Ceftobiprole Perspective: Current and Potential Future Indications
Abstract
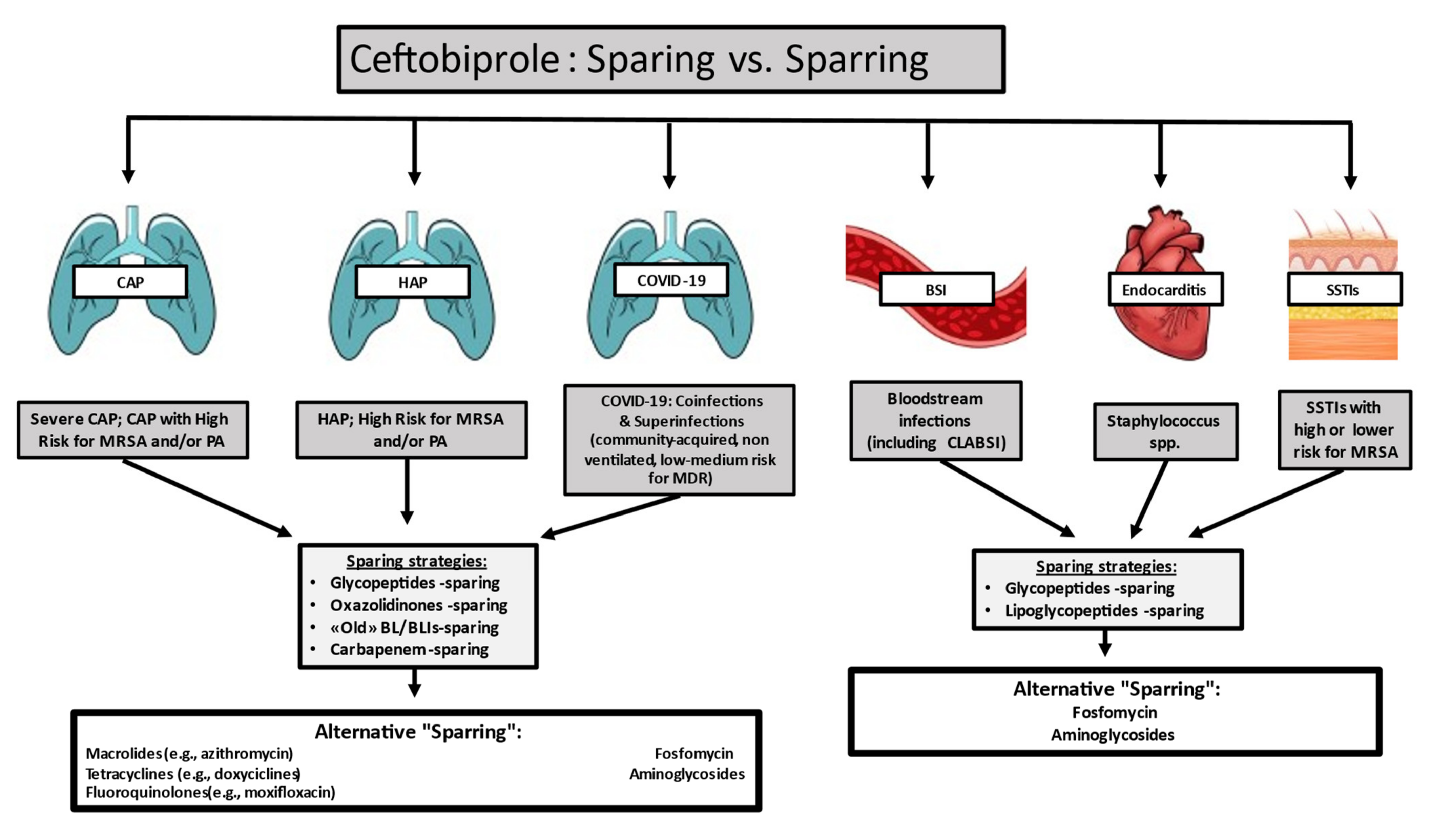
:1. Introduction
1.1. Ceftobiprole and Pneumonia
1.2. Ceftobiprole and Skin and Soft Tissue Infections
1.3. Ceftobiprole and Bacteremia
1.4. Ceftobiprole and Infective Endocarditis
2. Discussion
3. Materials and Methods
Author Contributions
Funding
Conflicts of Interest
References
- Nicholson, S.C.; Welte, T.; File, T.M., Jr.; Strauss, R.S.; Michiels, B.; Kaul, P.; Balis, D.; Arbit, D.; Amsler, K.; Noel, G.J. A randomised, double-blind trial comparing ceftobiprole medocaril with ceftriaxone with or without linezolid for the treatment of patients with community-acquired pneumonia requiring hospitalisation. Int. J. Antimicrob. Agents 2012, 39, 240–246. [Google Scholar] [CrossRef]
- Awad, S.S.; Rodriguez, A.H.; Chuang, Y.C.; Marjanek, Z.; Pareigis, A.J.; Reis, G.; Scheeren, T.W.L.; Sánchez, A.S.; Zhou, X.; Saulay, M.; et al. A phase 3 randomized double-blind comparison of ceftobiprole medocaril vs. ceftazidime plus linezolid for the treatment of hospital-acquired pneumonia. Clin. Infect. Dis. 2014, 59, 51–61. [Google Scholar] [CrossRef]
- Noel, G.J.; Bush, K.; Bagchi, P.; Ianus, J.; Strauss, R.S. A randomized, doubleblind trial comparing ceftobiprole medocaril with vancomycin plus ceftazidime for the treatment of patients with complicated skin and skin-structure infections. Clin. Infect. Dis. 2008, 46, 647–655. [Google Scholar] [CrossRef] [Green Version]
- Noel, G.J.; Strauss, R.S.; Amsler, K.; Heep, M.; Pypstra, R.; Solomkin, J.S. Results of a double-blind, randomized trial of ceftobiprole treatment of complicated skin and skin structure infections caused by gram-positive bacteria. Antimicrob. Agents Chemother. 2008, 52, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Giacobbe, D.R.; De Rosa, F.G.; Del Bono, V.; Grossi, P.A.; Pea, F.; Petrosillo, N.; Rossolini, G.M.; Tascini, C.; Tumbarello, M.; Viale, P.; et al. Ceftobiprole: Drug evaluation and place in therapy. Expert Rev. Anti. Infect. Ther. 2019, 17, 689–698. [Google Scholar] [CrossRef] [Green Version]
- Zhanel, G.G.; Lam, A.; Schweizer, F.; Thomson, K.; Walkty, A.; Rubinstein, E.; Gin, A.S.; Hoban, D.J.; Noreddin, A.M.; Karlowsky, J.A. Ceftobiprole: A review of a broad-spectrum and anti-MRSA cephalosporin. Am. J. Clin. Dermatol. 2008, 9, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.N.; Cheung, C.M.; Rybak, M.J. Activities of ceftobiprole, linezolid, vancomycin, and daptomycin against com-munity-associated and hospital-associated methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2008, 52, 2974–2976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, T.A.; Shang, W.; Amsler, K.M.; Bajaksouzian, S.; Jacobs, M.R.; Bush, K. Molecular characterisation of meticillin-resistant Staphylococcus aureus isolates from two ceftobiprole Phase 3 complicated skin and skin-structure infection clinical trials. Int. J. Antimicrob. Agents. 2009, 34, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.E.; Deshpande, L.M.; Costello, A.J.; Farrell, D.J.; Jones, R.N.; Flamm, R.K. Genotypic characterization of methicillin-resistant Staphylococcus aureus recovered at baseline from phase 3 pneumonia clinical trials for ceftobiprole. Microb. Drug Resist. 2016, 22, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Cillóniz, C.; Dominedò, C.; Garcia-Vidal, C.; Torres, A. Ceftobiprole for the treatment of pneumonia. Rev. Esp. Quimioter. 2019, 32 (Suppl. 3), 17–23. [Google Scholar]
- Liapikou, A.; Cilloniz, C.; Torres, A. Ceftobiprole for the treatment of pneumonia: A European perspective. Drug Des. Dev. Ther. 2015, 9, 4565–4572. [Google Scholar] [CrossRef] [Green Version]
- Scheeren, T.W.L.; Welte, T.; Saulay, M.; Engelhardt, M.; Santerre-Henriksen, A.; Hamed, K. Early improvement in severely ill patients with pneumonia treated with ceftobiprole: A retrospective analysis of two major trials. BMC Infect. Dis. 2019, 19, 195. [Google Scholar] [CrossRef] [Green Version]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of adults with hospitalacquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American thoracic society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Bassi, G.L.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European respiratory society (ERS), European society of intensive care medicine (ESICM), European society of clinical microbiology and infectious diseases (ESCMID) and asociación Latinoamericana del tórax (ALAT). Eur. Respir. J. 2017, 50, 1700582. [Google Scholar]
- Corcione, S.; Lupia, T.; Maraolo, A.E.; Mornese, P.S.; Gentile, I.; De Rosa, F.G. Carbapenem sparing strategy: Carbapenemase, treatment, and stewardship. Curr. Opin. Infect. Dis. 2019, 32, 663–673. [Google Scholar] [CrossRef]
- Rodríguez-Baño, J.; Gutiérrez-Gutiérrez, B.; Machuca, I.; Pascual, A. Treatment of infections caused by extend-ed-spectrumbeta-lactamase-, AmpC-, and carbapenemase-producing enterobacteriaceae. Clin. Microbiol. Rev. 2018, 31, e00079-17. [Google Scholar] [CrossRef] [Green Version]
- Torres, A.; Mouton, J.W.; Pea, F. Pharmacokinetics and dosing of ceftobiprole medocaril for the treatment of hospital- and community-acquired pneumonia in different patient populations. Clin. Pharmacokinet. 2016, 55, 1507–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodvold, K.A.; Nicolau, D.P.; Lodise, T.P.; Khashab, M.; Noel, G.J.; Kahn, J.B.; Gotfried, M.; Murray, S.A.; Nicholson, S.; Laohavaleeson, S.; et al. Identifying exposure targets for treatment of staphylococcal pneumonia with ceftobiprole. Antimicrob. Agents Chemother. 2009, 53, 3294–3301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Shin, W.I.; Pang, Y.X.; Meng, Y.; Lai, J.; You, C.; Zhao, H.; Lester, E.; Wu, T.; Pang, C.H. The first 75 days of Novel Coronavirus (SARS-CoV-2) outbreak: Recent advances, prevention, and treatment. Int. J. Environ. Res. Publ. Health 2020, 17, 2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- COVID-19 Map—Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html (accessed on 18 January 2021).
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and fungal co-infection in in-dividuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ge, Y.; Wu, T.; Zhao, K.; Chen, Y.; Wu, B.; Zhu, F.; Zhu, B.; Cui, L. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020, 285, 198005. [Google Scholar] [CrossRef] [PubMed]
- Contou, D.; Claudinon, A.; Pajot, O.; Micaëlo, M.; Longuet Flandre, P.; Dubert, M.; Cally, R.; Logre, E.; Fraissé, M.; Mentec, H.; et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann. Inten. Care. 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. COVID-19 Researchers Group. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Kolenda, C.; Ranc, A.G.; Boisset, S.; Caspar, Y.; Carricajo, A.; Souche, A.; Dauwalder, O.; Verhoeven, P.O.; Vandenesch, F.; Laurent, F.; et al. Assessment of respiratory bacterial coinfections among severe acute respiratory syndrome Coronavirus 2-positive patients hospitalized in intensive care units using conven-tional culture and biofire, filmarray pneumonia panel plus assay. Open Forum Infect. Dis. 2020, 22, 7. [Google Scholar] [CrossRef]
- Adler, H.; Ball, R.; Fisher, M.; Mortimer, K.; Vardhan, M.S. Low rate of bacterial co-infection in patients with COVID-19. Lancet Microbe. 2020, 1, e62. [Google Scholar] [CrossRef]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. The official publication of the European society of clinical microbiology and infectious diseases. Clin. Microbiol. Infect. 2020, 26, 1395–1399. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Hang, Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Lupia, T.; Corcione, S.; Mornese Pinna, S.; De Rosa, F.G. New cephalosporins for the treatment of pneumonia in internal medicine wards. J. Thorac. Dis. 2020, 12, 3747–3763. [Google Scholar] [CrossRef]
- Overcash, J.S.; Kim, C.; Keech, R.; Gumenchuk, I.; Ninov, B.; Gonzalez-Rojas, Y.; Waters, M.; Simeonov, S.; Engelhardt, M.; Saulay, M. Ceftobiprole compared with vancomycin plus aztreonam in the treatment of acute bacterial skin and skin structure infections: Results of a phase 3, randomized, double-blind trial (TARGET). An official publication of the infectious diseases society of America. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridki, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Infectious diseases society of America. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus in-fections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55, Erratum in Clin. Infect. Dis. 2011, 53, 319. Epub 4 January 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Wilson, B.; Gould, I.M. Current and future treatment options for community-associated MRSA infection. Expert Opin. Pharmacother. 2018, 19, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.R. Methicillin-resistant Staphylococcus aureus infections. Prim. Care 2013, 40, 637–654. [Google Scholar] [CrossRef] [PubMed]
- Culos, K.A.; Cannon, J.P.; Grim, S.A. Alternative agents to vancomycin for the treatment of methicillin-resistant S. aureus infections. Am. J. Ther. 2013, 20, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M.; Andrasevic, A.T.; Bassetti, M.; Bouza, E.; Chastre, J.; Cornaglia, G.; Esposito, S.; French, G.; Giamarellou, H.; Gyssens, I.C.; et al. European survey of antibiotic management of methicillin-resistant Staphylococcus aureus infection: Current clinical opinion and practice. Clin. Microbiol. Infect. 2010, 16 (Suppl. 1), 3–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moise, P.A.; Culshaw, D.L.; Wong-Beringer, A.; Bensman, J.; Lamp, K.C.; Smith, W.J.; Bauer, K.; Goff, D.A.; Adamson, R.; Leuthner, K.; et al. Comparative effectiveness of vancomycin versus daptomycin for MRSA bacteremia with vancomycin MIC >1 mg/L: A multicenter evaluation. Clin. Ther. 2016, 38, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Mootz, M.L.; Britt, R.S.; Mootz, A.A.; Lee, G.C.; Reveles, K.R.; Evoy, K.E.; Teng, C.; Frei, C.R. Comparative-effectiveness of ceftaroline and daptomycin as first-line MRSA therapy for patients with sepsis admitted to hospitals in the United States veterans health care system. Hosp. Pract. 2019, 47, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Rosanova, M.T.; Aguilar, P.S.; Sberna, N.; Lede, R. Efficacy and safety of ceftaroline: Systematic review and meta-analysis. Ther. Adv. Infect. Dis. 2018, 6, 2049936118808655. [Google Scholar] [CrossRef] [PubMed]
- McDanel, J.S.; Perencevich, E.N.; Diekema, D.J.; Herwaldt, L.A.; Smith, T.C.; Chrischilles, E.A.; Dawson, J.D.; Jiang, L.; Goto, M.; Schweizer, M.L. Comparative effectiveness of be-ta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin. Infect. Dis. 2015, 61, 361–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweizer, M.L.; Furuno, J.P.; Harris, A.D.; Johnson, J.K.; Shardell, M.D.; McGregor, J.C.; Thom, K.A.; Cosgrove, S.E.; Sakoulas, G.; Perencevich, E.N. Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin-susceptible Staphylococcus aureus bacteremia. BMC Infect. Dis. 2011, 11, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, D.; Wong, T.; Romney, M.; Leung, V. Comparative effectiveness of β-lactam versus vancomycin empiric therapy in patients with methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flamm, R.K.; Duncan, L.R.; Shortridge, D.; Smart, J.I.; Hamed, K.; Mendes, R.E.; Sader, H.S. Ceftobiprole activity when tested against contemporary bacteria causing bloodstream infections in the US (2016). Open Forum Infect. Dis. 2017, 4 (Suppl. 1), S368. [Google Scholar] [CrossRef] [Green Version]
- Pfaller, M.A.; Mendes, R.E.; Sader, H.S.; Streit, J.; Smart, J.I.; Hamed, K.A.; Duncan, L.R.; Flamm, R.K. Antimicrobial activity of ceftobiprole when tested against Gram-positive cocci causing serious infections (2016–2017): Endocarditis, diabetic foot, and bone/joint infections. In Proceedings of the ASM Microbe, Atlanta, GA, USA, 7–11 June 2018. [Google Scholar]
- Pfaller, M.A.; Flamm, R.K.; Duncan, L.R.; Streit, J.M.; Castanheira, M.; Sader, H.S. Antimicrobial activity of ceftobiprole and comparator agents when tested against contemporary Gram-positive and -negative organisms collected from Europe (2015). Diagn. Microbiol. Infect. Dis. 2018, 91, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.; Morata, L. Ceftobripole: Experience in staphylococcal bacteremia. Rev EspQuimioter. 2019, 32 (Suppl. 3), 24–28. [Google Scholar]
- Rello, J.; Rahav Scheeren, T.; Saulay, M.; Engelhardt, M.; Welte, T. Pooled analysis of clinical cure and mortality with ceftobiprole medocaril versus comparators in staphylococcal bacteremia in complicated skin infections, community- and hospital-acquired pneumonia. ECCMID 2016, O-318. [Google Scholar]
- Durante-Mangoni, E.; Andini, R.; Mazza, M.C.; Sangiovanni, F.; Bertolino, L.; Ursi, M.P.; Paradiso, L.; Karruli, A.; Esposito, C.; Murino, P.; et al. Real life experience with ceftobiprole in a tertiary-care hospital. J. Glob. Antimicrob. Resist. 2020, 22, 386–390. [Google Scholar] [CrossRef]
- Hamed, K.; Engelhardt, M.; Jones, M.E.; Saulay, M.; Holland, T.L.; Seifert, H.; Fowler, V.C., Jr. Ceftobiprole versus daptomycin in Staphylococcus aureus bacteremia: A novel protocol for a double-blind, Phase III trial. Future Microbiol. 2020, 15, 35–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habib, G.; Erba, P.A.; Lung, B.; Donal, E.; Cosyns, B.; Laroche, C.; Popescu, B.A.; Prendergast, B.; Tornos, P.; Sadeghpour, A.; et al. EURO-ENDO Investigators. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: A pro-spective cohort study. Eur. Heart J. 2019, 40, 3222–3232. [Google Scholar] [CrossRef] [Green Version]
- Pallotto, C.; Martinelli, L.; Baldelli, F.; Bucaneve, G.; Cecchini, E.; Malincarne, L.; Pasticci, M.B. Trends in infective endocarditis in a medi-um-sized University Hospital in Italy: Analysis of 232 cases. Infez. Med. 2014, 22, 124–131. [Google Scholar] [PubMed]
- Falcone, M.; Tiseo, G.; Durante-Mangoni, E.; Ravasio, V.; Barbaro, F.; Ursi, M.P.; Pasticci, M.B.; Bassetti, M.; Grossi, P.; Venditti, M.; et al. Risk factors and outcomes of endocarditis due to non-HACEK Gram-negative bacilli: Data from the prospective multicenter Italian endocarditis study cohort. Antimicrob. Agents Chemother. 2018, 62, e02208-7. [Google Scholar] [CrossRef] [Green Version]
- Tattevin, P.; Basuino, L.; Bauer, D.; Diep, B.A.; Chambers, H.F. Ceftobiprole is superior to vancomycin, daptomycin and linezolid for treatment of experimental endocarditis in rabbits caused by methicillin-resistant S. aureus. Antimicrob. Agents Chemother. 2010, 54, 610–613. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, J.; Abbanat, D.; Shang, W.; He, W.; Amsler, K.; Hastings, J.; Queenan, A.M.; Melton, J.L.; Barron, A.M.; Flamm, R.K.; et al. Synergistic activity of ceftobiprole and vancomycin in a rat model of infective endocarditis caused by methicillin-resistant and glycopeptide-intermediate S. aureus. Antimicrob. Agents Chemother. 2012, 56, 1476–1484. [Google Scholar] [CrossRef] [Green Version]
- Entenza, J.M.; Veloso, T.R.; Vouillmoz, J.; Giddey, M.; Majcherczyk, P.; Moreillon, P. In vivo synergism of ceftobiprole and vanco-mycin against experimental endocarditis due to vancomycin-intermediate S. aureus. Antimicrob. Agents Chemother. 2011, 55, 3977–3984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, F.; Efthymiou, A.; Van Delden, C.; Erard, V. Ceftobiprole as an ultimate successful therapy for MRSE prosthetic endo-vascular infection judged to be medically untreatable in a profound immunocompromised patient: A case report. In Proceedings of the Joint Annual Meeting 2018, Swiss Societies for Infectious Diseases (SSI), Hospital Hygiene (SSHH), Tropical Medicine and Parasitology (SSTMP) and Tropical and Travel Medicine (SSTTM), Interlaken, Switzerland, 13–14 September 2018. Poster P88. [Google Scholar]
- Rouse, M.S.; Steckelberg, J.M.; Patel, R. In vitro activity of ceftobiprole, daptomycin, linezolid and vancomycin against methi-cillin-resistant staphylococci associated with endocarditis and bone and joint infection. Diagn. Microbiol. Infect. Dis. 2007, 58, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, R.; Rodriguez-Esteban, M.A.; Garia-Carus, E.; Telenti, M.; Fernandez, J. In vitro activity of ceftaroline and ceftobiprole against clinical isolates of Gram-positive bacteria from infective endocarditis: Are these drugs potential options for the initial management of this disease? Diagn. Microbiol. Infect. Dis. 2020, 98, 115153. [Google Scholar] [CrossRef] [PubMed]
- Peiffer-Smadja, N.; Guillotel, E.; Luque-Paz, D.; Maataoui, N.; Lescure, F.X.; Cattoir, V. In vitro bactericidal activity of amoxicillin combined with different cephalosporins against endocarditis-associated Enterococcus faecalis clinical isolates. J. Antimicrob. Chemother. 2019, 74, 3511–3514. [Google Scholar] [CrossRef] [PubMed]
- Entenza, J.M.; Hohl, P.; Heinze-Krauss, I.; Glauser, M.P.; Moreillon, P. BAL9141, a novel extended-spectrum cephalosporin active against methicillin-resistant Staphylococcus aureus in treatment of experimental endocarditis. Antimicrob. Agents Chemother. 2002, 46, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, E.; Al Mansour, S.; Bosaeed, M.; Alharbi, A.; Alsaedy, A.; Aljohani, S.; Alalwan, B.; Alothman, A. Ceftobiprole for treatment of MRSA blood stream infection: A case series. Infect. Drug Resist. 2020, 13, 2667–2672. [Google Scholar] [CrossRef]
- Tascini, C.; Attanasio, V.; Ripa, M.; Carozza, A.; Pallotto, C.; Bernardo, M.; Francisci, D.; Oltolini, C.; Palmiero, G.; Scarpellini, P. Ceftobiprole for the treatment of infecive endocarditis: A case series. J. Glob. Antimicrob. Resist. 2020, 20, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Oltolini, C.; Castiglioni, B.; Tassan Din, C.; Castiglioni, A.; Ossi, C.; La Canna, G.; Pajoro, U.; Scarpellini, P. Methicillin-resistant Staphylococcus aureus endocarditis: First report of daptomycin plus ceftobiprole combination as salvage therapy. Int. J. Antimicrob. Agents. 2016, 47, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Campanile, F.; Bongiorno, D.; Mongelli, G.; Zanghì, G.; Stefani, S. Bactericidal activity of ceftobiprole combined with different antibiotics against selected gram positive isolates. Diagn. Microbiol. Infect. Dis. 2019, 93, 77–81. [Google Scholar] [CrossRef]
- Barber, K.E.; Werth, B.L.; Ireland, C.E.; Stone, N.E.; Nonejuie, P.; Sakoulas, G.; Pogliano, J.; Rybak, M.J. Potent synergy of ceftobiprole plus daptomycin against multiple strains of Staphylococcus aureus with various resistance phenotypes. J. Antimicrob. Chemother. 2014, 69, 3006–3010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stucki, A.; Cottagnoud, M.; Acosta, F.; Egerman, U.; Läuffer, J.; Cottagnoud, P. Evaluation of ceftobiprole activity against a variety of gram-negative pathogens, including Escherichia coli, Haemophilus influenzae (β-lactamase positive and β-lactamase negative), and Klebsiella pneumoniae, in a rabbit meningitis model. Antimicrob. Agents Chemother. 2012, 56, 921. [Google Scholar] [CrossRef] [PubMed]
- Plata-Menchaca, E.P.; Ferrer, R.; Rodríguez, J.C.R.; Morais, R.; Póvoa, P. Antibiotic treatment in patients with sepsis: A narrative review. Hosp. Pract. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Vazquez-Grande, G.; Kumar, A. Optimizing antimicrobial therapy of sepsis and septic shock: Focus on antibiotic combination therapy. Semin. Respir. Crit. Care Med. 2015, 36, 154–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intens. Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef] [PubMed]
- Brunkhorst, F.M.; Oppert, M.; Marx, G.; Bloos, F.; Ludewig, K.; Putensen, C.; Nierhaus, A.; Jaschinski, U.; Meier-Hellmann, A.; Weyland, A.; et al. Effect of empirical treatment with moxifloxacin and meropenem vs meropenem on sepsis-related organ dysfunction in patients with severe sepsis: A randomized trial. Jama 2012, 307, 2390–2399. [Google Scholar] [CrossRef]
- Paul, M.; Silbiger, I.; Grozinsky, S.; Soares-Weiser, K.; Leibovici, L. Beta lactam antibiotic monotherapy versus beta lac-tam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst. Rev. 2014, 7, Cd003344. [Google Scholar]
- Ong, D.S.Y.; Frencken, J.F.; Klein Klouwenberg, P.M.C.; Juffermans, N.; van der Poll, T.; Bonten, M.J.M.; Cremer, O.L.; MARS Consortium. Short-course adjunctive gentamicin as empirical therapy in patients with severe sepsis and septic shock: A prospective observational cohort study. Clin. Infect Dis. 2017, 64, 1731–1736. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupia, T.; Pallotto, C.; Corcione, S.; Boglione, L.; De Rosa, F.G. Ceftobiprole Perspective: Current and Potential Future Indications. Antibiotics 2021, 10, 170. https://doi.org/10.3390/antibiotics10020170
Lupia T, Pallotto C, Corcione S, Boglione L, De Rosa FG. Ceftobiprole Perspective: Current and Potential Future Indications. Antibiotics. 2021; 10(2):170. https://doi.org/10.3390/antibiotics10020170
Chicago/Turabian StyleLupia, Tommaso, Carlo Pallotto, Silvia Corcione, Lucio Boglione, and Francesco Giuseppe De Rosa. 2021. "Ceftobiprole Perspective: Current and Potential Future Indications" Antibiotics 10, no. 2: 170. https://doi.org/10.3390/antibiotics10020170
APA StyleLupia, T., Pallotto, C., Corcione, S., Boglione, L., & De Rosa, F. G. (2021). Ceftobiprole Perspective: Current and Potential Future Indications. Antibiotics, 10(2), 170. https://doi.org/10.3390/antibiotics10020170







