Assessing the Impact of COVID-19 on Antimicrobial Stewardship Activities/Programs in the United Kingdom
Abstract
1. Introduction
2. Results and Discussion
2.1. Demographics of Respondents
2.2. Impact of COVID-19 on Antimicrobial Stewardship (AMS) Activities/Initiatives
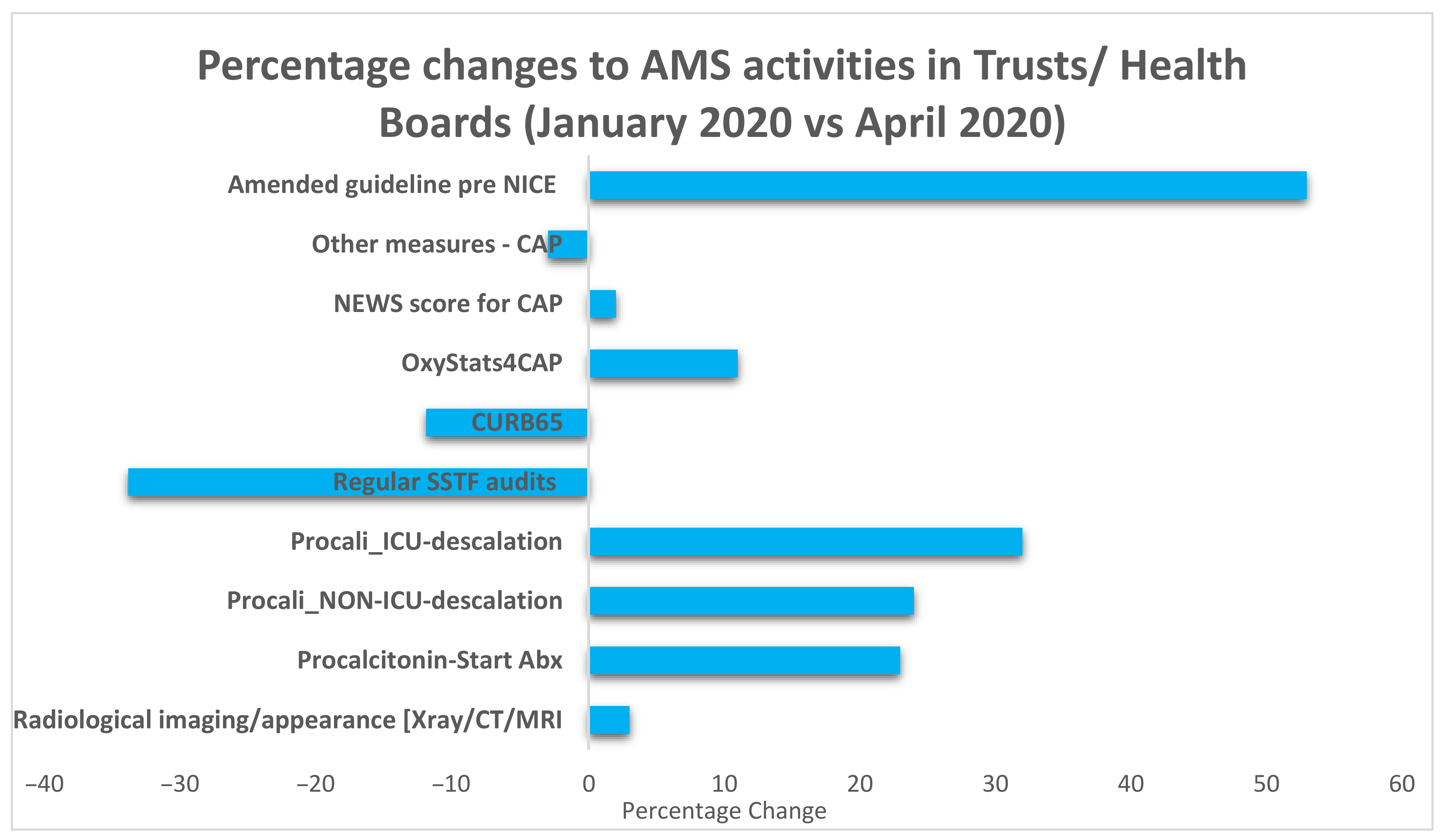
2.3. COVID-19 Specific Changes to the Management of Pneumonia
2.4. Participation in COVID-19 Clinical Trials*
2.5. Update of Local Guidelines and Implementation of National Guidelines
2.6. Communication Methods within Secondary Care Settings (n = 95)
2.7. Staff Changes during COVID-19 Epidemic
3. Materials and Methods
3.1. Respondent Eligibility
3.2. Data Management
3.3. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phelan, A.L.; Katz, R.; Gostin, L.O. The Novel Coronavirus Originating in Wuhan, China: Challenges for Global Health Governance. JAMA J. Am. Med. Assoc. 2020, 323, 709–710. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Huttner, B.D.; Catho, G.; Pano-Pardo, J.R.; Pulcini, C.; Schouten, J. COVID-19: Don’t neglect antimicrobial stewardship principles! Clin. Microbiol. Infect. 2020, 26, 808–810. [Google Scholar] [CrossRef]
- Mazdeyasna, H.; Nori, P.; Patel, P.; Doll, M.; Godbout, E.; Lee, K.; Noda, A.J.; Bearman, G.; Stevens, M.P. Antimicrobial Stewardship at the Core of COVID-19 Response Efforts: Implications for Sustaining and Building Programs. Curr. Infect. Dis. Rep. 2020, 22, 1–6. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Castro-Sanchez, E.; Charani, E.; Davies, F.; Satta, G.; Ellington, M.J.; Holmes, A.H. COVID-19 and the potential long-term impact on antimicrobial resistance. J. Antimicrob. Chemother. 2020, 75, 1681–1684. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and fungal co-infection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L.S. Bacterial and fungal coinfection among hospitalised patients with COVID-19: A retrospective cohort study in a UK secondary care setting. Clin. Microbiol. Infect. 2020, 26. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26. [Google Scholar] [CrossRef]
- Klein, E.Y.; Monteforte, B.; Gupta, A.; Jiang, W.; May, L.; Hsieh, Y.H.; Dugas, A. The frequency of influenza and bacterial coinfection: A systematic review and meta-analysis. Influenza Other Respir. Viruses 2016, 10, 394–403. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Cardona-Ospina, J.A.; Gutiérrez-Ocampo, E.; Villamizar-Peña, R.; Holguin-Rivera, Y.; Escalera-Antezana, J.P.; Alvarado-Arnez, L.E.; Bonilla-Aldana, D.K.; Franco-Paredes, C.; Henao-Martinez, A.F.; et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2020, 34, 101623. [Google Scholar] [CrossRef]
- Xiong, Y.; Sun, D.; Liu, Y.; Fan, Y.; Zhao, L.; Li, X.; Zhu, W. Clinical and High-Resolution CT Features of the COVID-19 Infection: Comparison of the Initial and Follow-up Changes. Investig. Radiol. 2020, 55, 332–339. [Google Scholar] [CrossRef]
- Cox, M.J.; Loman, N.; Bogaert, D.; O’Grady, J. Co-infections: Potentially lethal and unexplored in COVID-19. Lancet Microbe 2020, 1, e11. [Google Scholar] [CrossRef]
- Morris, D.E.; Cleary, D.W.; Clarke, S.C. Secondary Bacterial Infections Associated with Influenza Pandemics. Front. Microbiol. 2017, 8, 1041. [Google Scholar] [CrossRef]
- Schauwvlieghe, A.F.A.D.; Rijnders, B.J.A.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van de Veerdonk, F.L.; Gommers, D.; et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet. Respir. Med. 2018, 6, 782–792. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Deeb, A.M.; Al-Hameed, F.; Mandourah, Y.; Almekhlafi, G.A.; Sindi, A.A.; Al-Omari, A.; Shalhoub, S.; Mady, A.; Alraddadi, B.; et al. Macrolides in critically ill patients with Middle East Respiratory Syndrome. Int. J. Infect. Dis. 2019, 81, 184–190. [Google Scholar] [CrossRef]
- Gautret, P.; Lagier, J.C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Sevestre, J.; Mailhe, M.; Doudier, B.; Aubry, C.; Amrane, S.; et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med. Infect. Dis. 2020, 34. [Google Scholar] [CrossRef]
- Cavalcanti, A.B.; Zampieri, F.G.; Rosa, R.G.; Azevedo, L.C.P.; Veiga, V.C.; Avezum, A.; Damiani, L.P.; Marcadenti, A.; Kawano-Dourado, L.; Lisboa, T.; et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Arshad, S.; Kilgore, P.; Chaudhry, Z.S.; Jacobsen, G.; Wang, D.D.; Huitsing, K.; Brar, I.; Alangaden, G.J.; Ramesh, M.S.; McKinnon, J.E.; et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int. J. Infect. Dis. 2020, 97, 396–403. [Google Scholar] [CrossRef]
- Stevens, R.W.; Estes, L.; Rivera, C. Practical Implementation of COVID-19 Patient Flags into an Antimicrobial Stewardship Program’s Prospective Review. Infect. Control Hosp. Epidemiol. 2020, 41, 1–2. [Google Scholar] [CrossRef]
- PHE Fingertips. AMR Local Indicators. Available online: https://fingertips.phe.org.uk/profile/amr-local-indicators (accessed on 30 November 2020).
- Department of Health and Social Care. Drug Shortages Update. Supply Issues Update for Primary and Secondary Care: March/April 2020. Available online: https://www.lmc.org.uk/visageimages/Covid-19/Supply%20issues%20update%20for%20primary%20and%20secondary%20care%20March%20April%202020.pdf21 (accessed on 30 November 2020).
- Knight, M.; Evans, D.; Vancheeswaran, R.; van der Watt, M.; Smith, A.N.; Oliver, C.; Kelso, P.; Spencer, C.; Barlow, A. A Virtual Hospital Model Can Help Tackle the Covid-19 Pandemic. Available online: https://www.hsj.co.uk/technology-and-innovation/a-virtual-hospital-model-can-help-tackle-the-covid-19-pandemic/7027340.article (accessed on 30 November 2020).
- Lynch, C.; Mahida, N.; Gray, J. Antimicrobial stewardship: A COVID casualty? J. Hosp. Infect. 2020, 106, 401–403. [Google Scholar] [CrossRef]
- Monnet, D.L.; Harbarth, S. Will coronavirus disease (COVID-19) have an impact on antimicrobial resistance? Eurosurveillance 2020, 25, 2001886. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). COVID-19 Rapid Guideline: Managing Suspected or Confirmed Pneumonia in Adults in the Community (NG 165). Available online: https://www.nice.org.uk/guidance/ng165/ (accessed on 28 November 2020).
- National Institute for Health and Care Excellence (NICE). COVID-19 Rapid Guideline: Antibiotics for Pneumonia in Adults in Hospital (NG 173). Available online: https://www.nice.org.uk/guidance/ng173/ (accessed on 28 November 2020).
- Thorlund, K.; Dron, L.; Park, J.; Hsu, G.; Forrest, J.I.; Mills, E.J. A real-time dashboard of clinical trials for COVID-19. Lancet Digit. Health 2020, 2, e286–e287. [Google Scholar] [CrossRef]
- Cytlel. Global Coronavirus COVID-19 Clinical Trial Tracker. Available online: https://www.covid-trials.org/ (accessed on 28 November 2020).
- Martin, E.; Philbin, M.; Hughes, G.; Bergin, C.; Talento, A.F. Antimicrobial stewardship challenges and innovative initiatives in the acute hospital setting during the COVID-19 pandemic. J. Antimicrob. Chemother. 2021. [Google Scholar] [CrossRef]
- Goff, D.A.; Ashiru-Oredope, D.; Cairns, K.A.; Eljaaly, K.; Gauthier, T.P.; Langford, B.J.; Mahmoud, S.F.; Messina, A.P.; Michael, U.C.; Saad, T.; et al. Global contributions of pharmacists during the COVID-19 pandemic. J. Am. Coll. Clin. Pharm. 2020, 1–13. [Google Scholar] [CrossRef]
- Khor, W.P.; Olaoye, O.; D’Arcy, N.; Krockow, E.M.; Elshenawy, R.A.; Rutter, V.; Ashiru-Oredope, D. The Need for Ongoing Antimicrobial Stewardship during the COVID-19 Pandemic and Actionable Recommendations. Antibiotics 2020, 9, 904. [Google Scholar] [CrossRef]


| Country | Number of Trusts/Health Boards with Responses | % of Respondents |
|---|---|---|
| England | 79 | 83.2 |
| Scotland | 5 | 5.3 |
| Wales | 7 | 7.4 |
| Northern Ireland | 4 | 4.2 |
| Type of hospital/organization | Number | % of respondents |
| Teaching | 25 | 26.3 |
| District/General | 39 | 41.1 |
| Acute Trust with multiple types of hospitals | 13 | 13.7 |
| Specialist | 7 | 7.4 |
| Others | 11 | 11.6 |
| Community Trust, Mental Health Trust, or Clinical Commissioning Groups (CCG)/Primary care/Primary Care Network | 0 | 0 |
| Reported estimated number of COVID-19 cases by respondents | Number of respondents | % of respondents |
| 0–50 | 4 | 4.2 |
| 51–200 | 10 | 10.5 |
| 201–500 | 16 | 16.8 |
| 501–1000 | 21 | 22.1 |
| 1000–2000 | 12 | 12.6 |
| >2000 | 4 | 4.2 |
| Unsure | 25 | 26.3 |
| Do not wish to answer | 3 | 3.2 |
| National Guidelines | Yes (%) | Already Aligned (%) | Still Discussing (%) | Don’t Plan to (%) | NA (%) |
|---|---|---|---|---|---|
| Update CAP guidelines following publication of NICE NG 165 (n = 95) | 29.5 | 42.1 | 9.5 | 11.6 | 7.4 |
| Update HAP guidelines following publication of NICE NG173 (n = 95) | 29.5 | 41.1 | 10.5 | 11.6 | 7.4 |
| NICE criteria on when to stop antibiotics been implemented/promoted (n = 95) | 36.8 | 27.4 | 24.2 | 5.3 | 6.3 |
| Other Activities–Yes Responses | Number | % |
|---|---|---|
| Does your Trust have electronic prescribing for inpatients? | 43 | 45.3 |
| Has face to face clinical pharmacy time per patient reduced? | 72 | 75.8 |
| Has your organization published a specific antibiotic guideline for COVID-19? | 62 | 65.3 |
| Have you collected data on antibiotic use in COVID-19 patients since March 2020? | 45 | 47.4 |
| Is there formal recommendation/guidance/communication to stop antibiotics if patient is COVID + ve and no evidence of bacterial infection? | 69 | 72.6 |
| Have you collected data on bacterial co-infections since March 2020? | 22 | 23.2 |
| Method of Communication within Organizations | Number | % |
|---|---|---|
| Intranet | 54 | 56.8 |
| Antibiotic App | 50 | 52.6 |
| Virtual meetings/teleconference | 34 | 35.8 |
| No specific cascade of messages on antibiotic use | 16 | 16.8 |
| Emails to staff | 13 | 13.7 |
| Grand rounds | 13 | 13.7 |
| Specific guidelines | 10 | 10.5 |
| Online learning, e.g., internal webinars | 7 | 7.4 |
| New Responsibilities during COVID-19 Response | Number | % |
|---|---|---|
| Secondment to other clinical specialties at any point for more than 0.5WTE of usual AMS activities time | 54 | 56.8 |
| Secondment to ICU | 42 | 44.2 |
| Secondment to general medicine | 44 | 46.3 |
| Secondment to technical services | 6 | 6.3 |
| Secondment to other roles within pharmacy | 29 | 30.5 |
| Secondment to other roles outside pharmacy | 5 | 5.3 |
| Additional Organization-Wide (External to Pharmacy) Roles AMS Pharmacy Teams Were Involved in as Part of the COVID-19 Response | Number | % |
|---|---|---|
| Communications | 67 | 70.5 |
| Development of treatment guidelines linked to COVID | 16 | 16.8 |
| Development of other guidelines | 48 | 50.5 |
| Managing drug shortages (excluding antimicrobials) | 29 | 30.5 |
| Managing antimicrobial drug shortages | 77 | 81.1 |
| Monitor compliance with antimicrobial treatment guidelines | 54 | 56.8 |
| Management of patient’s own drugs for COVID-19 patients | 53 | 55.8 |
| Providing infection prevention and control advice | 57 | 60.0 |
| Providing personal protective equipment (PPE) advice | 33 | 34.7 |
| Others (wider pharmacy management responsibilities) | 5 | 5.3 |
| Source of Significant Proportion of Learning/Training on COVID-19 | Number | % |
|---|---|---|
| I learned on my own time | 53 | 55.8 |
| I have learnt on the job | 35 | 36.8 |
| I have not been able to dedicate time to learn about COVID-19 specifically | 5 | 5.3 |
| I received formal training which my hospital mandated | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashiru-Oredope, D.; Kerr, F.; Hughes, S.; Urch, J.; Lanzman, M.; Yau, T.; Cockburn, A.; Patel, R.; Sheikh, A.; Gormley, C.; et al. Assessing the Impact of COVID-19 on Antimicrobial Stewardship Activities/Programs in the United Kingdom. Antibiotics 2021, 10, 110. https://doi.org/10.3390/antibiotics10020110
Ashiru-Oredope D, Kerr F, Hughes S, Urch J, Lanzman M, Yau T, Cockburn A, Patel R, Sheikh A, Gormley C, et al. Assessing the Impact of COVID-19 on Antimicrobial Stewardship Activities/Programs in the United Kingdom. Antibiotics. 2021; 10(2):110. https://doi.org/10.3390/antibiotics10020110
Chicago/Turabian StyleAshiru-Oredope, Diane, Frances Kerr, Stephen Hughes, Jonathan Urch, Marisa Lanzman, Ting Yau, Alison Cockburn, Rakhee Patel, Adel Sheikh, Cairine Gormley, and et al. 2021. "Assessing the Impact of COVID-19 on Antimicrobial Stewardship Activities/Programs in the United Kingdom" Antibiotics 10, no. 2: 110. https://doi.org/10.3390/antibiotics10020110
APA StyleAshiru-Oredope, D., Kerr, F., Hughes, S., Urch, J., Lanzman, M., Yau, T., Cockburn, A., Patel, R., Sheikh, A., Gormley, C., Chavda, A., Vaghela, T., Phillips, C., Reid, N., & Brady, A. (2021). Assessing the Impact of COVID-19 on Antimicrobial Stewardship Activities/Programs in the United Kingdom. Antibiotics, 10(2), 110. https://doi.org/10.3390/antibiotics10020110







