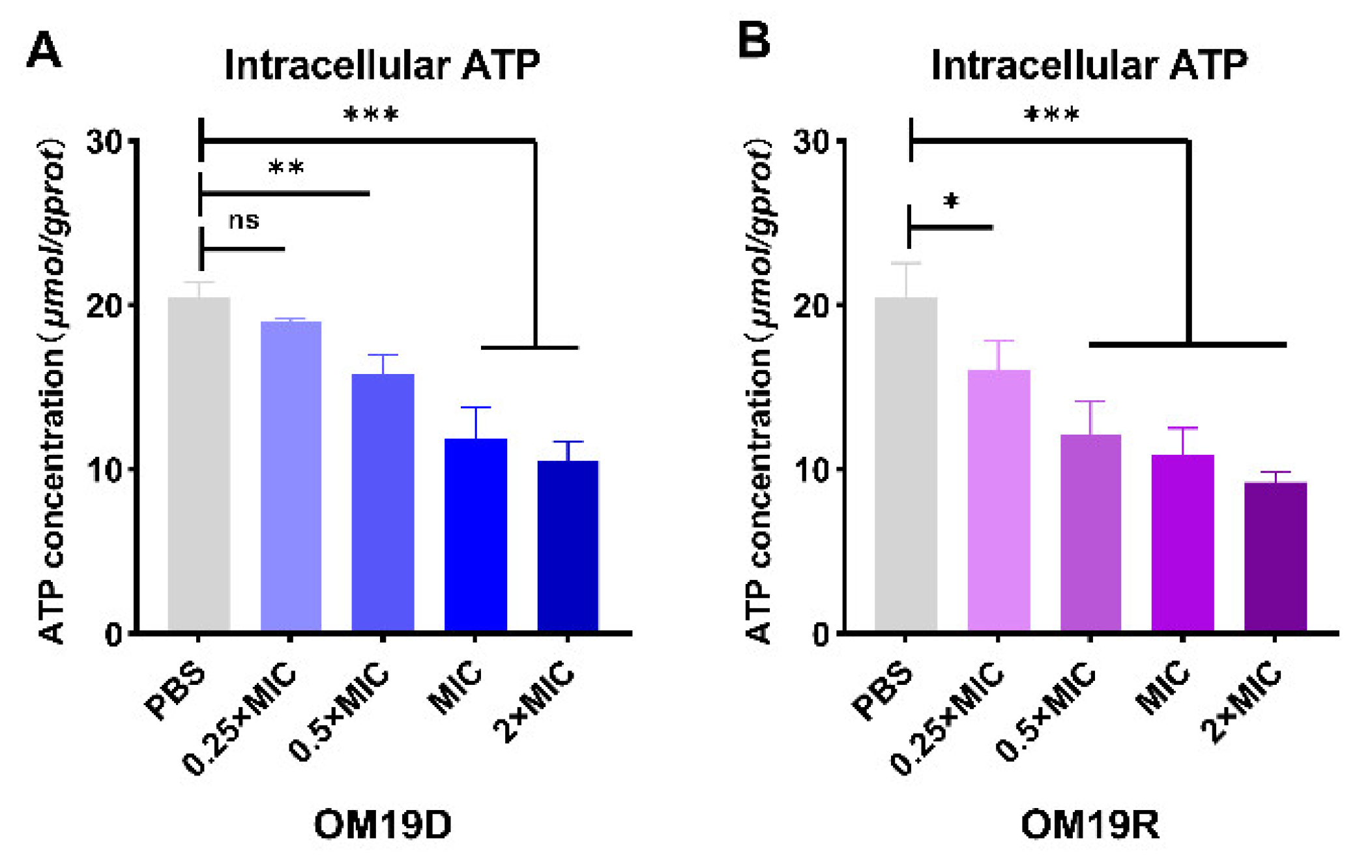
2.8.5. Intracellular ATP
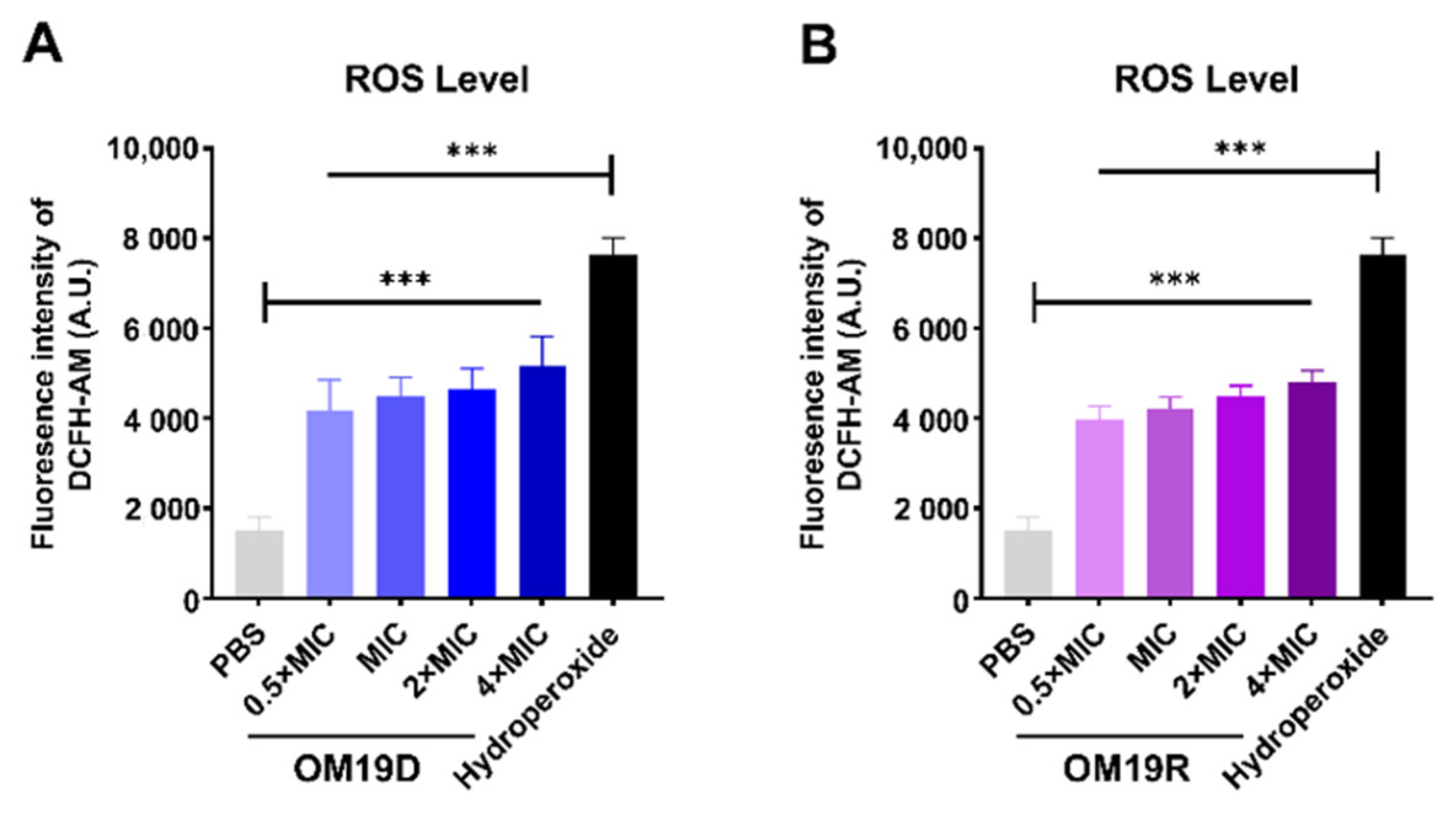
2.8.6. Total ROS Level
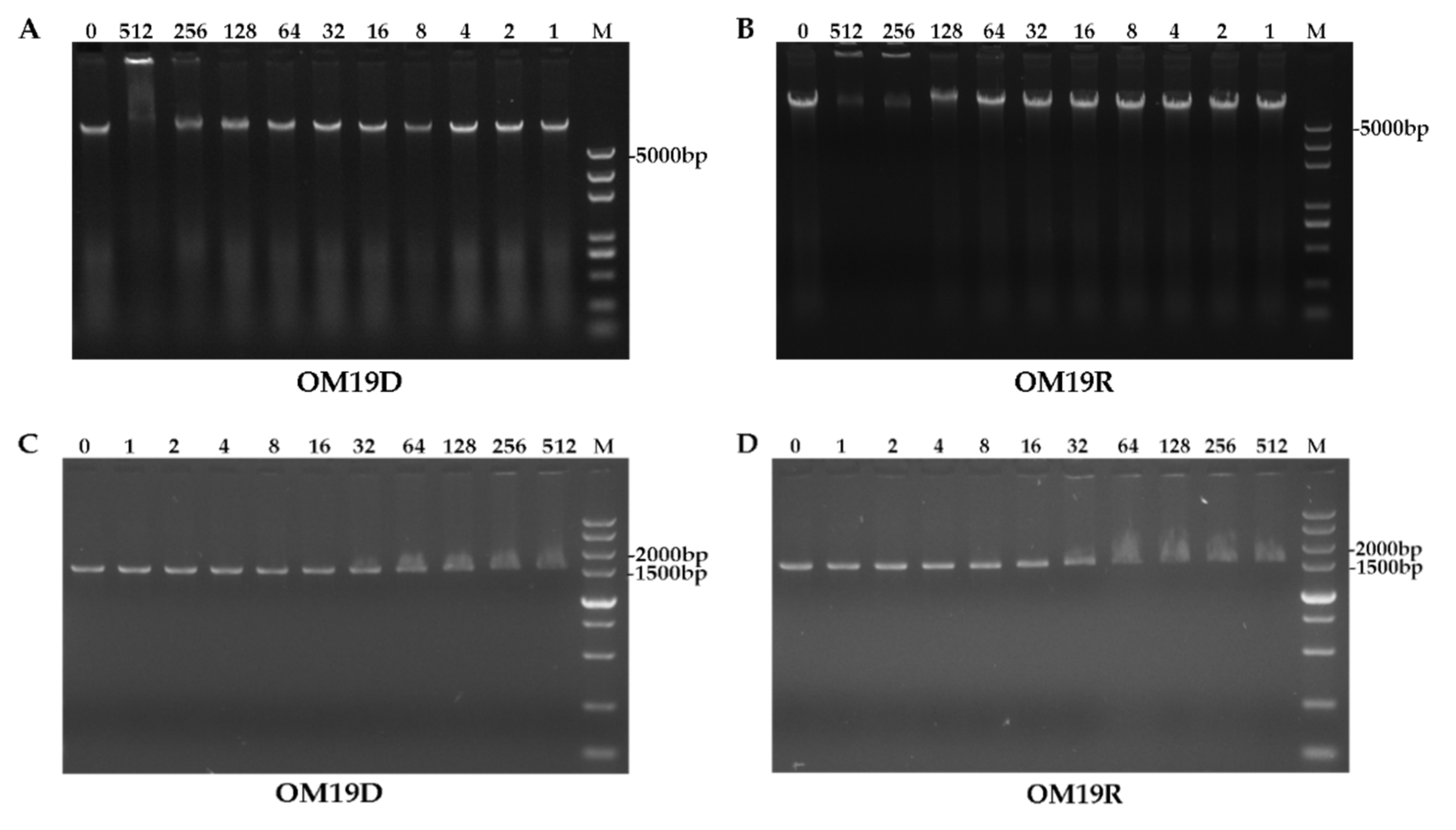
2.8.7. DNA Binding Assay
2.8.8. Quantification of Intracellular Protein Concentration
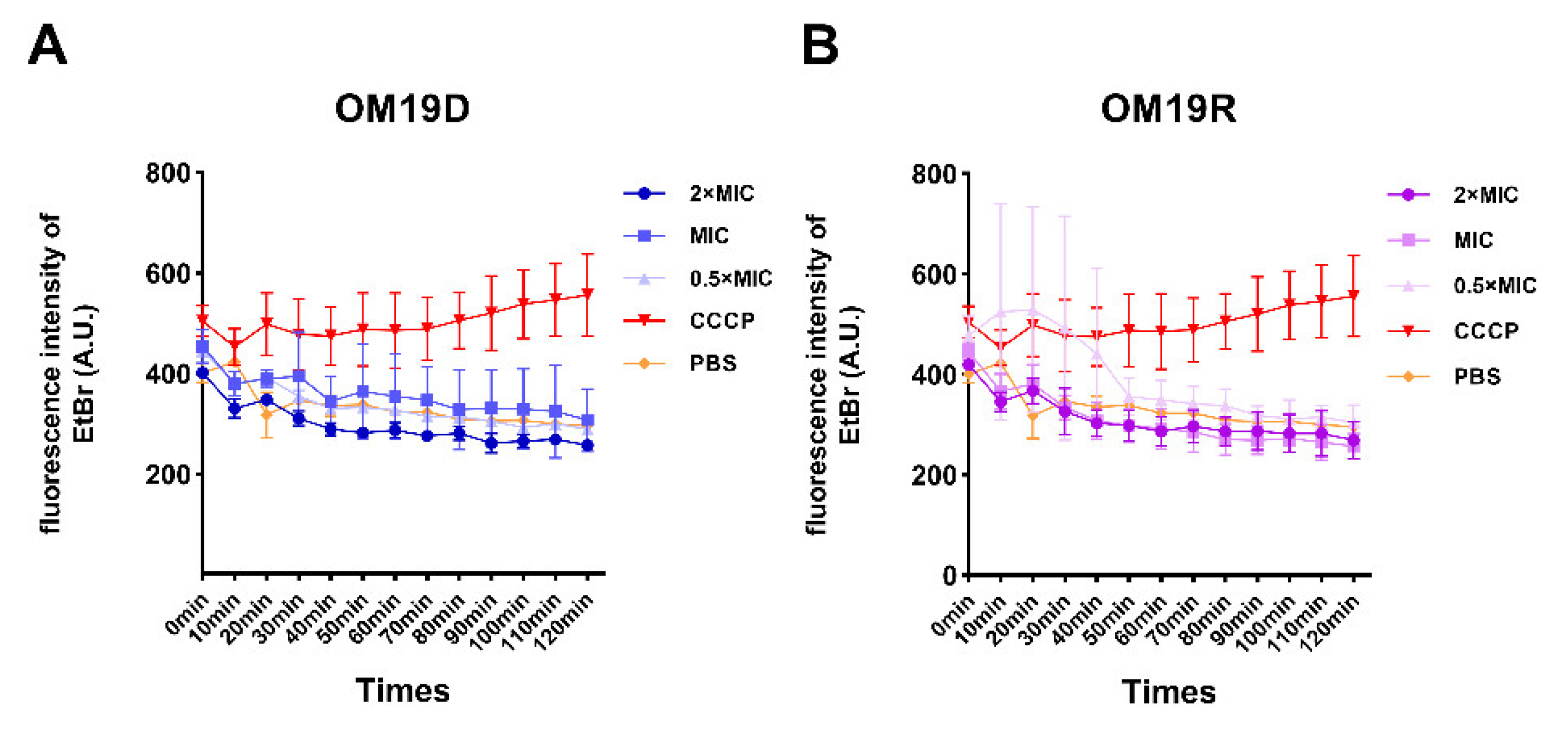
2.8.9. Non-Specific Efflux Pumps
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Bacterial Species
4.1.2. Reagent
4.2. Synthesis and Sequence Analysis of Peptides
4.3. MIC Measurements
4.4. CD Measurements
4.5. Typsin Resistance Assays
4.6. Physiological Salt Sensitivity Assays
4.7. Temperature and pH Sensitivity Assays
4.8. Serum and Plasma Sensitivity Assays
4.9. Measurement of Hemolysis Activity
4.10. Cytotoxicity Assays
4.11. Determination of AKP Outflow
4.12. Outer Membrane Permeability Assay
4.13. Inner Membrane Permeability Assay
4.14. Proton Motive Force Assay
4.14.1. Membrane Potential Assay
4.14.2. Intracellular pH Assay
4.15. Intracellular ATP Determination
4.16. Total ROS Measurement
4.17. Efflux Pump Assay
4.18. Quantification of Intracellular Protein
4.18.1. Test of Protein Concentration Change with Peptides Concentration Change
4.18.2. Test of Protein Concentration Change with Action Time
4.19. DNA Binding Assay
4.20. Growth Curves of Bacteria and Time-Kill Curve
4.20.1. The Bacterial Growth Curve
4.20.2. Time-Kill Curve
4.21. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Davies, J. Where have All the Antibiotics Gone? Can. J. Infect. Dis. Med. Microbiol. 2006, 17, 287–290. [Google Scholar] [CrossRef]
- Rosen, T. Antibiotic Resistance: An Editorial Review With Recommendations. J. Drugs Dermatol. 2011, 10, 724–733. [Google Scholar]
- Tan, P.; Fu, H.; Ma, X. Design, optimization, and nanotechnology of antimicrobial peptides: From exploration to applications. Nano Today 2021, 39, 101229. [Google Scholar] [CrossRef]
- Sato, H.; Feix, J.B. Peptide–membrane interactions and mechanisms of membrane destruction by amphipathic α-helical antimicrobial peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 1245–1256. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chou, S.; Yang, Z.; Yang, Y.; Wang, Z.; Song, J.; Dou, X.; Shan, A. Combating Drug-Resistant Fungi with Novel Imperfectly Amphipathic Palindromic Peptides. J. Med. Chem. 2018, 61, 3889–3907. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Liu, Y.; Tan, Z.; Ju, Y.; Yang, Y.; Dong, W. Antimicrobial activity, membrane interaction and stability of the D-amino acid substituted analogs of antimicrobial peptide W3R6. J. Photochem. Photobiol. B Biol. 2019, 200, 111645. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, J.H.; Kim, S.C.; Cho, J.H. De novo generation of short antimicrobial peptides with enhanced stability and cell specificity. J. Antimicrob. Chemother. 2013, 69, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Rozek, A.; Powers, J.-P.S.; Friedrich, C.L.; Hancock, R.E.W. Structure-Based Design of an Indolicidin Peptide Analogue with Increased Protease Stability. Biochemistry 2003, 42, 14130–14138. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Wang, J.; Peng, J.; Zhao, P.; Kong, Z.; Wang, K.; Yan, W.; Wang, R. D-amino acid substitution enhances the stability of antimicrobial peptide polybia-CP. Acta Biochim. Biophys. Sin. 2017, 49, 916–925. [Google Scholar] [CrossRef] [Green Version]
- Zhong, C.; Liu, T.; Gou, S.; He, Y.; Zhu, N.; Zhu, Y.; Wang, L.; Liu, H.; Zhang, Y.; Yao, J.; et al. Design and synthesis of new N-terminal fatty acid modified-antimicrobial peptide analogues with potent in vitro biological activity. Eur. J. Med. Chem. 2019, 182, 111636. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, J.; Yang, Z.; He, S.; Yang, Y.; Feng, X.; Dou, X.; Shan, A. Antimicrobial Peptides with High Proteolytic Resistance for Combating Gram-Negative Bacteria. J. Med. Chem. 2019, 62, 2286–2304. [Google Scholar] [CrossRef]
- Vlieghe, P.; Lisowski, V.; Martinez, J.; Khrestchatisky, M. Synthetic therapeutic peptides: Science and market. Drug Discov. Today 2010, 15, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Miura, C.; Ohta, T.; Ozaki, Y.; Tanaka, H.; Miura, T. Trypsin is a multifunctional factor in spermatogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 20972–20977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karstad, R.; Isaksen, G.; Brandsdal, B.R.O.; Svendsen, J.S.; Svenson, J. Unnatural amino acid side chains as S1, S1′, and S2′ probes yield cationic antimicrobial peptides with stability toward chymotryptic degradation. J. Med. Chem. 2010, 53, 5558–5566. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of Antimicrobial Peptide Action and Resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [Green Version]
- Bechinger, B.; Gorr, S.-U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J. Dent. Res. 2016, 96, 254–260. [Google Scholar] [CrossRef] [Green Version]
- Arias, M.; Piga, K.B.; Hyndman, M.E.; Vogel, H.J. Improving the Activity of Trp-Rich Antimicrobial Peptides by Arg/Lys Substitutions and Changing the Length of Cationic Residues. Biomolecules 2018, 8, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartoloni, M.; Jin, X.; Marcaida, M.J.; Banha, J.; Dibonaventura, I.; Bongoni, S.; Bartho, K.; Gräbner, O.; Sefkow, M.; Darbre, T.; et al. Bridged bicyclic peptides as potential drug scaffolds: Synthesis, structure, protein binding and stability. Chem. Sci. 2015, 6, 5473–5490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, R.P.; Gellman, S.H.; DeGrado, W.F. β-Peptides: From Structure to Function. Chem. Rev. 2001, 101, 3219–3232. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, J.; Gilon, C.; Hoffman, A.; Kessler, H. N-Methylation of Peptides: A New Perspective in Medicinal Chemistry. Acc. Chem. Res. 2008, 41, 1331–1342. [Google Scholar] [CrossRef]
- Räder, A.F.; Reichart, F.; Weinmüller, M.; Kessler, H. Improving oral bioavailability of cyclic peptides by N-methylation. Bioorg. Med. Chem. 2018, 26, 2766–2773. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Lai, Y.-D.; Chang, C.-H.; Tsai, Y.-C.; Tang, C.-C.; Jan, J.-S. Star-shaped polypeptides exhibit potent antibacterial activities. Nanoscale 2019, 11, 11696–11708. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Liu, Y.; Hu, Q.; Han, Y.; Hao, Z.; Shen, J.; Zhu, K. Programmable antibiotic delivery to combat methicillin-resistant Staphylococcus aureus through precision therapy. J. Control. Release 2020, 321, 710–717. [Google Scholar] [CrossRef]
- Lu, J.; Xu, H.; Xia, J.; Ma, J.; Xu, J.; Li, Y.; Feng, J. d- and Unnatural Amino Acid Substituted Antimicrobial Peptides with Improved Proteolytic Resistance and Their Proteolytic Degradation Characteristics. Front. Microbiol. 2020, 11, 563030. [Google Scholar] [CrossRef]
- Hamase, K.; Morikawa, A.; Etoh, S.; Tojo, Y.; Miyoshi, Y.; Zaitsu, K. Analysis of Small Amounts of D-Amino Acids and the Study of Their Physiological Functions in Mammals. Anal. Sci. 2009, 25, 961–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narancic, T.; Almahboub, S.A.; O’Connor, K.E. Unnatural amino acids: Production and biotechnological potential. World J. Microbiol. Biotechnol. 2019, 35, 67. [Google Scholar] [CrossRef]
- Pless, S.A.; Ahern, C.A. Unnatural Amino Acids as Probes of Ligand-Receptor Interactions and Their Conformational Consequences. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 211–229. [Google Scholar] [CrossRef]
- Stromstedt, A.A.; Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Evaluation of Strategies for Improving Proteolytic Resistance of Antimicrobial Peptides by Using Variants of EFK17, an Internal Segment of LL-37. Antimicrob. Agents Chemother. 2009, 53, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Liu, J.; Cui, Q.; Jia, B.-Y.; Pei, Z.-H.; Odah, K.A.; Wang, Y.-M.; Dong, W.-L.; Kong, L.-C.; Ma, H.-X. Design and Characterization of a Novel Hybrid Antimicrobial Peptide OM19R Based on Oncocin and MDAP-2. Int. J. Pept. Res. Ther. 2019, 26, 1839–1846. [Google Scholar] [CrossRef]
- Jia, B.-Y.; Wang, Y.-M.; Zhang, Y.; Wang, Z.; Wang, X.; Muhammad, I.; Kong, L.-C.; Pei, Z.-H.; Ma, H.-X.; Jiang, X.-Y.; et al. High Cell Selectivity and Bactericidal Mechanism of Symmetric Peptides Centered on d-Pro–Gly Pairs. Int. J. Mol. Sci. 2020, 21, 1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, F.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antibacterial Activity and Mechanism of Linalool against Shewanella putrefaciens. Molecules 2021, 26, 245. [Google Scholar] [CrossRef]
- Abad, J.P. Proton Motive Force; Proton Motive Force: Heidelberg, Germany, 2015. [Google Scholar]
- Wang, G.; Li, X.; Wang, Z. APD2: The updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 2008, 37, D933–D937. [Google Scholar] [CrossRef] [Green Version]
- Kang, X.; Dong, F.; Shi, C.; Liu, S.; Sun, J.; Chen, J.; Li, H.; Xu, H.; Lao, X.; Zheng, H. DRAMP 2.0, an updated data repository of antimicrobial peptides. Sci. Data 2019, 6, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molchanova, N.; Hansen, P.R.; Franzyk, H. Advances in Development of Antimicrobial Peptidomimetics as Potential Drugs. Molecules 2017, 22, 1430. [Google Scholar] [CrossRef] [Green Version]
- Svenson, J.; Stensen, W.; Brandsdal, B.-O.; Haug, B.E.; Monrad, J.; Svendsen, J.S. Antimicrobial Peptides with Stability toward Tryptic Degradation. Biochemistry 2008, 47, 3777–3788. [Google Scholar] [CrossRef]
- Moreton, R.C. United States pharmacopeia-national formulary. J. Excip. Food Chem. 2016, 6, 925. [Google Scholar]
- Rabanal, F.; Cajal, Y. Recent advances and perspectives in the design and development of polymyxins. Nat. Prod. Rep. 2017, 34, 886–908. [Google Scholar] [CrossRef] [PubMed]
- Baltz, R.H.; Miao, V.; Wrigley, S.K. Natural products to drugs: Daptomycin and related lipopeptide antibiotics. Nat. Prod. Rep. 2005, 22, 717–741. [Google Scholar] [CrossRef]
- Lai, Z.; Tan, P.; Zhu, Y.; Shao, C.; Shan, A.; Li, L. Highly Stabilized alpha-Helical Coiled Coils Kill Gram-Negative Bacteria by Multicomplementary Mechanisms under Acidic Condition. ACS Appl. Mater. Interfaces 2019, 11, 22113–22128. [Google Scholar] [CrossRef]
- Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Antimicrobial peptides: Key components of the innate immune system. Crit. Rev. Biotechnol. 2011, 32, 143–171. [Google Scholar] [CrossRef] [Green Version]
- Askoura, M.; Mottawea, W.; Abujamel, T.; Taher, I. Efflux pump inhibitors (EPIs) as new antimicrobial agents against Pseudomonas aeruginosa. Libyan J. Med. 2011, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015.
- Wang, J.; Chou, S.; Xu, L.; Zhu, X.; Dong, N.; Shan, A.; Chen, Z. High specific selectivity and Membrane-Active Mechanism of the synthetic centrosymmetric α-helical peptides with Gly-Gly pairs. Sci. Rep. 2015, 5, 15963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, C.; Li, W.; Tan, P.; Shan, A.; Dou, X.; Ma, D.; Liu, C. Symmetrical Modification of Minimized Dermaseptins to Extend the Spectrum of Antimicrobials with Endotoxin Neutralization Potency. Int. J. Mol. Sci. 2019, 20, 1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, N.; Zhu, X.; Chou, S.; Shan, A.; Li, W.; Jiang, J. Antimicrobial potency and selectivity of simplified symmetric-end peptides. Biomaterials 2014, 35, 8028–8039. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jia, Y.; Yang, K.; Li, R.; Xiao, X.; Zhu, K.; Wang, Z. Metformin Restores Tetracyclines Susceptibility against Multidrug Resistant Bacteria. Adv. Sci. 2020, 7, 1902227. [Google Scholar] [CrossRef]
- Ding, X.; Yang, C.; Moreira, W.; Yuan, P.; Periaswamy, B.; De Sessions, P.F.; Zhao, H.; Tan, J.; Lee, A.; Ong, K.X.; et al. A Macromolecule Reversing Antibiotic Resistance Phenotype and Repurposing Drugs as Potent Antibiotics. Adv. Sci. 2020, 7, 2001374. [Google Scholar] [CrossRef] [PubMed]
- Maisuria, V.B.; Hosseinidoust, Z.; Tufenkji, N. Polyphenolic extract from maple syrup potentiates antibiotic susceptibility and reduces biofilm formation of pathogenic bacteria. Appl. Environ. Microbiol. 2015, 81, 3782–3792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, I.; Hansen, J.E.; Jana, B.; Molchanova, N.; Oddo, A.; Thulstrup, P.W.; Damborg, P.; Guardabassi, L.; Hansen, P.R. Structure–Activity Study, Characterization, and Mechanism of Action of an Antimicrobial Peptoid D2 and Its d- and l-Peptide Analogues. Molecules 2019, 24, 1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.; Liu, Y.; Huang, X.; Ding, S.; Wang, Y.; Shen, J.; Zhu, K. A broad-spectrum antibiotic adjuvant reverses multidrug-resistant Gram-negative pathogens. Nat. Microbiol. 2020, 5, 1040–1050. [Google Scholar] [CrossRef]







| Peptides | Sequence 1 | Theoretical MW | Measured MW 2 | Net Charge |
|---|---|---|---|---|
| OM19D | VDkPPYLPrPrPIrrPGGr-NH2 | 2227.65 | 2226.63 | 6 |
| OM19R | VDKPPYLPRPRPIRRPGGR-NH2 | 2227.65 | 2226.63 | 6 |
| Bacterial Species | OM19D | OM19R |
|---|---|---|
| Sensitive strains | ||
| Escherichia coli ATCC25922 | 2 | 1 |
| Escherichia coli k88 | 4 | 2 |
| Salmonella enterica ATCC 14028 | 4 | 2 |
| Salmonella typhimurium CMCC50115 | 2 | 1 |
| Shigella flexneri ATCC12022 | 2 | 1 |
| Shigella dysenteriae CMCC51252 | 4 | 2 |
| Klebsiella pneumoniae ATCC25955 | >128 | >128 |
| Staphylococcus aureus ATCC25923 | >128 | >128 |
| Clinical isolated strains | ||
| Escherichia coli SN5 | 2 | 8 |
| Escherichia coli S1N1 | 2 | 8 |
| Escherichia coli w136 | 4 | 1 |
| Escherichia coli w122 | 8 | 2 |
| Escherichia coli w123 | 4 | 1 |
| Escherichia coli w124 | 4 | 1 |
| Escherichia coli QY | 4 | 4 |
| Shigella flexneri QY1 | 8 | 8 |
| Methicillin-resistant Staphylococcus aureus HP | >128 | >128 |
| Peptides | Control 1 | Trypsin (mg/mL) | Trypsin (10mg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 5 | 2.5 | 1.25 | 0.625 | 1 h | 2 h | 4 h | 8 h | ||
| OM19D | 2 | 16 | 16 | 8 | 8 | 4 | 16 | 16 | 16 | 32 |
| OM19R | 1 | >128 | >128 | 64 | 64 | 64 | >128 | >128 | >128 | >128 |
| Melittin | 2 | >128 | >128 | 64 | 32 | 16 | >128 | >128 | >128 | >128 |
| Peptides | Control 1 | Physiological Salts 2 | |||||
|---|---|---|---|---|---|---|---|
| NaCl | KCl | NH4Cl | MgCl2 | ZnCl2 | FeCl3 | ||
| OM19D | 2 | 4 | 2 | 2 | 4 | 2 | 4 |
| OM19R | 1 | 4 | 2 | 2 | 4 | 2 | 2 |
| Melittin | 2 | 4 | 2 | 2 | 2 | 2 | 2 |
| Peptides | Control 1 | Temperature | pH | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 °C | 42 °C | 65 °C | 100 °C | pH4 | pH6 | pH8 | pH10 | ||
| OM19D | 2 | 2 | 2 | 2 | 2 | 2 | 4 | 2 | 4 |
| OM19R | 1 | 1 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 1 |
| Melittin | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Peptides | Control 1 | Serum Concentrations (%) | Plasma Concentrations (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | 100 | 50 | 25 | 12.5 | ||
| OM19D | 2 | 32 | 32 | 16 | 8 | 64 | 64 | 32 | 16 |
| OM19R | 1 | 32 | 32 | 32 | 16 | 64 | 64 | 64 | 32 |
| Melittin | 2 | >128 | 64 | 64 | 32 | >128 | >128 | >128 | >128 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Zhang, M.; Muhammad, I.; Cui, Q.; Zhang, H.; Jia, Y.; Xu, Q.; Kong, L.; Ma, H. An Antibacterial Peptide with High Resistance to Trypsin Obtained by Substituting d-Amino Acids for Trypsin Cleavage Sites. Antibiotics 2021, 10, 1465. https://doi.org/10.3390/antibiotics10121465
Zhao X, Zhang M, Muhammad I, Cui Q, Zhang H, Jia Y, Xu Q, Kong L, Ma H. An Antibacterial Peptide with High Resistance to Trypsin Obtained by Substituting d-Amino Acids for Trypsin Cleavage Sites. Antibiotics. 2021; 10(12):1465. https://doi.org/10.3390/antibiotics10121465
Chicago/Turabian StyleZhao, Xiaoou, Mengna Zhang, Inam Muhammad, Qi Cui, Haipeng Zhang, Yu Jia, Qijun Xu, Lingcong Kong, and Hongxia Ma. 2021. "An Antibacterial Peptide with High Resistance to Trypsin Obtained by Substituting d-Amino Acids for Trypsin Cleavage Sites" Antibiotics 10, no. 12: 1465. https://doi.org/10.3390/antibiotics10121465
APA StyleZhao, X., Zhang, M., Muhammad, I., Cui, Q., Zhang, H., Jia, Y., Xu, Q., Kong, L., & Ma, H. (2021). An Antibacterial Peptide with High Resistance to Trypsin Obtained by Substituting d-Amino Acids for Trypsin Cleavage Sites. Antibiotics, 10(12), 1465. https://doi.org/10.3390/antibiotics10121465






