Farnesane-Type Sesquiterpenoids with Antibiotic Activity from Chiliadenus lopadusanus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Identification of Bio-Active Metabolites
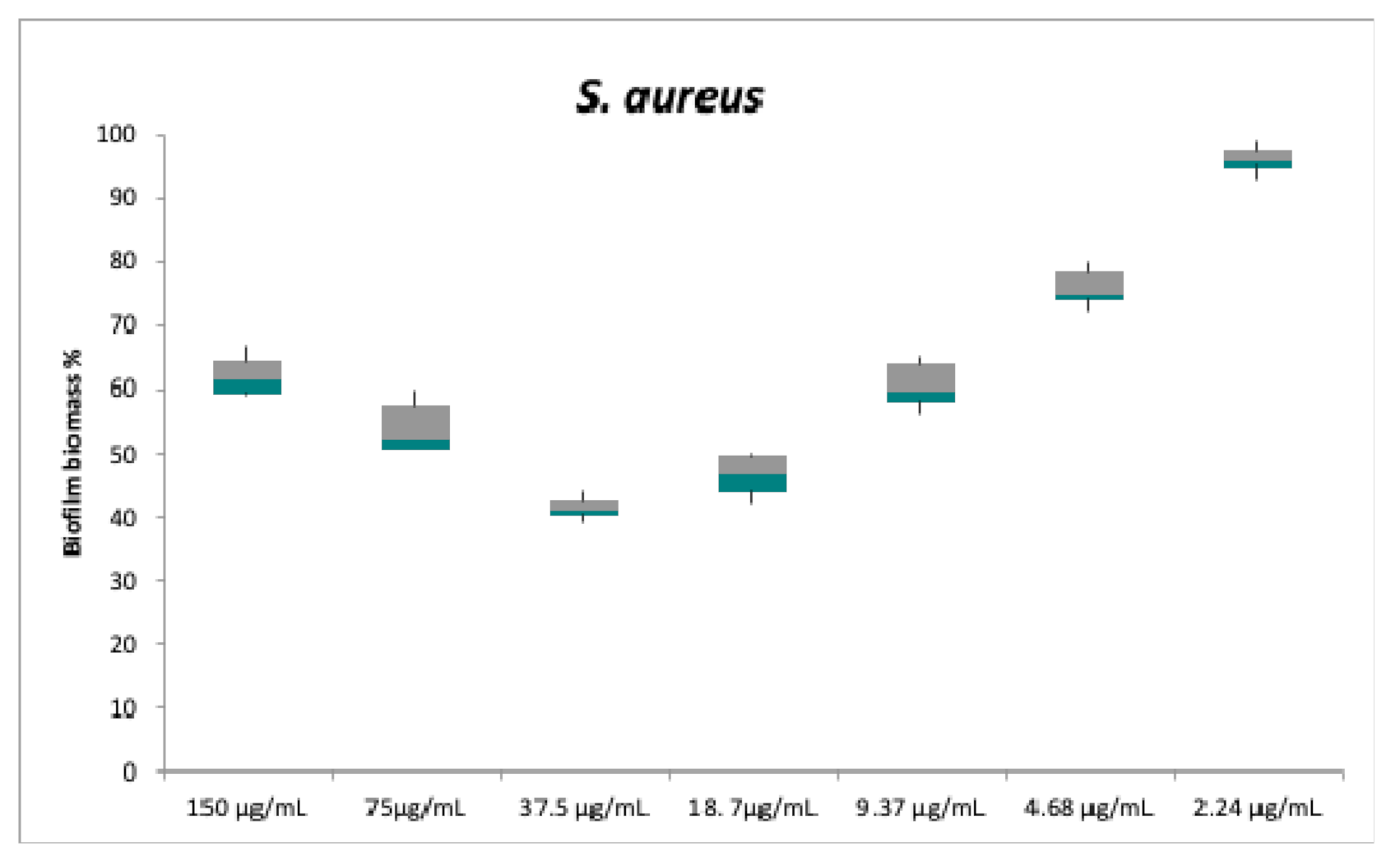
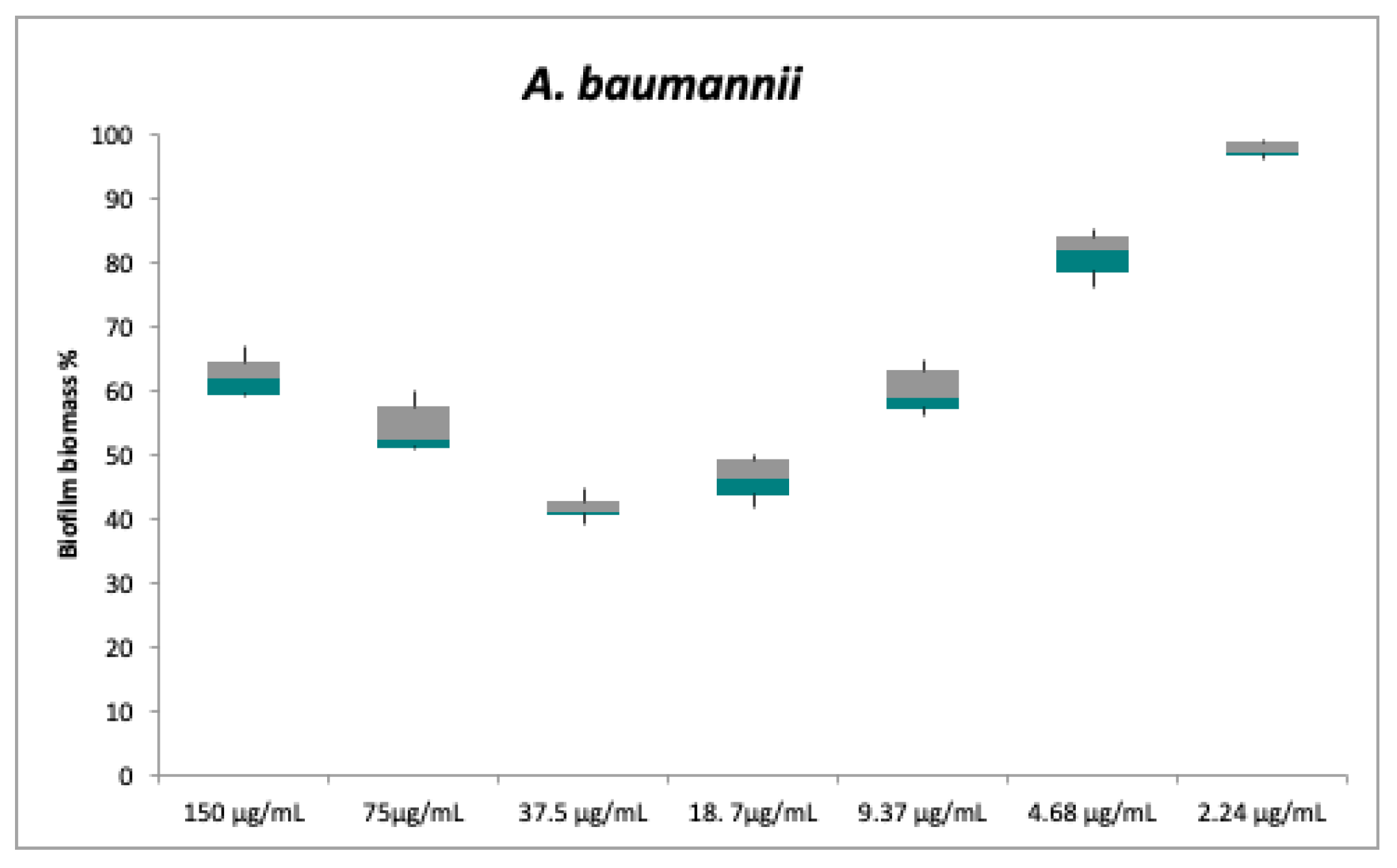
2.2. Antibacterial and Anti-Biofilm Activities
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Isolation of Fungal Metabolites
3.3.1. 9-Hydroxynerolidol (1)
3.3.2. 9-Oxoxynerolidol (2)
3.3.3. Chiliadenol B (3)
3.4. Test Bacterial Strains and Culture Conditions
3.5. Antimicrobial Assays
3.6. Biofilm Formation Inhibition Assay
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO); Antimicrobial Resistance. No Time to Wait: Securing the Future from Drug-Resistant Infections. Report to the Secretary-General of the United Nations. Available online: https://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG_final_report_EN.pdf?ua=1 (accessed on 8 April 2020).
- Da Costa, P.M.; Loureiro, L.; Matos, A.J. Transfer of multidrug-resistant bacteria between intermingled ecological niches: The interface between humans, animals and the environment. Int. J. Environ. Res. Public Health 2013, 1, 278–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argudín, M.A.; Deplano, A.; Meghraoui, A.; Dodémont, M.; Heinrichs, A.; Denis, O.; Nonhoff, C.; Roisin, S. Bacteria from animals as a pool of antimicrobial resistance genes. Antibiotics 2017, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority, European Centre for Disease Prevention and Control. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017. EFSA J. 2019, 17, 5598. [Google Scholar]
- Hoelzer, K.; Wong, N.; Thomas, J.; Talkington, K.; Jungman, E.; Coukell, A. Antimicrobial drug use in food-producing animals and associated human health risks: What, and how strong, is the evidence? BMC Vet. Res. 2017, 13, 211. [Google Scholar] [CrossRef]
- Marturano, J.E.; Lowery, T.J. ESKAPE pathogens in bloodstream infections are associated with higher cost and mortality but can be predicted using diagnoses upon admission. Open Forum Infect. Dis. 2019, 6, ofz503. [Google Scholar] [CrossRef]
- Giraldi, G.; Montesano, M.; Napoli, C.; Frati, P.; La Russa, R.; Santurro, A.; Scopetti, M.; Orsi, G.B. Healthcare-associated infections due to multidrug-resistant organisms: A surveillance study on extra hospital stay and direct costs. Curr. Pharm. Biotechnol. 2019, 20, 643–652. [Google Scholar] [CrossRef]
- Yelin, I.; Kishony, R. Antibiotic resistance. Cell 2018, 172, 1136. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The Who Priority List of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Nielsen, T.B.; Brass, E.P.; Gilbert, D.N.; Bartlett, J.G.; Spellberg, B. Sustainable discovery and development of antibiotics—is a nonprofit approach the future? N. Engl. J. Med. 2019, 381, 503–505. [Google Scholar]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Dg, B.; Lister, T.; May-Dracka, T.L. New natural products as new leads for antibacterial drug discovery. Bioorganic Med. Chem. Lett. 2014, 24, 413–418. [Google Scholar]
- Betts, J.W.; Hornsey, M.; La Ragione, R.M. Novel antibacterials: Alternatives to traditional antibiotics. Adv. Microb. Physiol. 2018, 73, 123–169. [Google Scholar] [PubMed]
- Roscetto, E.; Masi, M.; Esposito, M.; Di Lecce, R.; Delicato, A.; Maddau, L.; Calabrò, V.; Evidente, A.; Catania, M.R. Anti-Biofilm activity of the fungal phytotoxin sphaeropsidin A against clinical isolates of antibiotic-resistant bacteria. Toxins 2020, 12, 444. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products, 3rd ed.; John Wiley and Sons Ltd.: Chicester, UK, 2009. [Google Scholar]
- Osbourn, A.E.; Lanzotti, V. Plant-Derived Natural Products; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Roberto, L.; Catania, M.R.; Chiaviello, A.; De Natale, A.; Roscetto, E.; Pinto, G.; Pollio, A.; Ingenito, A.; Palumbo, G. Screening and scoring of antimicrobial and biological activities of italian vulnerary plants against major oral pathogenic bacteria. Evid. Based Complement. Alternat. Med. 2013, 316280. [Google Scholar] [CrossRef] [Green Version]
- Pollio, A.; Zarrelli, A.; Romanucci, V.; Di Mauro, A.; Barra, F.; Pinto, G.; Crescenzi, E.; Roscetto, M.; Palumbo, G. Polyphenolic profile and targeted bioactivity of methanolic extracts from Mediterranean ethnomedicinal plants on human cancer cell lines. Molecules 2016, 21, 395. [Google Scholar] [CrossRef] [Green Version]
- Romulo, A.; Ea, Z.; Rondevaldova, J.; Kokoska, L. Screening of in vitro antimicrobial activity of plants used in traditional Indonesian medicine. Pharm. Biol. 2018, 56, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, I.C.D.O.; Mariano, E.G.A.; Careli, R.T.; Morais-Costa, F.; De Sant’Anna, F.M.; Pinto, M.S.; De Souza, M.R.; Duarte, E.R. Plants of the Cerrado with antimicrobial effects against Staphylococcus spp. and Escherichia coli from cattle. BMC Vet. Res. 2018, 14, 32. [Google Scholar]
- Oo, O.; Pa, S.; Ps, F. Antimicrobial and antiprotozoal activities of twenty-four Nigerian medicinal plant extracts. S. Afr. J. Bot. 2018, 117, 240–246. [Google Scholar]
- Ferrazzano, G.F.; Cantile, T.; Roberto, L.; Ingenito, A.; Catania, M.R.; Roscetto, E.; Palumbo, G.; Zarrelli, A.; Pollio, A. Determination of the in vitro and in vivo antimicrobial activity on salivary Streptococci and Lactobacilli and chemical characterisation of the phenolic content of a Plantago lanceolata infusion. BioMed Res. Int. 2015, 2015, 286817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocquet, L.; Sahpaz, S.; Bonneau, N.; Beaufay, C.; Mahieux, S.; Samaillie, J.; Roumy, V.; Jacquin, J.; Bordage, S.; Hennebelle, T.; et al. Phenolic compounds from Humulus lupulus as natural antimicrobial products: New weapons in the fight against methicillin resistant Staphylococcus aureus, Leishmania mexicana and Trypanosoma brucei strains. Molecules 2019, 24, 1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naz, R.; Roberts, T.H.; Bano, A.; Nosheen, A.; Yasmin, H.; Hassan, M.N.; Keyani, R.; Ullah, S.; Khan, W.; Anwar, Z. GC-MS analysis, antimicrobial, antioxidant, antilipoxygenase and cytotoxic activities of Jacaranda mimosifolia methanol leaf extracts and fractions. PLoS ONE 2020, 15, e0236319. [Google Scholar] [CrossRef] [PubMed]
- Zito, P.; Sajeva, M.; Scirica, E.; Bruno, M.; Maggio, A.; Senatore, F. Essential oils of Chiliadenus lopadusanus (Asteraceae). Nat. Prod. Commun. 2013, 8, 1159–1162. [Google Scholar] [CrossRef] [Green Version]
- Sacco, T.; Maffei, M. Essential oil of Chiliadenus lopadusanus growing spontaneously in Lampedusa Island (Italy). Planta Med. 1987, 53, 582. [Google Scholar] [CrossRef]
- Hammerschmidt, F.J.; Clark, A.M.; Soliman, F.M.; El-Kashoury, E.S.A.; El-Kawy, M.M.A.; El-Fishawy, A.M. Chemical composition and antimicrobial activity of essential oils of Jasonia candicans and Jasonia montana. Planta Med. 1993, 59, 68–70. [Google Scholar] [CrossRef]
- Satyal, P.; Paudel, P.; Kafle, A.; Pokharel, S.K.; Lamichhane, B.; Dosoky, N.S.; Moriarity, D.M.; Setzer, W.N. Bioactivities of volatile components from Nepalese Artemisia species. Nat. Prod. Commun. 2012, 7, 1651–1658. [Google Scholar] [CrossRef] [Green Version]
- Guillén, M.D.; Ibargoitia, M.L. Volatile components obtained from the leaves of Jasonia glutinosa. Food Chem. 1996, 56, 155–158. [Google Scholar] [CrossRef]
- Romero, V.; Costas-Mora, I.; Lavilla, I.; Bendicho, C. Cold vapor-solid phase microextraction using amalgamation in different Pd-based substrates combined with direct thermal desorption in a modified absorption cell for the determination of Hg by atomic absorption spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2011, 66, 156–162. [Google Scholar] [CrossRef]
- Avato, P.; Raffo, F.; Aldouri, N.A.; Vartanian, S.T. Essential oils of Varthemia iphionoides from Jordan. Flavour Fragr. J. 2004, 19, 559–561. [Google Scholar] [CrossRef]
- Hegazy, M.E.F.; Matsusa, H.; Nakamura, S.; Hussein, T.A.; Yoshikawa, M.; Parè, P.W. Chemical constituents and their antibacterial and antifungal activity from the Egyptian herbal medicine Chiliadenus montanus. Phytochemistry 2014, 103, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Stoessl, A.; Stothers, J.B.; Ward, E.W.B. The structures of some stress metabolites from Solanum melongena. Can. J. Chem. 1975, 53, 3351–3358. [Google Scholar] [CrossRef]
- Hiroi, M.; Takaoka, D. 9-oxonerolidol from camphor leaf oil. Chem. Lett. 1972, 1, 1213–1214. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar]
- Aleksic Sabo, V.; Nikolic, I.; Mimica-Dukic, N.; Knezevic, P. Anti-Acinetobacter baumannii activity of selected phytochemicals alone, in binary combinations and in combinations with conventional antibiotics. Nat. Prod. Res. 2020, 1–4. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef]
- Gomes, F.I.A.; Teixeira, P.; Azeredo, J.; Oliveira, R. Effect of farnesol on planktonic and biofilm cells of Staphylococcus epidermidis. Curr. Microbiol. 2009, 59, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.B.; Yu, X.Q.; Wang, B.; Liu, A.H.; Zhao, T.S.; Guo, Y.W.; Huang, H.L.; Mao, S.C. Structurally diverse halosesquiterpenoids from the red alga Laurencia composita Yamada. Fitoterapia 2020, 146, 104716. [Google Scholar] [CrossRef]
- Evidente, A.; Kornienko, A.; Cimmino, A.; Andolfi, A.; Lefranc, F.; Mathieu, V.; Kiss, R. Fungal metabolites with anticancer activity. Nat. Prod. Rep. 2014, 31, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Nocera, P.; Reveglia, P.; Cimmino, A.; Evidente, A. Fungal metabolites antagonists towards plant pests and human pathogens: Structure-activity relationship studies. Molecules 2018, 23, 834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.R.; Fu, C.S.; Yang, W.J.; Wang, X.L.; Feng, D.; Wang, X.N.; Ren, D.-M.; Lou, H.-X.; Shen, T. Investigation of constituents from Cinnamomum camphora (L.) J. Presl and evaluation of their anti-inflammatory properties in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Ethnopharmacol. 2018, 221, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Tian, X. Quorum sensing and bacterial social interactions in biofilms. Sensors 2012, 12, 2519–2538. [Google Scholar] [CrossRef]
- Rukayadi, Y.; Hwang, J.K. In vitro activity of xanthorrhizol against Streptococcus mutans biofilms. Lett. Appl. Microbiol. 2006, 42, 400–404. [Google Scholar] [CrossRef]
- Alves, S.; Duarte, A.; Sousa, S.; Domingues, F.C. Study of the major essential oil compounds of Coriandrum sativum against Acinetobacter baumannii and the effect of linalool on adhesion, biofilms and quorum sensing. Biofouling 2016, 32, 155–165. [Google Scholar] [CrossRef]
- Leyva Del Rio, D.; Sartori, N.; Tomblin, N.B.; Phark, J.H.; Pardi, V.; Murata, R.M.; Duarte, S., Jr. Bioactive dental adhesive system with tt-farnesol: Effects on dental biofilm and bonding properties. Front. Bioeng. Biotechnol. 2020, 8, 865. [Google Scholar] [CrossRef]
- Pammi, M.; Liang, R.; Hicks, J.M.; Barrish, J.; Versalovic, J. Farnesol decreases biofilms of Staphylococcus epidermidis and exhibits synergy with nafcillin and vancomycin. Pediatr. Res. 2011, 70, 578–583. [Google Scholar] [CrossRef] [Green Version]
- Berger, S.; Braun, S. 200 and More Basic NMR Experiments—A Practical Course, 1st ed.; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Cirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by Staphylococci. APMIS 2007, 115, 891–899. [Google Scholar]




| Strains | Plant Extract (500 µg/mL) | Compound 1 MIC | Compound 2 MIC | Compound 3 MIC | CO MIC | TE MIC |
|---|---|---|---|---|---|---|
| S. aureus | 100% inhibition | 75 ± 1.8 | 150 ± 3.8 | nd | - | 1 ± 0.1 |
| A. baumannii | 100% inhibition | 150 ± 4.2 | 150 ± 3.2 | nd | 0.78 ± 0.0 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masi, M.; Roscetto, E.; Cimmino, A.; Catania, M.R.; Surico, G.; Evidente, A. Farnesane-Type Sesquiterpenoids with Antibiotic Activity from Chiliadenus lopadusanus. Antibiotics 2021, 10, 148. https://doi.org/10.3390/antibiotics10020148
Masi M, Roscetto E, Cimmino A, Catania MR, Surico G, Evidente A. Farnesane-Type Sesquiterpenoids with Antibiotic Activity from Chiliadenus lopadusanus. Antibiotics. 2021; 10(2):148. https://doi.org/10.3390/antibiotics10020148
Chicago/Turabian StyleMasi, Marco, Emanuela Roscetto, Alessio Cimmino, Maria Rosaria Catania, Giuseppe Surico, and Antonio Evidente. 2021. "Farnesane-Type Sesquiterpenoids with Antibiotic Activity from Chiliadenus lopadusanus" Antibiotics 10, no. 2: 148. https://doi.org/10.3390/antibiotics10020148
APA StyleMasi, M., Roscetto, E., Cimmino, A., Catania, M. R., Surico, G., & Evidente, A. (2021). Farnesane-Type Sesquiterpenoids with Antibiotic Activity from Chiliadenus lopadusanus. Antibiotics, 10(2), 148. https://doi.org/10.3390/antibiotics10020148










