Pharmacovigilance Data as a Trigger to Identify Antimicrobial Resistance and Inappropriate Use of Antibiotics: A Study Using Reports from The Netherlands Pharmacovigilance Centre
Abstract
:1. Introduction
1.1. Antimicrobial Resistance (AMR) and Antimicrobial Stewardship
1.2. The Access, Watch and Reserve (AWaRe) Classification for Availability and Appropriate Use
1.3. Pharmacovigilance and Antimicrobial Resistance
1.4. Pharmacovigilance in The Netherlands
1.5. Study Objective
2. Material and Methods
Data Source and Search Strategy
- (a)
- Reports on Antibiotics classified under the Anatomical Therapeutic Chemical (ATC) Classification ATC J01 or ATC J04.
- (b)
- Reports coded with at least one of the following MedDRA (version 21.1) Preferred terms and codes included the following: pathogen resistance (10034133); drug ineffective (10013709); treatment failure (10066901); drug resistance (10059866); therapeutic product ineffective (10060769); therapy non-responder (10051082); decreased activity (10011953); drug ineffective for unapproved indication (10051118); therapeutic response decreased (10043414); multiple drug resistance (10048723); off label use (10053762); medication error (10027091); product use in unapproved indication (10076476); contraindicated product administered (10078504)
3. Results
3.1. ADR Reports with AMR-Relevant Codes
3.2. Most Frequently Used PTs in Cases of Suspected Resistance or Use-Related Issues
3.3. Applying the AWaRe Classification to the Reports
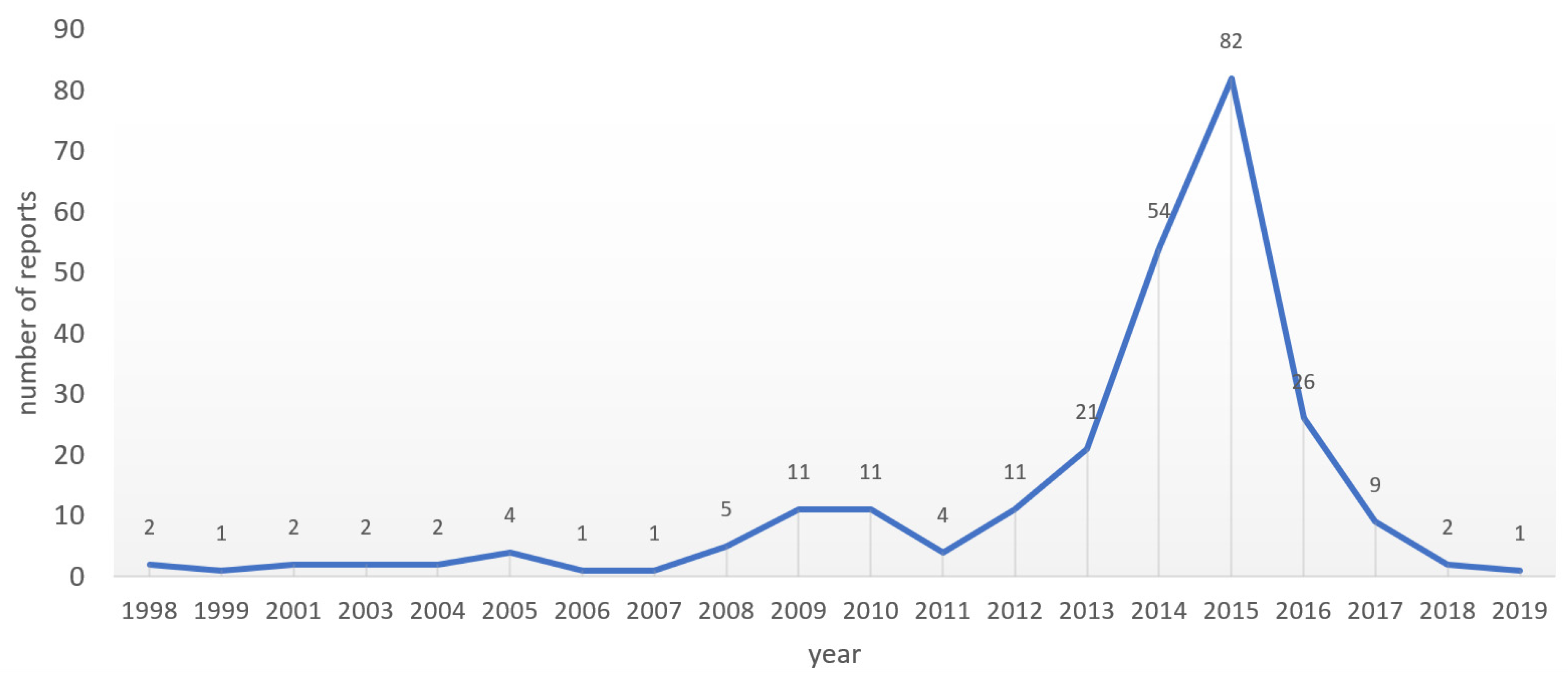
3.4. The 2015 Peak in Numbers of AMR-Relevant ADR Reports to Lareb
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 4 July 2021).
- CDC. About Antibiotic Resistance. Available online: https://www.cdc.gov/drugresistance/about.html (accessed on 4 July 2021).
- Ashiru-Oredope, D.; Doble, A.; Akpan, M.R.; Hansraj, S.; Shebl, N.A.; Ahmad, R.; Hopkins, S. Antimicrobial Stewardship Programmes in Community Healthcare Organisations in England: A Cross-Sectional Survey to Assess Implementation of Programmes and National Toolkits. Antibiotics 2018, 7, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doron, S.; Davidson, L.E. Antimicrobial stewardship. Mayo Clin. Proc. 2011, 86, 1113–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamdan, S.; El-Dahiyat, F. Implementation and evaluation of an antimicrobial stewardship program across nine hospitals in the United Arab Emirates: A qualitative study. J. Pharm. Pract. Res. 2020, 50, 124–131. [Google Scholar] [CrossRef]
- Jonas, O.B.; Irwin, A.; Berthe, F.C.J.; Le Gall, F.G.; Marquez, P.V. Drug-Resistant Infections: A Threat to Our Economic Future (Vol. 2): Final Report (English); HNP/Agriculture Global Antimicrobial Resistance Initiative; World Bank Group: Washington, DC, USA, 2017; Available online: http://documents.worldbank.org/curated/en/323311493396993758/final-report (accessed on 21 July 2021).
- Shirazi, O.U.; Ab Rahman, N.S.; Zin, C.S. A Narrative Review of Antimicrobial Stewardship Interventions within In-Patient Settings and Resultant Patient Outcomes. J. Pharm. BioAllied Sci. 2020, 12, 369–380. [Google Scholar] [CrossRef] [PubMed]
- May, L.; Martín Quirós, A.; Ten Oever, J.; Hoogerwerf, J.; Schoffelen, T.; Schouten, J. Antimicrobial stewardship in the emergency department: Characteristics and evidence for effectiveness of interventions. Clin. Microbiol. Infect. 2021, 27, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Acquisto, N.M.; May, L. Collaborative Antimicrobial Stewardship in the Emergency Department. Infect. Dis. Clin. N. Am. 2020, 34, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Buckel, W.R.; Kaye, K.S.; Patel, P.K. Collaborative Antimicrobial Stewardship: Working with Hospital and Health System Administration. Infect. Dis. Clin. N. Am. 2020, 34, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Olans, R.D.; Hausman, N.B.; Olans, R.N. Nurses and Antimicrobial Stewardship: Past, Present, and Future. Infect. Dis. Clin. N. Am. 2020, 34, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Wall, S. Prevention of antibiotic resistance—An epidemiological scoping review to identify research categories and knowledge gaps. Global Health Action 2019, 12 (Suppl. 1), 1756191. [Google Scholar] [CrossRef]
- WHO. New Report Calls for Urgent Action to Avert Antimicrobial Resistance Crisis. Available online: https://www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis (accessed on 21 July 2021).
- Sharland, M.; Pulcini, C.; Harbarth, S.; Zeng, M.; Gandra, S.; Mathur, S.; Magrini, N. Classifying antibiotics in the WHO Essential Medicines List for optimal use—Be AWaRe. Lancet Infect. Dis. 2018, 18, 18–20. [Google Scholar] [CrossRef] [Green Version]
- WHO. AWaRe Policy Brief. Available online: https://adoptaware.org/assets/pdf/aware_policy_brief.pdf (accessed on 18 July 2021).
- Pharmacovigilance: Overview|European Medicines Agency (europa.eu). Available online: https://www.ema.europa.eu/en/human-regulatory/overview/pharmacovigilance-overview (accessed on 13 June 2021).
- UMC. Glossary of Pharmacovigilance Terms. Available online: https://www.who-umc.org/global-pharmacovigilance/publications/glossary/ (accessed on 13 July 2021).
- UMC. Antimicrobial Resistance—An Overlooked Adverse Event. Available online: https://www.who-umc.org/media/2775/web_uppsalareports_issue74.pdf (accessed on 13 June 2021).
- Agrawal, V.; Shrivastava, T.P.; Adusumilli, P.K.; Vivekanandan, K.; Thota, P.; Bhushan, S. Pivotal role of Pharmacovigilance Programme of India in containment of antimicrobial resistance in India. Perspect. Clin. Res. 2019, 10, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Bairy, L.K.; Nayak, V.A.A.; Kunder, S.K. Advances in pharmacovigilance initiatives surrounding antimicrobial resistance-Indian perspective. Expert Opin. Drug Saf. 2016, 15, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Kuzmina, A.V.; Asetskaya, I.L.; Zyryanov, S.K.; Polivanov, V.A. Detecting medication errors associated with the use of beta-lactams in the Russian Pharmacovigilance database. BMC Pharmacol. Toxicol. 2021, 22, 5. [Google Scholar] [CrossRef] [PubMed]
- Ruíz-Garzón, J.A.; Calderón-Ospina, C.A. Consideraciones acerca del reporte y la evaluación del fallo terapéutico en farmacovigilancia/Considerations regarding the reporting and evaluation of therapeutic failure in pharmacovigilance. Rev. Fac. Med. 2019, 67, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Habarugira, J.M.V.; Figueras, A. Antimicrobial stewardship: Can we add pharmacovigilance networks to the toolbox? Eur. J. Clin. Pharmacol. 2021, 77, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Habarugira, J.M.V.; Figueras, A. Pharmacovigilance network as an additional tool for the surveillance of antimicrobial resistance. Pharmacoepidemiol. Drug Saf. 2021, 30, 1123–1131. [Google Scholar] [CrossRef]
- Sharma, M.; Baghel, R.; Thakur, S.; Adwal, S. Surveillance of adverse drug reactions at an adverse drug reaction monitoring centre in Central India: A 7-year surveillance study. BMJ Open 2021, 11, e052737. [Google Scholar] [CrossRef] [PubMed]
- UMC. Members of the WHO Programme for International Drug Monitoring. Available online: https://www.who-umc.org/global-pharmacovigilance/who-programme-for-international-drug-monitoring/who-programme-members/ (accessed on 13 June 2021).
- Härmark, L.; van Hunsel, F.; Grundmark, B. ADR Reporting by the General Public: Lessons Learnt from the Dutch and Swedish Systems. Drug Saf. 2015, 38, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Van Grootheest, A.C.; Passier, J.L.; van Puijenbroek, E.P. Meldingen van bijwerkingen rechtstreeks door patiënten: Gunstige ervaringen van het eerste jaar [Direct reporting of side effects by the patient: Favourable experience in the first year]. Ned. Tijdschr. Geneeskd. 2005, 149, 529–533. (In Dutch) [Google Scholar] [PubMed]
- De Langen, J.; van Hunsel, F.; Passier, A.; de Jong-van den Berg, L.; van Grootheest, K. Adverse drug reaction reporting by patients in the Netherlands: Three years of experience. Drug Saf. 2008, 31, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Guideline on Good Pharmacovigilance Practices (GVP) Module V—Risk Management Systems (Rev 2) (europa.eu). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-module-v-risk-management-systems-rev-2_en.pdf (accessed on 21 July 2021).
| RIOLE Categories (Number of Reports; %) | PTs in the RIOLE Category (Number of Reports; %) | Most Reported Antibiotics per PT (Number of Reports; %) |
|---|---|---|
| Suggesting AMR (98; 39%) | drug ineffective (71; 72%), | aztreonam (9; 13%) |
| amoxicillin + Beta-lactamase inhibitor (6; 8%) | ||
| doxycycline (6; 8%) | ||
| pathogen resistance (14; 14%) | ceftazidime (5; 36%) | |
| ciprofloxacin (2; 14%) | ||
| linezolid (2; 14%) | ||
| drug resistance (13; 13%) | tobramycin (3; 23%) | |
| ciprofloxacin (2; 15%) | ||
| Suggesting use-related issues (119; 47%) | off-label use (91; 76%) | tobramycin (53; 58%) |
| colistin (24; 26%) | ||
| doxycycline (6; 7%) | ||
| product use in unapproved indication (28; 15%) | tobramycin (27; 96%) | |
| Suggesting both AMR and use-related issues (35; 14%) | Combinations of PTs Suggesting both AMR and use-related issues (35; 14%) | ciprofloxacin (7; 20%) azithromycin (3; 9%) |
| TOTAL = 252; 100% | - | - |
| AWaRe Categories (Number of Reports; %) | Most Reported Antibiotics in the AWaRe Category (Number of Reports; %) | Most Used PTs in the AWaRE Category (Number of Reports; %) * |
|---|---|---|
| Access (40; 16%) | doxycycline (14; 35%) | drug ineffective (25; 63%) off label use (6; 15%) |
| amoxicillin + Beta-lactamase inhibitor (7; 18%) | ||
| sulfamethoxazole + trimethoprim (4; 10%) | ||
| Watch (137; 54%) | tobramycin (89; 78%) | Off label use (57; 42%) Product use in unapproved indication (27; 20%) drug ineffective (20; 15%) pathogen resistance (8; 6%) |
| ciprofloxacin (16; 33%) | ||
| azithromycin (8; 17%) | ||
| moxifloxacin (7; 15%) | ||
| Reserve (45; 19%) | colistin (30; 91%) | off label use (25; 76%) |
| aztreonam (12; 11%) | ||
| Combination of different classes (17; 6%) | concomitant from different classes | drug ineffective (7; 41%) drug resistance (3; 18%) |
| Other or not classified (13; 5%) | drug ineffective (8; 61%) | |
| TOTAL (252; 100%) | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habarugira, J.M.V.; Härmark, L.; Figueras, A. Pharmacovigilance Data as a Trigger to Identify Antimicrobial Resistance and Inappropriate Use of Antibiotics: A Study Using Reports from The Netherlands Pharmacovigilance Centre. Antibiotics 2021, 10, 1512. https://doi.org/10.3390/antibiotics10121512
Habarugira JMV, Härmark L, Figueras A. Pharmacovigilance Data as a Trigger to Identify Antimicrobial Resistance and Inappropriate Use of Antibiotics: A Study Using Reports from The Netherlands Pharmacovigilance Centre. Antibiotics. 2021; 10(12):1512. https://doi.org/10.3390/antibiotics10121512
Chicago/Turabian StyleHabarugira, Jean Marie Vianney, Linda Härmark, and Albert Figueras. 2021. "Pharmacovigilance Data as a Trigger to Identify Antimicrobial Resistance and Inappropriate Use of Antibiotics: A Study Using Reports from The Netherlands Pharmacovigilance Centre" Antibiotics 10, no. 12: 1512. https://doi.org/10.3390/antibiotics10121512







