Global Prevalence of Nosocomial Multidrug-Resistant Klebsiella pneumoniae: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Results
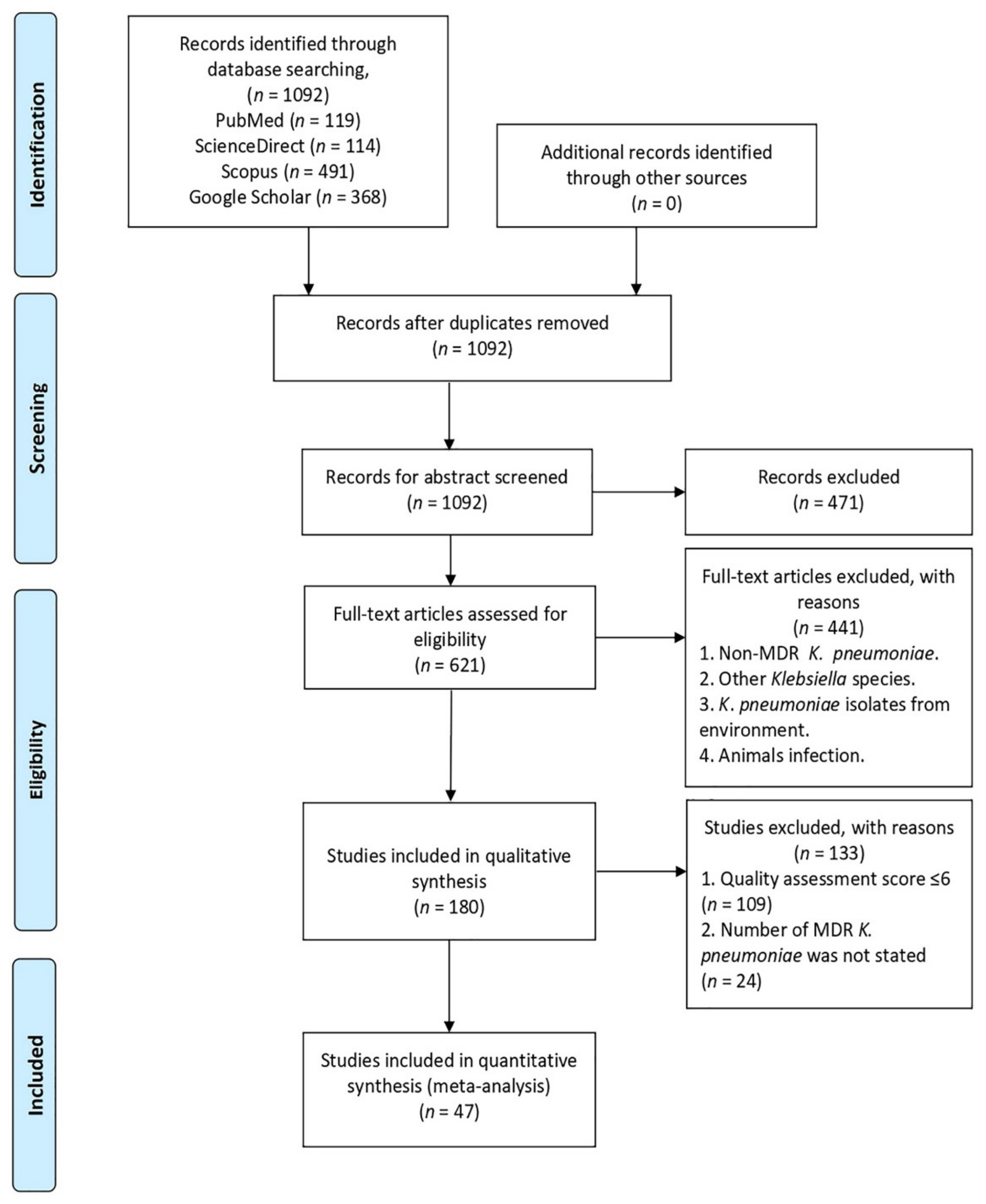
2.1. Search and Screening Results
2.2. Characteristics of the Included Studies
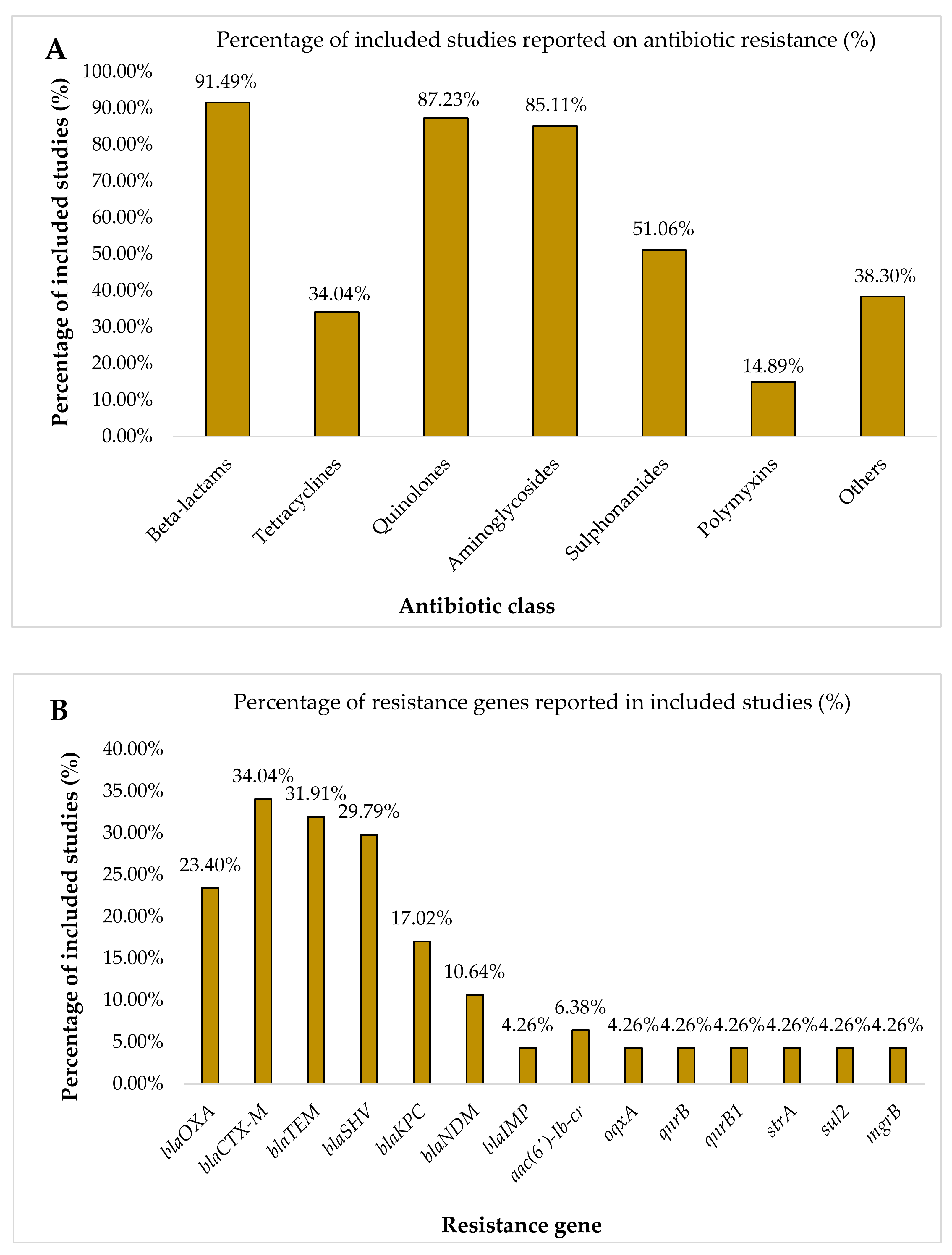
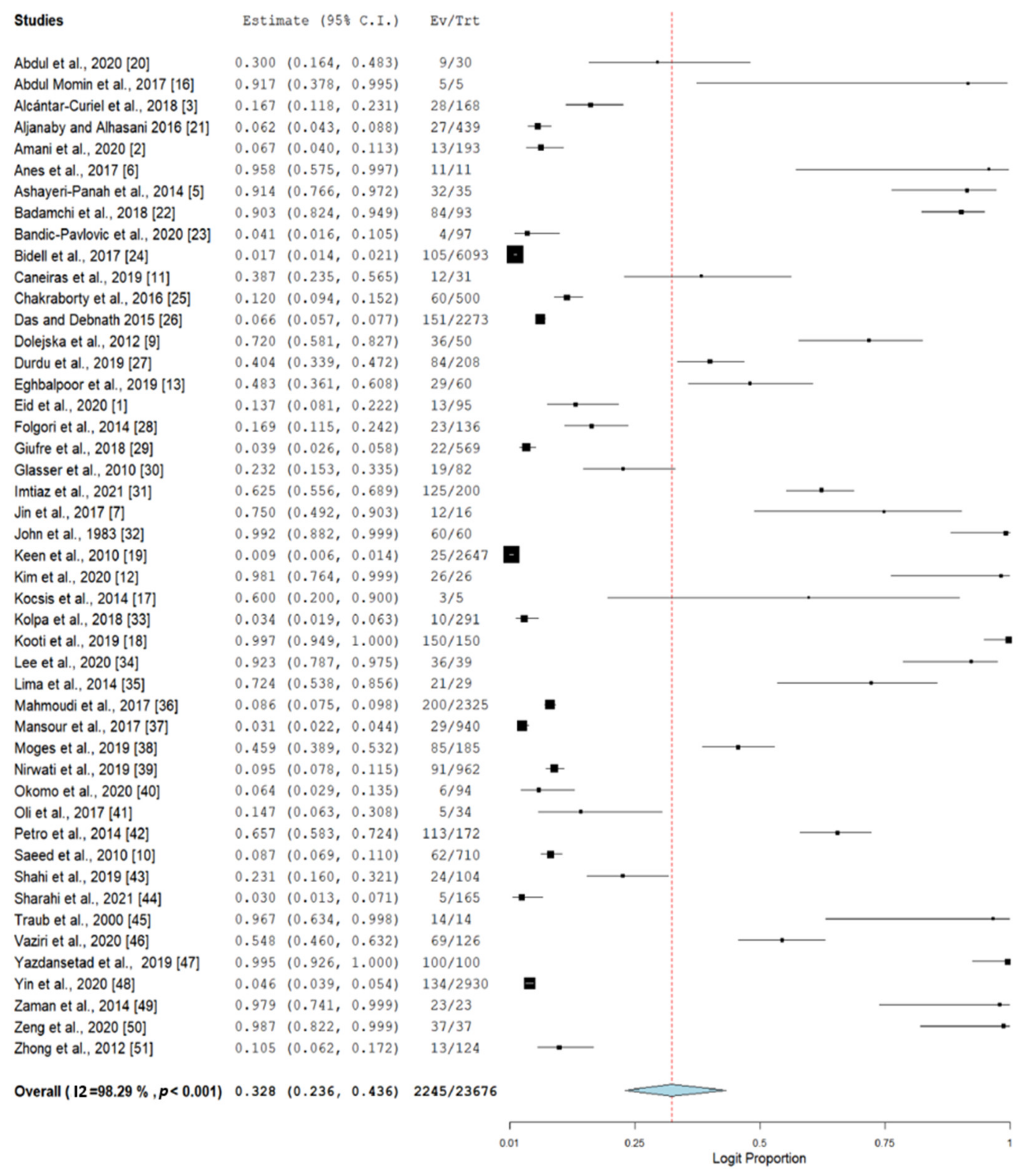
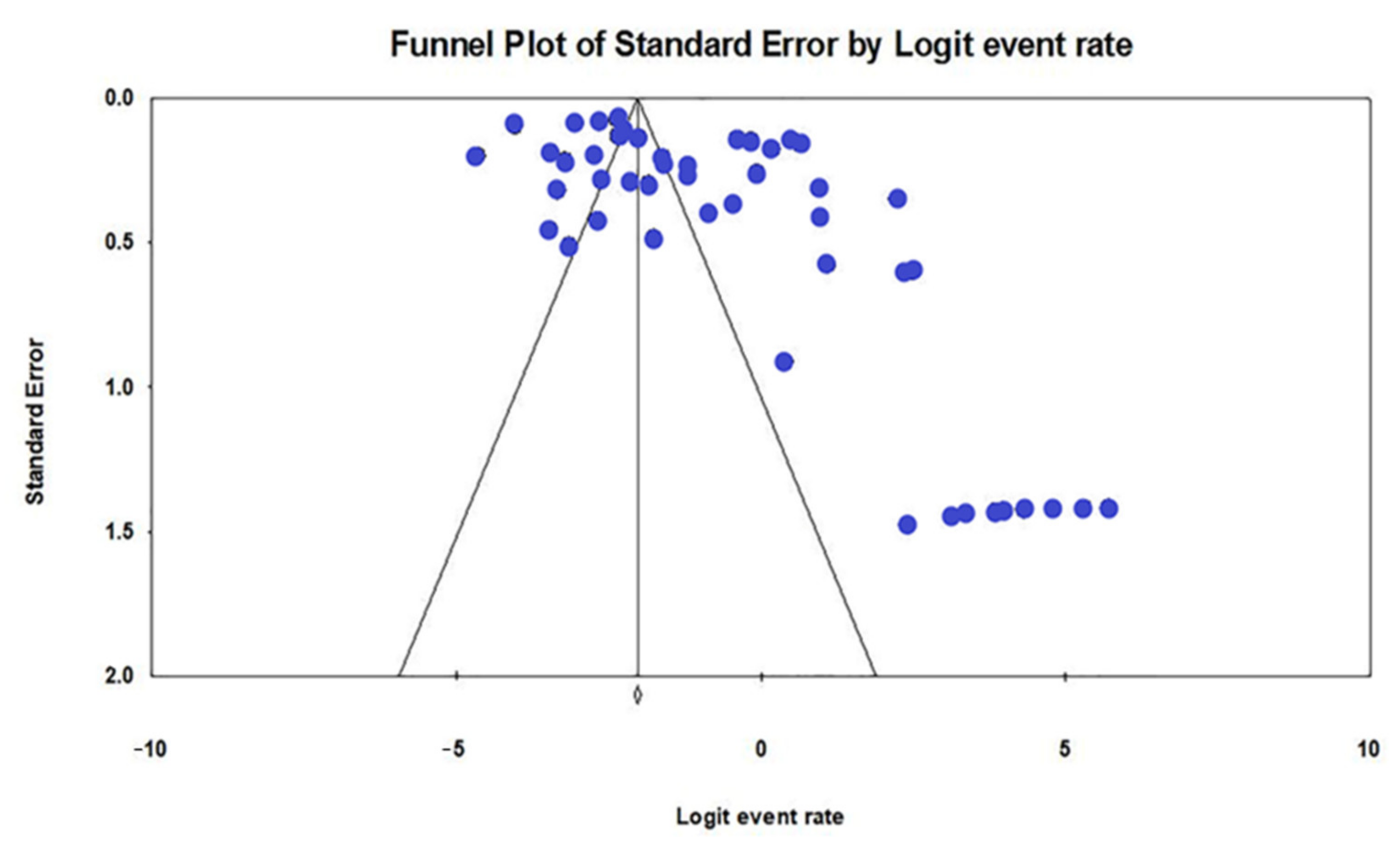
2.3. Prevalence of Nosocomial MDR K. pneumoniae
| Author ID | Country | Number of Isolates | Number of K. pneumoniae | Number of MDR K. pneumoniae | Resistance Profile to Antibiotic Class | Genes Encoded for Antibiotic Resistance |
|---|---|---|---|---|---|---|
| Abdul et al. 2020 [20] | Iraq | 30 | 14 | 9 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides. | NR |
| Abdul Momin et al. 2017 [16] | Brunei | 5 | 5 | 5 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides. | blaOXA-232, blaCTX-M-15, blaTEM-1b, blaSHV-11 |
| Alcántar-Curiel et al. 2018 [3] | Mexico | 168 | 168 | 28 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides. | NR |
| Aljanaby and Alhasani 2016 [21] | Iraq | 439 | 32 | 27 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Chloramphenicol, Nitrofurantoin. | NR |
| Amani et al. 2020 [2] | Iran | 193 | 36 | 13 | Beta-lactams, Aminoglycosides, Chloramphenicol, Nitrofurantoin. | oqxA |
| Anes et al. 2017 [6] | United Kingdom | 11 | 11 | 11 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Sulphonamides, Chloramphenicol. | blaCTX-M-15, blaSHV-12, blaTEM-1B, oqxAB, qnrB |
| Ashayeri-Panah et al. 2014 [5] | Iran | 35 | 35 | 32 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides, Polymyxin, Nitrofurantoin. | blaSHV |
| Badamchi et al. 2018 [22] | Iran | 93 | 93 | 84 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides, Rifampin. | blaKPC |
| Bandic-Pavlovic et al. 2020 [23] | Croatia | 97 | 8 | 4 | Beta-lactams, Quinolones, Aminoglycosides. | blaCTX-M-15, blaOXA-48 |
| Bidell et al. 2017 [24] | United States of America | 6093 | 1039 | 105 | Beta-lactams, Quinolones. | NR |
| Caneiras et al. 2019 [11] | Portugal | 31 | 31 | 12 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Fosfomycin. | blaTEM-10, blaTEM-24, blaCTX-M-15, blaKPC-3, blaSHV-11 |
| Chakraborty et al. 2016 [25] | Bangladesh | 500 | 108 | 60 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Sulphonamides. | NR |
| Das and Debnath 2015 [26] | India | 2273 | 671 | 151 | NR | NR |
| Dolejska et al. 2012 [9] | Czech Republic | 50 | 36 | 36 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Sulphonamides, Chloramphenicol. | blaCTX-M-15, blaTEM-1, blaOXA-1, aac(6’)-Ib-cr, qnrB1, strA, sul2, tet(A), aac(3’)-II |
| Durdu et al. 2019 [27] | Turkey | 208 | 208 | 84 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Sulphonamides, Polymyxin. | NR |
| Eghbalpoor et al. 2019 [13] | Iran | 60 | 60 | 29 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides. | blaCTX-M, blaTEM, blaSHV |
| Eid et al. 2020 [1] | Egypt | 95 | 22 | 13 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Chloramphenicol. | NR |
| Folgori et al. 2014 [28] | Italy | 136 | 37 | 23 | NR | blaKPC, blaOXA-48 |
| Giufre et al. 2018 [29] | Italy | 569 | 52 | 22 | Beta-lactams, Quinolones, Sulphonamides. | blaCTX-M-14, blaCTX-M-15, blaTEM-24, blaTEM-52, blaSHV-12, blaKPC-3 |
| Glasser et al. 2010 [30] | United States of America | 82 | 22 | 19 | Beta-lactams, Quinolones, Aminoglycosides. | NR |
| Imtiaz et al. 2021 [31] | Pakistan | 200 | 200 | 125 | Beta-lactams, Quinolones, Aminoglycosides, Polymyxin. | blaTEM, blaSHV, blaCTX-M-14, blaCTX-M-15, blaOXA, blaNDM-1, blaKPC, blaOXA-48 type, mcr-1, mcr-2 |
| Jin et al. 2017 [7] | China | 16 | 16 | 12 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides, Fosfomycin. | blaCTX-M-14, blaCTX-M-15, blaDHA-1, blaIMP-4, blaIMP-8, blaNDM-1, blaTEM-1 |
| John et al. 1983 [32] | United States of America | 60 | 60 | 60 | NR | NR |
| Keen et al. 2010 [19] | United States of America | 2647 | 695 | 25 | NR | NR |
| Kim et al. 2020 [12] | South Korea | 26 | 26 | 26 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Sulphonamides, Polymyxin, Chloramphenicol, Fosfomycin, Nitrofurans. | aph(3’)-VIa, armA, aac(6’)-Ib-cr, aadA2, aadA1, aac(3)-IId, strA, strB, blaOXA-1, blaTEM-1A, blaOXA-9, blaCTX-M-15, blaSHV-28, blaNDM-1, blaOXA-232, catB3, catA1, cmlA1, mph(E), msr(E), ere(A), qnrB1, oqxA, oqxB, dfrA12, dfrA1, sul1, sul2, ARR-3, fosA, mgrB, phoP |
| Kocsis et al. 2014 [17] | Italy | 5 | 3 | 3 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides. | blaKPC-3, blaTEM-1, blaOXA-9, blaSHV-11, aac(6’)Ib |
| Kolpa et al. 2018 [33] | Poland | 291 | 44 | 10 | Beta-lactams, Quinolones, Aminoglycosides. | NR |
| Kooti et al. 2019 [18] | Iran | 150 | 150 | 150 | Beta-lactams, Quinolones, Aminoglycosides. | blaIMP, blaVIM |
| Lee et al. 2020 [34] | Malaysia | 39 | 36 | 36 | Beta-lactams, Quinolones, Aminoglycosides. | blaTEM, blaSHV, blaOXA-1, blaCTX-M-1, blaCTX-M-9 |
| Lima et al. 2014 [35] | Brazil | 29 | 29 | 21 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Sulphonamides, Chloramphenicol. | NR |
| Mahmoudi et al. 2017 [36] | Iran | 2325 | 263 | 200 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides. | NR |
| Mansour et al. 2017 [37] | Tunisia | 940 | 220 | 29 | Beta-lactams, Tetracyline, Quinolones, Aminoglycosides, Sulphonamides, Polymyxin, Trimethoprim. | mgrB, blaOXA-48, blaOXA-204, blaCMY-4, blaNDM-1, blaCMY-16, blaCTX-M-15 |
| Moges et al. 2019 [38] | Ethiopia | 185 | 97 | 85 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Sulphonamides, Chloramphenicol. | NR |
| Nirwati et al. 2019 [39] | Indonesia | 962 | 167 | 91 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides. | NR |
| Okomo et al. 2020 [40] | Gambia | 94 | 6 | 6 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Sulphonamides. | NR |
| Oli et al. 2017 [41] | Nigeria | 34 | 5 | 5 | Beta-lactams, Quinolones, Aminoglycosides. | NR |
| Petro et al. 2014 [42] | Tanzania | 172 | 113 | 113 | Beta-lactams. | NR |
| Saeed et al. 2010 [10] | Kingdom of Saudi Arabia | 710 | 96 | 62 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides, Polymyxin, Chloramphenicol. | NR |
| Shahi et al. 2019 [43] | Iran | 104 | 104 | 24 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides. | blaKPC-2 |
| Sharahi et al. 2021 [44] | Iran | 165 | 52 | 5 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Sulphonamides, Fosfomycin. | blaTEM, blaSHV, blaCTX-M, blaNDM-1, blaNDM-6 |
| Traub et al. 2000 [45] | Germany | 14 | 14 | 14 | Beta-lactams, Quinolones, Aminoglycosides, Polymyxin, Chloramphenicol, Fosfomycin + Glucose-6-phosphate, Nitrofurantoin, Rifampin | NR |
| Vaziri et al. 2020 [46] | Iran | 126 | 126 | 69 | Beta-lactams, Quinolones, Aminoglycosides. | qnrB, qnrS, aac(6′)-Ib-cr |
| Yazdansetad et al. 2019 [47] | Iran | 100 | 100 | 100 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides, Nitrofurantoin. | blaTEM, blaCTX-M, blaSHV |
| Yin et al. 2020 [48] | China | 2930 | 452 | 134 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Rifamycins. | |
| Zaman et al. 2014 [49] | Kingdom of Saudi Arabia | 23 | 23 | 23 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides. | blaOXA-48, blaOXA-D, blaTEM-1, blaSHV-1, blaSHV-11, blaSHV-12, blaCTX-M-14, blaCTX-M-15, aadB, dfrA7 |
| Zeng et al. 2020 [50] | China | 37 | 37 | 37 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides. | blaKPC-2, blaSHV, blaTEM, blaOXA-1, blaCTX-M-15, blaCTX-M-177, blaCTX-M-3, blaCTX-M-14 |
| Zhong et al. 2012 [51] | China | 124 | NR | 13 | Beta-lactams, Quinolones, Aminoglycosides. | NR |
| Subgroup | No. of Studies | Prevalence | 95% CI | p-Value | I2 (%) | Q | Heterogeneity Test | |
|---|---|---|---|---|---|---|---|---|
| DF | p-Value | |||||||
| Location | ||||||||
| Iraq | 2 | 14.0 | 2.5–50.5 | 0.053 | 94.38 | 17.793 | 1 | <0.001 |
| Mexico | 1 | 16.7 | 11.8–23.1 | - | - | - | - | - |
| Iran | 10 | 55.0 | 27.5–79.8 | 0.736 | 98.14 | 484.982 | 9 | <0.001 |
| United Kingdom | 1 | 95.8 | 57.5–99.7 | - | - | - | - | - |
| Portugal | 1 | 38.7 | 23.5–56.5 | - | - | - | - | - |
| Italy | 3 | 15.5 | 3.9–45.5 | 0.028 | 94.42 | 35.86 | 2 | <0.001 |
| Bangladesh | 1 | 12.0 | 9.4–15.2 | - | - | - | - | - |
| India | 1 | 6.6 | 5.7–7.7 | - | - | - | - | - |
| Czech republic | 1 | 72.0 | 58.1–82.7 | - | - | - | - | - |
| Egypt | 1 | 13.7 | 8.1–22.2 | - | - | - | - | - |
| USA | 4 | 11.6 | 2.5–40.6 | 0.016 | 98.12 | 159.439 | 3 | <0.001 |
| Pakistan | 1 | 62.5 | 55.6–68.9 | - | - | - | - | - |
| China | 4 | 38.6 | 9.5–79.0 | 0.612 | 96.36 | 82.437 | 3 | <0.001 |
| South Korea | 1 | 98.1 | 76.4–99.9 | - | - | - | - | - |
| Turkey | 1 | 40.4 | 33.9–47.2 | - | - | - | - | - |
| Malaysia | 1 | 92.3 | 78.7–97.5 | - | - | - | - | - |
| Brazil | 1 | 72.4 | 53.8–85.6 | - | - | - | - | - |
| Tunisia | 1 | 3.1 | 2.2–4.4 | - | - | - | - | - |
| Brunei | 1 | 91.7 | 37.8–99.5 | - | - | - | - | - |
| Indonesia | 1 | 9.5 | 7.8–11.5 | - | - | - | - | - |
| Gambia | 1 | 6.4 | 2.9–13.5 | - | - | - | - | - |
| Nigeria | 1 | 14.7 | 6.3–30.8 | - | - | - | - | - |
| Tanzania | 1 | 65.7 | 58.3–72.4 | - | - | - | - | - |
| Saudi Arabia | 2 | 64.3 | 0.4–99.9 | 0.849 | 94.64 | 18.640 | 1 | <0.001 |
| Germany | 1 | 96.7 | 63.4–99.8 | - | - | - | - | - |
| Croatia | 1 | 4.1 | 1.6–10.5 | - | - | - | - | - |
| Ethiopia | 1 | 45.9 | 38.9–53.2 | - | - | - | - | - |
| Poland | 1 | 3.4 | 1.9–6.3 | - | - | - | - | - |
| Region | ||||||||
| Asia | 11 | 39.6 | 22.1–60.3 | 0.324 | 98.35 | 607.235 | 10 | <0.001 |
| South America | 1 | 72.4 | 53.8–85.6 | - | - | - | - | - |
| North America | 5 | 12.9 | 3.1–40.3 | 0.014 | 98.45 | 258.186 | 4 | <0.001 |
| Europe | 9 | 31.2 | 11.5–61.2 | 0.213 | 95.84 | 192.35 | 8 | <0.001 |
| Africa | 4 | 28.7 | 11.5–55.5 | 0.114 | 95.78 | 71.023 | 3 | <0.001 |
| Middle East | 17 | 35.4 | 21.2–52.7 | 0.097 | 97.79 | 723.876 | 16 | <0.001 |
3. Discussion
4. Materials and Methods
4.1. Selection Criteria
4.2. Literature Search
4.3. Data Extraction and Quality Assessment
4.4. Data Synthesis and Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| No | Author ID | Checklist 1 | Overall Score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |||
| 1. | Abdul et al. 2020 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| 2. | Abdul Momin et al. 2017 | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 7 |
| 3. | Alcántar-Curiel et al. 2018 | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | 7 |
| 4. | Aljanaby and Alhasani 2016 | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | 7 |
| 5. | Amani et al. 2020 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| 6. | Anes et al. 2017 | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | 7 |
| 7. | Ashayeri-Panah et al. 2014 | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | 7 |
| 8. | Badamchi et al. 2018 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | 8 |
| 9. | Bandic-Pavlovic et al. 2020 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | 8 |
| 10 | Bidell et al. 2017 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | 8 |
| 11. | Caneiras et al. 2019 | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | 7 |
| 12. | Chakraborty et al. 2016 | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | 7 |
| 13. | Das and Debnath 2015 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| 14. | Dolejska et al. 2012 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 8 |
| 15. | Durdu et al. 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| 16. | Eghbalpoor et al. 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| 17. | Eid et al. 2020 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 8 |
| 18. | Folgori et al. 2014 | Yes | Yes | Yes | Yes | Yes | No | Yes | No | Yes | 7 |
| 19. | Giufre et al. 2018 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| 20. | Glasser et al. 2010 | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | 7 |
| 21. | Imtiaz et al. 2021 | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 7 |
| 22. | Jin et al. 2017 | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 7 |
| 23. | John et al. 1983 | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 7 |
| 24. | Keen et al. 2010 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| 25. | Kim et al. 2020 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| 26. | Kocsis et al. 2014 | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
| 27. | Kolpa et al. 2018 | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 7 |
| 28. | Kooti et al. 2019 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 8 |
| 29. | Lee et al. 2020 | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | 7 |
| 30. | Lima et al. 2014 | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | 7 |
| 31. | Mahmoudi et al. 2017 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| 32. | Mansour et al. 2017 | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 7 |
| 33. | Moges et al. 2019 | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 7 |
| 34. | Nirwati et al. 2019 | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | 7 |
| 35. | Okomo et al. 2020 | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | 7 |
| 36. | Oli et al. 2017 | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | 7 |
| 37. | Petro et al. 2014 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| 38. | Saeed et al. 2010 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 8 |
| 39. | Shahi et al. 2019 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 8 |
| 40. | Sharahi et al. 2021 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| 41. | Traub et al. 2000 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 8 |
| 42. | Vaziri et al. 2020 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| 43. | Yazdansetad et al. 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| 44. | Yin et al. 2020 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| 45. | Zaman et al. 2014 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 8 |
| 46. | Zeng et al. 2020 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | 8 |
| 47. | Zhong et al. 2012 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| Author ID | Antibiotic Resistance to the Beta-Lactams Class |
|---|---|
| Abdul et al. 2020 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems, Monobactams. |
| Abdul Momin et al. 2017 | Beta-lactams; Cephalosporins, Carbapenems. |
| Alcántar-Curiel et al. 2018 | Beta-lactams; Penicillins, Cephalosporins. |
| Aljanaby and Alhasani 2016 | Beta-lactams; Penicillins, Cephalosporins, Carbapenems. |
| Amani et al. 2020 | Beta-lactams; Penicillins, Cephalosporins, Carbapenems. |
| Anes et al. 2017 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems, Monobactams. |
| Ashayeri-Panah et al. 2014 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Monobactams. |
| Badamchi et al. 2018 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems. |
| Bandic-Pavlovic et al. 2020 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems. |
| Bidell et al. 2017 | Beta-lactams; Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems. |
| Caneiras et al. 2019 | Beta-lactams; Penicillins/Beta-lactamase inhibitor, Cephalosporins. |
| Chakraborty et al. 2016 | Beta-lactams; Penicillins, Cephalosporins. |
| Das and Debnath 2015 | Beta-lactams; NR |
| Dolejska et al. 2012 | Beta-lactams; Carbapenems. |
| Durdu et al. 2019 | Beta-lactams; Penicillins/Beta-lactamase inhibitor, Cephalosporins, Cephalosporins/Beta-lactamase inhibitor, Carbapenems. |
| Eghbalpoor et al. 2019 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems. |
| Eid et al. 2020 | Beta-lactams; Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems. |
| Folgori et al. 2014 | Beta-lactams; NR |
| Giufre et al. 2018 | Beta-lactams; Penicillins, Cephalosporins. |
| Glasser et al. 2010 | Beta-lactams; Penicillins/Beta-lactamase inhibitor, Cephalosporins. |
| Imtiaz et al. 2021 | Beta-lactams; Penicillins, Cephalosporins, Carbapenems, Monobactams. |
| Jin et al. 2017 | Beta-lactams; Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems, Monobactams. |
| John et al. 1983 | Beta-lactams; NR |
| Keen et al. 2010 | Beta-lactams; NR |
| Kim et al. 2020 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems, Monobactams. |
| Kocsis et al. 2014 | Beta-lactams; Cephalosporins, Carbapenems, Monobactams. |
| Kolpa et al. 2018 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Cephalosporins/Beta-lactamase inhibitor, Carbapenems. |
| Kooti et al. 2019 | Beta-lactams; Cephalosporins, Carbapenems, Monobactams. |
| Lee et al. 2020 | Beta-lactams; Penicillins, Cephalosporins. |
| Lima et al. 2014 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems, Monobactams. |
| Mahmoudi et al. 2017 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems. |
| Mansour et al. 2017 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems, Monobactams. |
| Moges et al. 2019 | Beta-lactams; Penicillins, Cephalosporins. |
| Nirwati et al. 2019 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems. |
| Okomo et al. 2020 | Beta-lactams; Penicillins, Cephalosporins. |
| Oli et al. 2017 | Beta-lactams; Penicillins, Cephalosporins. |
| Petro et al. 2014 | Beta-lactams; Penicillins/Beta-lactamase inhibitor. |
| Saeed et al. 2010 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems, Monobactams. |
| Shahi et al. 2019 | Beta-lactams; Cephalosporins, Carbapenems. |
| Sharahi et al. 2021 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems, Monobactams. |
| Traub et al. 2000 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems, Monobactams. |
| Vaziri et al. 2020 | Beta-lactams; Cephalosporins, Monobactams. |
| Yazdansetad et al. 2019 | Beta-lactams; Cephalosporins, Carbapenems. |
| Yin et al. 2020 | Beta-lactams; Penicillins/Beta-lactamase inhibitor, Cephalosporins, Cephalosporins/Beta-lactamase inhibitor, Carbapenems. |
| Zaman et al. 2014 | Beta-lactams; Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems. |
| Zeng et al. 2020 | Beta-lactams; Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems |
| Zhong et al. 2012 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins. |
References
- Eid, D.; Sayed, O.M.; Hozayen, W.G.; Azmy, A.F. Battling biofilm forming nosocomial pathogens using chitosan and pluronic F127. J. Pure Appl. Microbiol. 2020, 14, 1893–1903. [Google Scholar] [CrossRef]
- Amani, I.; Salehi, M.B.; Mansour, F.N. Frequency of prevalence of Klebsiella pneumoniae in clinical samples and the evaluation of the role of efflux pump in determining antibiotic resistance. Khazar J. Sci. Technol. 2020, 4, 41–64. [Google Scholar]
- Alcántar-Curiel, M.D.; Ledezma-Escalante, C.A.; Jarillo-Quijada, M.D.; Gayosso-Vázquez, C.; Morfín-Otero, R.; Rodríguez-Noriega, E.; Cedillo-Ramírez, M.L.; Santos-Preciado, J.I.; Girón, J.A. Association of antibiotic resistance, cell adherence, and biofilm production with the endemicity of nosocomial Klebsiella pneumoniae. BioMed. Res. Int. 2018, 2018, 7012958. [Google Scholar] [CrossRef]
- Ghashghaee, A.; Behzadifar, M.; Azari, S.; Farhadi, Z.; Bragazzi, N.L.; Behzadifar, M.; Shahri, S.S.S.; Ghaemmohamadi, M.S.; Ebadi, F.; Mohammadibakhsh, R.; et al. Prevalence of nosocomial infections in Iran: A systematic review and meta-analysis. Med. J. Islam. Repub. Iran 2018, 32, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Ashayeri-Panah, M.; Feizabadi, M.M.; Eftekhar, F. Correlation of multi-drug resistance, integron and blaESBL gene carriage with genetic fingerprints of extended-spectrum β-lactamase producing Klebsiella pneumoniae. Jundishapur J. Microbiol. 2014, 7, e8747. [Google Scholar] [CrossRef] [PubMed]
- Anes, J.; Hurley, D.; Martins, M.; Fanning, S. Exploring the genome and phenotype of multi-drug resistant Klebsiella pneumoniae of clinical origin. Front. Microbiol. 2017, 8, 1913. [Google Scholar] [CrossRef]
- Jin, Y.; Dong, C.; Shao, C.; Wang, Y.; Liu, Y. Molecular epidemiology of clonally related metallo-ß-lactamase-producing Klebsiella pneumoniae isolated from newborns in a hospital in Shandong, China. Jundishapur J. Microbiol. 2017, 10, e14046. [Google Scholar] [CrossRef]
- Marwah, P.; Chawla, D.; Chander, J.; Guglani, V.; Marwah, A. Bacteriological profile of neonatal sepsis in a tertiary-care hospital of Northern India. Indian Pediatr. 2015, 52, 158–159. [Google Scholar] [PubMed]
- Dolejska, M.; Brhelova, E.; Dobiasova, H.; Krivdova, J.; Jurankova, J.; Sevcikova, A.; Dubska, L.; Literak, I.; Cizek, A.; Vavrina, M.; et al. Dissemination of IncFIIK-type plasmids in multiresistant CTX-M-15-producing Enterobacteriaceae isolates from children in hospital paediatric oncology wards. Int. J. Antimicrob. Agents 2012, 40, 510–515. [Google Scholar] [CrossRef]
- Saeed, N.K.; Kambal, A.M.; El-Khizzi, N.A. Antimicrobial-resistant bacteria in a general intensive care unit in Saudi Arabia. Saudi Med. J. 2010, 31, 179–187. [Google Scholar]
- Caneiras, C.; Lito, L.; Melo-Cristino, J.; Duarte, A. Community-and hospital-acquired Klebsiella pneumoniae urinary tract infections in Portugal: Virulence and antibiotic resistance. Microorganisms 2019, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, S.; Kim, J.; Bae, S. Tracking short-term changes in the genetic diversity and antimicrobial resistance of OXA-232-producing Klebsiella pneumoniae ST14 in clinical settings. Clin. Microbiol. Infect. 2020, 26, 78–86. [Google Scholar] [CrossRef]
- Eghbalpoor, F.; Habibi, M.; Azizi, O.; Karam, M.R.A.; Bouzari, S. Antibiotic resistance, virulence and genetic diversity of Klebsiella pneumoniae in community- and hospital-acquired urinary tract infections in Iran. Acta Microbiol. Immunol. Hung. 2019, 66, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Wang, L.; Zeng, C.; Fu, L.; Liu, Z.; Hu, J. Clinical and microbiological characteristics of nosocomial, healthcare-associated, and community-acquired Klebsiella pneumoniae infections in Guangzhou, China. Antimicrob. Resist. Infect. Control 2021, 10, 1–11. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559312/ (accessed on 10 August 2021).
- Abdul Momin, M.H.F.; Liakopoulos, A.; Phee, L.M.; Wareham, D.W. Emergence and nosocomial spread of carbapenem-resistant OXA-232-producing Klebsiella pneumoniae in Brunei Darussalam. J. Glob. Antimicrob. Resist. 2017, 9, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, E.; Cascio, G.L.; Piccoli, M.; Cornaglia, G. KPC-3 carbapenemase harbored in FIIk plasmid from Klebsiella pneumoniae ST512 and Escherichia coli ST43 in the same patient. Microb. Drug Resist. 2014, 20, 377–382. [Google Scholar] [CrossRef]
- Kooti, S.; Zamani, K.; Sisakht, M.T.; Mansury, D.; Motamedifar, M. Phenotypic and genotypic detection of antibiotic resistance among metallo-beta-lactamases producing Klebsiella pneumoniae strains isolated from patients in intensive care units in Shiraz, Iran. Gene Rep. 2019, 17, 100522. [Google Scholar] [CrossRef]
- Keen, E.F.; Robinson, B.J.; Hospenthal, D.R.; Aldous, W.K.; Wolf, S.E.; Chung, K.K.; Murray, C.K. Prevalence of multidrug-resistant organisms recovered at a military burn center. Burns 2010, 36, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Jabar, H.H.; Abd, A.K.H.; Abdulamir, A.S. Efficacy of combinations of piperacilline/tazobactam, ceftazidime, amikacin and bacteriophage against Enterobacteriaceae sepsis in neonates: In vitro study. Sys. Rev. Pharm. 2020, 11, 165–170. [Google Scholar]
- Aljanaby, A.A.J.; Alhasani, A.H.A. Virulence factors and antibiotic susceptibility patterns of multidrug resistance Klebsiella pneumoniae isolated from different clinical infections. Afr. J. Microbiol. Res. 2016, 10, 829–843. [Google Scholar]
- Badamchi, A.; Farahani, R.K.; Naghadalipoor, M.; Etemadi, M.R.; Tabatabaie, A. Phenotypic and genotypic characterization of antibiotic resistance in Klebsiella pneumoniae isolated from patients admitted to a third-level hospital in Tehran, Iran. Curr. Pediatr. Rev. 2018, 22, 258–262. [Google Scholar]
- Bandić-pavlović, D.; Zah-bogović, T.; Žižek, M.; Bielen, L.; Bratić, V.; Hrabač, P.; Slačanac, D.; Mihaljević, S.; Bedenić, B. Gram-negative bacteria as causative agents of ventilator-associated pneumonia and their respective resistance mechanisms. J. Chemother. 2020, 32, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Bidell, M.R.; Opraseuth, M.P.; Yoon, M.; Mohr, J.; Lodise, T.P. Effect of prior receipt of antibiotics on the pathogen distribution and antibiotic resistance profile of key Gram-negative pathogens among patients with hospital-onset urinary tract infections. BMC Infect. Dis. 2017, 17, 176. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Mohsina, K.; Sarker, P.K.; Alam, M.Z.; Abdul Karim, M.I.; Abu Sayem, S.M. Prevalence, antibiotic susceptibility profiles and ESBL production in Klebsiella pneumoniae and Klebsiella oxytoca among hospitalized patients. Period. Biol. 2016, 118, 53–58. [Google Scholar] [CrossRef]
- Das, P.K.; Debnath, J. Prevalence and antibiotic susceptibility pattern of Klebsiella pneumoniae isolated from various clinical specimen in a tertiary care hospital of Tripura. Scholars Acad. J. Biosci. 2015, 3, 931–935. [Google Scholar]
- Durdu, B.; Koc, M.M.; Hakyemez, I.N.; Akkoyunlu, Y.; Daskaya, H.; Gultepe, B.S.; Aslan, T. Risk factors affecting patterns of antibiotic resistance and treatment efficacy in extreme drug resistance in intensive care unit-acquired Klebsiella pneumoniae infections: A 5-year analysis. Med. Sci. Monit. 2019, 25, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Folgori, L.; Livadiotti, S.; Carletti, M.; Bielicki, J.; Pontrelli, G.; Ciofi Degli Atti, M.L.; Bertaina, C.; Lucignano, B.; Ranno, S.; Carretto, E.; et al. Epidemiology and Clinical Outcomes of Multidrug-resistant, Gram-negative Bloodstream Infections in a European Tertiary Pediatric Hospital During a 12-month Period. J. Pediatr. Infect. Dis. 2014, 33, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Giufrè, M.; Accogli, M.; Ricchizzi, E.; Barbanti, F.; Farina, C.; Fazii, P.; Mattei, R.; Sarti, M.; Barozzi, A.; Buttazzi, R.; et al. Multidrug-resistant infections in long-term care facilities: Extended-spectrum β-lactamase–producing Enterobacteriaceae and hypervirulent antibiotic resistant Clostridium difficile. Diagn. Microbiol. Infect. Dis. 2018, 91, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Glasser, J.S.; Guymon, C.H.; Mende, K.; Wolf, S.E.; Hospenthal, D.R.; Murray, C.K. Activity of topical antimicrobial agents against multidrug-resistant bacteria recovered from burn patients. Burns 2010, 36, 1172–1184. [Google Scholar] [CrossRef]
- Imtiaz, W.; Syed, Z.; Rafaque, Z.; Andrews, S.C.; Dasti, J.I. Analysis of antibiotic resistance and virulence traits (genetic and phenotypic) in Klebsiella pneumoniae clinical isolates from Pakistan: Identification of significant levels of carbapenem and colistin resistance. Infect. Drug Resist. 2021, 14, 227–236. [Google Scholar] [CrossRef]
- John, J.F.; McKee, K.T.; Twitty, J.A.; Schaffner, W. Molecular epidemiology of sequential nursery epidemics caused by multiresistant Klebsiella pneumoniae. J. Pediatr. 1983, 102, 825–830. [Google Scholar] [CrossRef]
- Kołpa, M.; Wałaszek, M.; Gniadek, A.; Wolak, Z.; Dobroś, W. Incidence, Microbiological Profile and Risk Factors of Healthcare-Associated Infections in Intensive Care Units: A 10 Year Observation in a Provincial Hospital in Southern Poland. Int. J. Environ. Res. Public Health 2018, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Q.; Ahmad Kamar, A.; Velayuthan, R.D.; Chong, C.W.; The, C.S.J. Clonal relatedness in the acquisition of intestinal carriage and transmission of multidrug resistant (MDR) Klebsiella pneumoniae and Escherichia coli and its risk factors among preterm infants admitted to the neonatal intensive care unit (NICU). Pediatr. Neonatol. 2021, 62, 129–137. [Google Scholar] [CrossRef]
- Lima, A.M.S.; de Melo, M.E.S.; Alves, L.C.; Brayner, F.A.; Lopes, A.C.S. Investigation of class 1 integrons in Klebsiella pneumoniae clinical and microbiota isolates belonging to different phylogenetic groups in Recife, State of Pernambuco. Rev. Soc. Bras. Med. Trop. 2014, 47, 165–169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahmoudi, S.; Mahzari, M.; Banar, M.; Pourakbari, B.; Haghi Ashtiani, M.T.; Mohammadi, M.; Valian, S.K.; Mamishi, S. Antimicrobial resistance patterns of gram-negative bacteria isolated from bloodstream infections in an Iranian referral paediatric hospital: A 5.5-year study. J. Glob. Antimicrob. Resist. 2017, 11, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Mansour, W.; Haenni, M.; Saras, E.; Grami, R.; Mani, Y.; Khalifa, A.B.H.; el Atrouss, S.; Kheder, M.; Hassen, M.F.; Boujâafar, N.; et al. Outbreak of colistin-resistant carbapenemase-producing Klebsiella pneumoniae in Tunisia. J. Glob. Antimicrob. Resist. 2017, 10, 88–94. [Google Scholar] [CrossRef]
- Moges, F.; Eshetie, S.; Abebe, W.; Mekonnen, F.; Dagnew, M.; Endale, A.; Amare, A.; Feleke, T.; Gizachew, M.; Tiruneh, M. High prevalence of extended-spectrum beta-lactamase-producing Gram-negative pathogens from patients attending Felege Hiwot Comprehensive Specialized Hospital, Bahir Dar, Amhara region. PLoS ONE 2019, 14, e0215177. [Google Scholar] [CrossRef]
- Nirwati, H.; Sinanjung, K.; Fahrunissa, F.; Wijaya, F.; Napitupulu, S.; Hati, V.P.; Hakim, M.S.; Meliala, A.; Aman, A.T.; Nuryastuti, T. Biofilm formation and antibiotic resistance of Klebsiella pneumoniae isolated from clinical samples in a tertiary care hospital, Klaten, Indonesia. BMC Proc. 2019, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Okomo, U.; Senghore, M.; Darboe, S.; Bojang, E.; Zaman, S.M.A.; Hossain, M.J.; Nwakanma, D.; Le Doare, K.; E Holt, K.; Hos, N.J.; et al. Investigation of sequential outbreaks of Burkholderia cepacia and multidrug-resistant extended spectrum β-lactamase producing Klebsiella species in a West African tertiary hospital neonatal unit: A retrospective genomic analysis. Lancet Microbe. 2020, 1, e119–e129. [Google Scholar] [CrossRef]
- Oli, A.N.; Eze, D.E.; Gugu, T.H.; Ezeobi, I.; Maduagwu, U.N.; Ihekwereme, C.P. Multi-antibiotic resistant extended-spectrum beta-lactamase producing bacteria pose a challenge to the effective treatment of wound and skin infections. Pan Afr. Med. J. 2017, 27, 66. [Google Scholar] [CrossRef] [PubMed]
- Petro, D.; Mushi, M.F.; Moremi, N.; Iddi, S.; Mirambo, M.; Seni, J.; Mshana, S.E. In vitro susceptibility of multi-drug resistant Pseudomonas aeruginosa and extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolated from clinical specimens at Bugando Medical Centre, Tanzania to piperacillin-Tazobactam. Tanzan. J. Health Res. 2014, 16, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Shahi, A.; Hasani, A.; Rezaee, M.A.; Jafarabadi, M.A.; Hasani, A.; Kafil, H.S.; Memar, M.Y.; Soltani, E. Klebsiella pneumoniae carbapenemase production among K. pneumoniae isolates and its concern on antibiotic susceptibility. Microbiol. Res. 2019, 10, 8–11. [Google Scholar] [CrossRef]
- Sharahi, J.Y.; Hashemi, A.; Ardebili, A.; Davoudabadi, S. Molecular characteristics of antibiotic-resistant Escherichia coli and Klebsiella pneumoniae strains isolated from hospitalized patients in Tehran, Iran. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Traub, W.H.; Schwarze, I.; Bauer, D. Nosocomial outbreak of cross-infection due to multiple-antibiotic-resistant Klebsiella pneumoniae: Characterization of the strain and antibiotic susceptibility studies. Chemotherapy 2000, 46, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, S.; Afsharian, M.; Mansouri, F.; Azizi, M.; Nouri, F.; Madadi-Goli, N.; Afshar, Z.M.; Zamanian, M.H.; Alvandi, A.; Ahmadi, K. Frequency of qnr and aac(6′)Ib-cr genes among ESBL-producing Klebsiella pneumoniae strains isolated from burn patients in Kermanshah, Iran. Jundishapur J. Microbiol. 2020, 13, e100348. [Google Scholar] [CrossRef]
- Yazdansetad, S.; Alkhudhairy, M.K.; Najafpour, R.; Farajtabrizi, E.; Al-Mosawi, R.M.; Saki, M.; Jafarzadeh, E.; Izadpour, F.; Ameri, A. Preliminary survey of extended-spectrum β-lactamases (ESBLs) in nosocomial uropathogen Klebsiella pneumoniae in north-central Iran. Heliyon 2019, 5, e02349. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhao, C.; Li, H.; Jin, L.; Wang, Q.; Wang, R.; Zhang, Y.; Zhang, J.; Wang, H.; Yang, C.; et al. Clinical and microbiological characteristics of adults with hospital-acquired pneumonia: A 10-year prospective observational study in China. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 40, 683–690. [Google Scholar] [CrossRef]
- Uz Zaman, T.; Aldrees, M.; Al Johani, S.M.; Alrodayyan, M.; Aldughashem, F.A.; Balkhy, H.H. Multi-drug carbapenem-resistant Klebsiella pneumoniae infection carrying the OXA-48 gene and showing variations in outer membrane protein 36 causing an outbreak in a tertiary care hospital in Riyadh, Saudi Arabia. Int. J. Infect. Dis. 2014, 28, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhang, J.; Li, C.; Fu, Y.; Zhao, Y.; Wang, Y.; Zhao, J.; Guo, Y.; Zhang, X. The determination of gyrA and parC mutations and the prevalence of plasmid-mediated quinolone resistance genes in carbapenem resistant Klebsiella pneumonia ST11 and ST76 strains isolated from patients in Heilongjiang Province, China. Infect. Genet. Evol. 2020, 82, 104319. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Men, T.Y.; Li, H.; Peng, Z.H.; Gu, Y.; Ding, X.; Xing, T.-H.; Fan, J.-W. Multidrug-resistant gram-negative bacterial infections after liver transplantation-spectrum and risk factors. J. Infect. 2012, 64, 299–310. [Google Scholar] [CrossRef]
- Abayneh, M.; Worku, T. Prevalence of multidrug-resistant and extended-spectrum beta-lactamase (ESBL)-producing gram-negative bacilli: A meta-analysis report in Ethiopia. Drug Target Insights 2020, 14, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Abrar, S.; Hussain, S.; Khan, R.A.; Ain, N.U.; Haider, H.; Riaz, S. Prevalence of extended-spectrum-β-lactamase-producing Enterobacteriaceae: First systematic meta-analysis report from Pakistan. Antimicrob. Resist. Infect. Control 2018, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Sonda, T.; Kumburu, H.; van Zwetselaar, M.; Alifrangis, M.; Lund, O.; Kibiki, G.; Aarestrup, F.M. Meta-analysis of proportion estimates of extended-spectrum-beta-lactamase-producing Enterobacteriaceae in East Africa hospitals. Antimicrob. Resist. Infect. Control 2016, 5, 18. [Google Scholar] [CrossRef]
- Mansouri, F.; Sheibani, H.; Masroor, M.J.; Afsharian, M. Extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae and urinary tract infections in pregnant/postpartum women: A systematic review and meta-analysis. Int. J. Clin. Pract. 2019, 73, e13422. [Google Scholar] [CrossRef]
- Cantón, R.; Novais, A.; Valverde, A.; Machado, E.; Peixe, L.; Baquero, F.; Coque, T. Prevalence and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 2008, 14, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Navon-Venezia, S.; Kondratyeva, K.; Carattoli, A. Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 252–275. [Google Scholar] [CrossRef] [PubMed]
- Mączyńska, B.; Paleczny, J.; Oleksy-Wawrzyniak, M.; Choroszy-Król, I.; Bartoszewicz, M. In vitro susceptibility of multi-drug resistant Klebsiella pneumoniae strains causing nosocomial infections to fosfomycin. A comparison of determination methods. Pathogens 2021, 10, 512. [Google Scholar] [CrossRef]
- Hou, X.H.; Song, X.Y.; Ma, X.B.; Zhang, S.Y.; Zhang, J.Q. Molecular characterization of multidrug-resistant Klebsiella pneumoniae isolates. Braz. J. Microbiol. 2015, 46, 759–768. [Google Scholar] [CrossRef]
- Hou, S.Y.; Wu, D.; Feng, X.H. Polymyxin monotherapy versus polymyxin-based combination therapy against carbapenem-resistant Klebsiella pneumoniae: A systematic review and meta-analysis. J. Glob. Antimicrob. Resist. 2020, 23, 197–202. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wang, J.; Wang, R.; Cai, Y. Double-carbapenem therapy in the treatment of multidrug resistant gram-negative bacterial infections: A systematic review and meta-analysis. BMC Infect. Dis. 2020, 20, 408. [Google Scholar] [CrossRef]
- Khan, H.A.; Baig, F.K.; Mehboob, R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac. J. Trop. Biomed. 2017, 7, 478–482. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Cochrane, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Asri, N.A.; Ahmad, S.; Mohamud, R.; Mohd Hanafi, N.; Mohd Zaidi, N.F.; Irekeola, A.A.; Shueb, R.H.; Yee, L.C.; Mohd Noor, N.; Mustafa, F.H.; et al. Global Prevalence of Nosocomial Multidrug-Resistant Klebsiella pneumoniae: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10, 1508. https://doi.org/10.3390/antibiotics10121508
Mohd Asri NA, Ahmad S, Mohamud R, Mohd Hanafi N, Mohd Zaidi NF, Irekeola AA, Shueb RH, Yee LC, Mohd Noor N, Mustafa FH, et al. Global Prevalence of Nosocomial Multidrug-Resistant Klebsiella pneumoniae: A Systematic Review and Meta-Analysis. Antibiotics. 2021; 10(12):1508. https://doi.org/10.3390/antibiotics10121508
Chicago/Turabian StyleMohd Asri, Nur Ain, Suhana Ahmad, Rohimah Mohamud, Nurmardhiah Mohd Hanafi, Nur Fatihah Mohd Zaidi, Ahmad Adebayo Irekeola, Rafidah Hanim Shueb, Leow Chiuan Yee, Norhayati Mohd Noor, Fatin Hamimi Mustafa, and et al. 2021. "Global Prevalence of Nosocomial Multidrug-Resistant Klebsiella pneumoniae: A Systematic Review and Meta-Analysis" Antibiotics 10, no. 12: 1508. https://doi.org/10.3390/antibiotics10121508
APA StyleMohd Asri, N. A., Ahmad, S., Mohamud, R., Mohd Hanafi, N., Mohd Zaidi, N. F., Irekeola, A. A., Shueb, R. H., Yee, L. C., Mohd Noor, N., Mustafa, F. H., Yean, C. Y., & Yusof, N. Y. (2021). Global Prevalence of Nosocomial Multidrug-Resistant Klebsiella pneumoniae: A Systematic Review and Meta-Analysis. Antibiotics, 10(12), 1508. https://doi.org/10.3390/antibiotics10121508








