Mechanism of Antifungal Activity by 5-Aminoimidazole-4-Carbohydrazonamide Derivatives against Candida albicans and Candida krusei
Abstract
:1. Introduction
2. Results
2.1. Antifungal Susceptibility to Imidazole Derivatives
2.2. Interference with Ergosterol Synthesis
2.3. Antioxidants’ Influence on Antifungal Activity
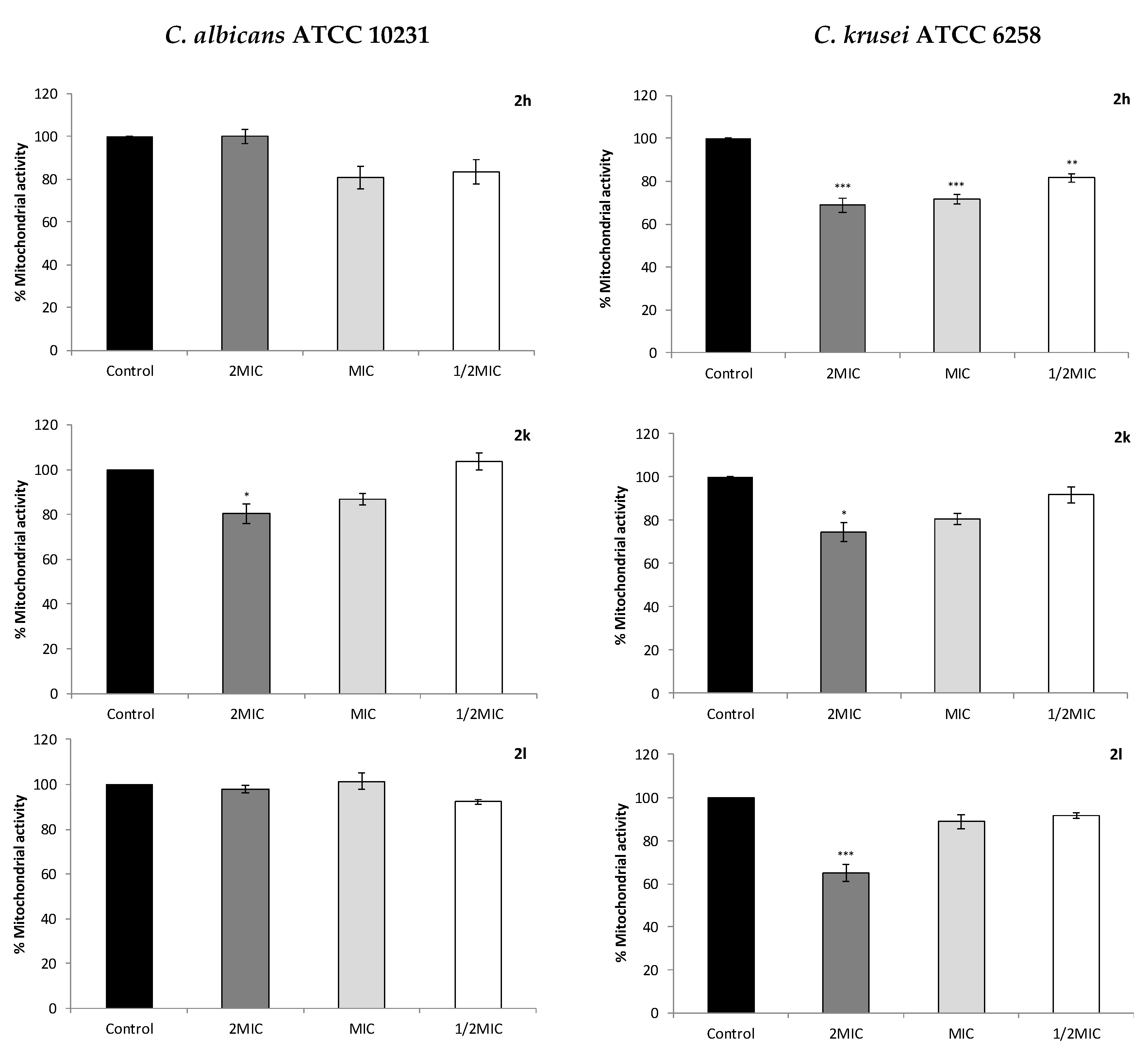
2.4. Effect on Cell Mitochondrial Function
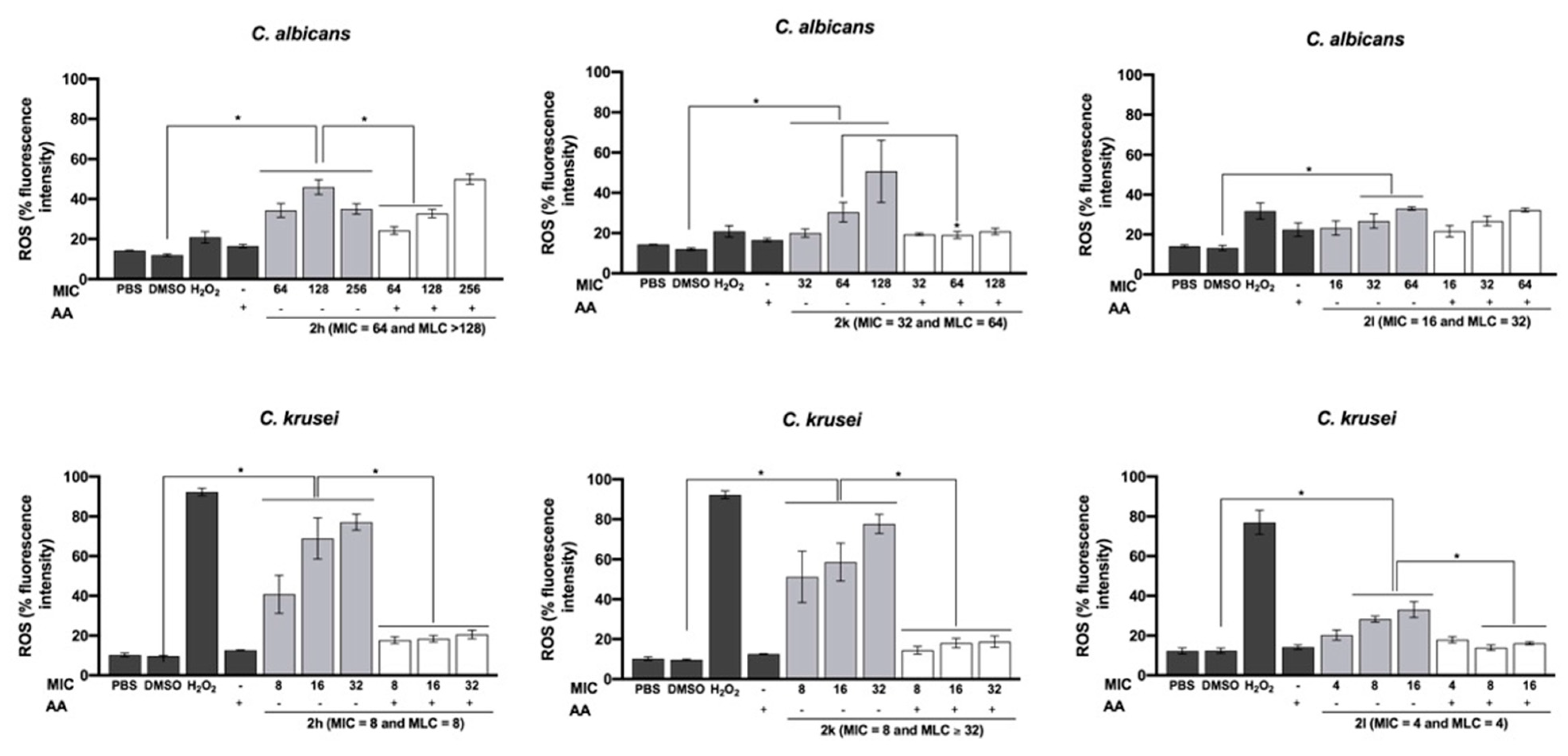
2.5. Effect on Total Intracellular ROS Production
2.6. C. albicans Virulence Mechanisms: Interference with Germ Tube Formation
2.7. Interaction Test by Checkboard Microdilution Assay
3. Discussion
4. Materials and Methods
4.1. Standards and Reagents
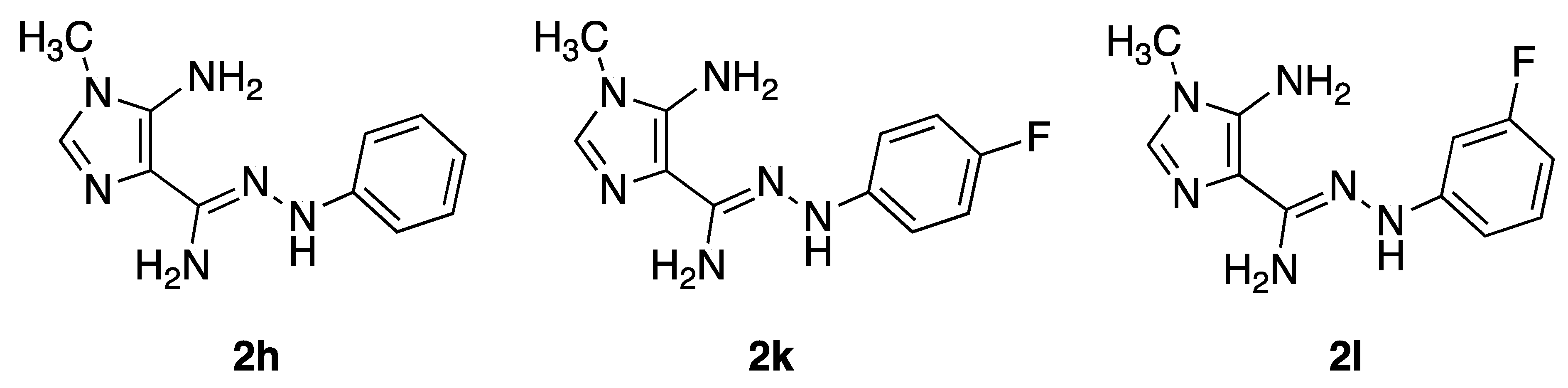
4.2. Compounds
4.3. Fungal Organisms
4.4. Susceptibility Tests
4.5. Cell Membrane Ergosterol
4.6. Cell Metabolic Viability
4.7. Total Intracellular ROS Measurement
4.8. Germ Tube Inhibition Assay
4.9. Checkboard Microdilution Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chin, V.K.; Lee, T.Y.; Rusliza, B.; Chong, P.P. Dissecting Candida albicans infection from the perspective of C. albicans virulence and omics approaches on host-pathogen interaction: A review. Int. J. Mol. Sci. 2016, 17, 1643. [Google Scholar] [CrossRef] [Green Version]
- Pendleton, K.M.; Huffnagle, G.B.; Dickson, R.P. The significance of Candida in the human respiratory tract: Our evolving understanding. Pathog. Dis. 2017, 75, ftx029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, J.E.; Rossi, D.C.P.; Jabes, D.L.; Barbosa, D.A.; Cunha, F.F.M.; Nunes, L.R.; Arruda, D.C.; Taborda, C.P. In vitro and in vivo inhibitory activity of limonene against different isolates of Candida spp. J. Fungi. 2020, 6, 183. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.N.; Ramos, L.S.; Oliveira, S.S.C.; Magalhães, L.B.; Squizani, E.D.; Kmetzsch, L.; Vainstein, M.H.; Branquinha, M.H.; Santos, A.L.S. Insights into the multi-azole resistance profile in Candida haemulonii species complex. J. Fungi. 2020, 6, 215. [Google Scholar] [CrossRef] [PubMed]
- Delattin, N.; Cammue, B.; Thevissen, K. Reactive oxygen species-inducing antifungal agents and their activity against fungal biofilms. Future Med. Chem. 2014, 6, 77–90. [Google Scholar] [CrossRef]
- Gintjee, T.J.; Donnelley, M.A.; Thompson, G.R., 3rd. Aspiring antifungals: Review of current antifungal pipeline developments. J. Fungi. 2020, 6, 28. [Google Scholar] [CrossRef] [Green Version]
- Goughenour, D.K.; Rappleye, C.A. Antifungal therapeutics for dimorphic fungal pathogens. Virulence 2017, 8, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Ami, R.B.; Lewis, R.E.; Kontoyiannis, D.P. Immunopharmacology of modern antifungals. Clin. Infect. Dis. 2008, 47, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Lopes, G.; Pinto, E.; Andrade, P.B.; Valentão, P. Antifungal activity of phlorotannins against dermatophytes and yeasts: Approaches to the mechanism of action and influence on Candida albicans virulence factor. PLoS ONE 2013, 8, e72203. [Google Scholar] [CrossRef]
- Posch, W.; Steger, M.; Wilflingseder, D.; Lass-Flörl, C. Promising immunotherapy against fungal diseases. Expert. Opin. Biol. Ther. 2017, 17, 861–870. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, D.; Kondo, K.; Uehara, N.; Otokozawa, S.; Tsuji, N.; Yagihashi, A.; Wtanabe, N. Endogenous reactive oxygen species is an important mediator of miconazole antifungal effect. Antimicrob. Agents Chemother. 2002, 46, 3113–3117. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Manoharlal, R.; Puri, N.; Prasad, R. Antifungal curcumin induces reactive oxygen species and triggers an early apoptosis but prevents hyphae development by targeting the global repressor TUP1 in Candida albicans. Biosci. Rep. 2010, 30, 391–404. [Google Scholar] [CrossRef]
- Lipovsky, A.; Gedanken, A.; Nitzan, Y.; Lubart, R. Antifungal activity of ZnO nanoparticles-the role of ROS mediated cell injury. Nanotechnology 2011, 22, 105101. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Z.; Li, Y.; Lu, C.; Wang, H.; Shen, Y.; Du, L. HSAF-induced antifungal effects in Candida albicans through ROS-mediated apoptosis. RSC Adv. 2016, 6, 30895–30904. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.I.; Gabriel, C.; Cerqueira, F.; Maia, M.; Pinto, E.; Sousa, J.C.; Medeiros, R.; Proença, M.F.; Dias, A.M. Synthesis and antimicrobial activity of novel 5-aminoimidazole-4-carboxamidrazones. Bioorg. Med. Chem. Lett. 2014, 24, 4699–4702. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C.; Grenho, L.; Cerqueira, F.; Medeiros, R.; Dias, A.M.; Ribeiro, A.I.; Proença, M.F.; Fernandes, M.H.; Sousa, J.C.; Monteiro, F.J.; et al. Inhibitory effect of 5-aminoimidazole-4-aarbohydrazonamides against Candida spp. biofilm on nanohydroxyapatite substrate. Mycopatholologia 2019, 184, 775–786. [Google Scholar] [CrossRef] [PubMed]
- McLennan, H.R.; Esposti, M.D. The contribution of mitochondrial respiratory complexes to the production of reactive oxygen species. J. Bioenerg. Biomembr. 2000, 32, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Jamiu, A.T.; Albertyn, J.; Sebolai, O.M.; Pohl, C.H. Update on Candida krusei, a potential multidrug-resistant pathogen. Med. Mycol. 2021, 59, 14–30. [Google Scholar] [CrossRef]
- Vale-Silva, L.A.; Coste, A.T.; Ischer, F.; Parker, J.E.; Kelly, S.L.; Pinto, E.; Sanglard, D. Azole resistance by loss of function of the sterol Δ5,6-desaturase gene (ERG3) in Candida albicans does not necessarily decrease virulence. Antimicrob. Agents Chemother. 2012, 56, 1960–1968. [Google Scholar] [CrossRef] [Green Version]
- De Cremer, K.; De Brucker, K.; Staes, I.; Peeters, A.; Van den Driessche, F.; Coenye, T.; Cammue, B.P.A.; Thevissen, K. Stimulation of superoxide production increases fungicidal action of miconazole against Candida albicans biofilms. Sci. Rep. 2016, 6, 27463. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jia, X.M.; Jia, J.-H.; Li, M.-B.; Cao, Y.-Y.; Gao, P.-H.; Liao, W.-Q.; Cao, Y.-B.; Jiang, Y.-Y. Ascorbic acid decreases the antifungal effect of fluconazole in the treatment of candidiasis. Clin. Exp. Pharmacol. Physiol. 2009, 36, e40–e46. [Google Scholar] [CrossRef] [PubMed]
- Vandenbosch, D.; Braeckmans, K.; Nelis, H.J.; Coenye, T. Fungicidal activity of miconazole against Candida spp. biofilms. J. Antimicrob. Chemother. 2010, 65, 694–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almshawit, H.; Macreadie, I. Fungicidal effect of thymoquinone involves generation of oxidative stress in Candida glabrata. Microbiol. Res. 2017, 195, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.-J.; Njus, D.; Schlegel, H.B. A theoretical study of ascorbic acid oxidation and HOO•/O2•− radical scavenging. Org. Biomol. Chem. 2017, 15, 4417–4431. [Google Scholar] [CrossRef]
- Como, J.; Dismukes, W. Systemic antifungal drugs: Azole antifungal drugs. In Clinical Mycology; Dismukes, W.E., Pappas, P.G., Sobel, J.D., Eds.; Oxford University Press: New York, NY, USA, 2003; pp. 64–87. ISBN 9780195148091. [Google Scholar]
- Ruy, F.; Vercesi, A.E.; Kowaltowski, A.J. Inhibition of specific electron transport pathways leads to oxidative stress and decreased Candida albicans proliferation. J. Bioenerg. Biomembr. 2006, 38, 129–135. [Google Scholar] [CrossRef]
- Sánchez, N.S.; Königsberg, M. Using yeast to easily determine mitochondrial functionality with 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenyltetrazolium bromide (MTT) assay. Biochem. Mol. Biol. Educ. 2006, 34, 209–212. [Google Scholar] [CrossRef]
- Martchenko, M.; Alarco, A.-M.; Harcus, D.; Whiteway, M. Superoxide dismutases in Candida albicans: Transcriptional regulation and functional characterization of the hyphal-induced SOD5 gene. Mol. Biol. Cell. 2004, 15, 456–467. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Liao, K.; Wang, D. Honokiol induces superoxide production by targeting mitochondrial respiratory chain complex I in Candida albicans. PLoS ONE. 2017, 12, e0184003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamarre, C.; LeMay, J.D.; Deslauriers, N.; Bourbonnais, Y. Candida albicans expresses an unusual cytoplasmic manganese-containing superoxide dismutase (SOD3 gene product) upon the entry and during the stationary phase. J. Biol. Chem. 2001, 276, 43784–43791. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Quijas, M.D.; Zazueta-Sandoval, R.; Obregón-Herrera, A.; López-Romero, E.; Cuéllar-Cruz, M. Effect of oxidative stress on cell wall morphology in four pathogenic Candida species. Mycol. Progr. 2015, 14, 8. [Google Scholar] [CrossRef]
- Mahl, C.D.; Behling, C.S.; Hackenhaar, F.S.; Silva, M.N.C.; Putti, J.; Salomon, T.B.; Alves, S.H.; Fuentefria, A.; Benfato, M.S. Induction of ROS generation by fluconazole in Candida glabrata: Activation of antioxidant enzymes and oxidative DNA damage. Diagn. Microbiol. Infect. Dis. 2015, 82, 203–208. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Koide, K.; Watanabe, K.; Morita, Y.; Mizuguchi, I.; Akashi, T. The expression of the pathogenic yeast Candida albicans catalase gene in response to hydrogen peroxide. Microbiol. Immunol. 1999, 43, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Abegg, M.A.; Alabarse, P.V.G.; Schuller, Á.K.; Benfato, M.S. Glutathione levels in and total antioxidant capacity of Candida sp. cells exposed to oxidative stress caused by hydrogen peroxide. Rev. Soc. Bras. Med. Trop. 2012, 45, 620–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, A.P. Dimorphism and virulence in Candida albicans. Curr. Opin. Microbiol. 1998, 1, 687–692. [Google Scholar] [CrossRef]
- Martins, V.P.; Dinamarco, T.M.; Curti, C.; Uyemura, S.A. Classical and alternative components of the mitochondrial respiratory chain in pathogenic fungi as potential therapeutic targets. J. Bioenerg. Biomembr. 2011, 43, 81–88. [Google Scholar] [CrossRef]
- Saville, S.P.; Lazzell, A.L.; Bryant, A.P.; Fretzen, A.; Monreal, A.; Solberg, E.O.; Monteagudo, C.; Lopez-Ribot, J.L.; Milne, G.T. Inhibition of filamentation can be used to treat disseminated candidiasis. Antimicrob. Agents Chemother. 2006, 50, 3312–3316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonough, J.A.; Bhattacherjee, V.; Sadlon, T.; Hostetter, M.K. Involvement of Candida albicans NADH dehydrogenase complex I in filamentation. Fungal Genet. Biol. 2002, 36, 117–127. [Google Scholar] [CrossRef]
- CLSI, Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; M27-A3 and third informational supplement M27-S3; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- Pinto, E.; Afonso, C.; Duarte, S.; Vale-Silva, L.; Costa, E.; Sousa, E.; Pinto, M. Antifungal activity of xanthones: Evaluation of their effect on ergosterol biosynthesis by high-performance liquid chromatography. Chem. Biol. Drug Des. 2011, 77, 212–222. [Google Scholar] [CrossRef]
- Arthington-Skaggs, B.A.; Jradi, H.; Desai, T.; Morrison, C.J. Quantitation of ergosterol content: Novel method for determination of fluconazole susceptibility of Candida albicans. J. Clin. Microbiol. 1999, 37, 3332–3337. [Google Scholar] [CrossRef] [Green Version]
- Marichal, P.; Gorrens, J.; Van Cutsem, J.; Vanden Bossche, H. Culture media for the study of the effects of azole derivatives on germ tube formation and hyphal growth of C. albicans. Mykosen 1986, 29, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Sheehan, D.J.; Hitchcock, C.A.; Ghannoum, M.A. Combination treatment of invasive fungal infections. Clin. Microbiol. Rev. 2005, 18, 163–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Compounds | Candida Strains | MIC (µg/mL) | MLC (µg/mL) |
|---|---|---|---|
| 2h | C. albicans ATCC | 32–64 | >128 |
| C. albicans H37 | 64 | >128 | |
| C. albicans M1 | 64 | >128 | |
| C. dubliniensis CD1 | 64 | >128 | |
| C. krusei ATCC | 4–8 | 8 | |
| 2k | C. albicans ATCC | 32 | 64 |
| C. albicans H37 | 32 | 64 | |
| C. albicans M1 | 16 | 64 | |
| C. dubliniensis CD1 | 16 | 32 | |
| C. krusei ATCC | 8 | ≥32 | |
| 2l | C. albicans ATCC | 16 | 32 |
| C. albicans H37 | 16 | ≥32 | |
| C. albicans M1 | 8 | 16 | |
| C. dubliniensis CD1 | 8 | ≥16 | |
| C. krusei ATCC | 4 | 4 | |
| Fluconazole | C. albicans ATCC | 1 | >128 |
| C. albicans H37 | 64 | >128 | |
| C. albicans M1 | 2 | 128 | |
| C. dubliniensis CD1 | 1 | >128 | |
| C. krusei ATCC | 64 | 64–128 |
| Compounds/ Species | MIC (µg/mL) | |||||
|---|---|---|---|---|---|---|
| 2h | 2h + AA | 2k | 2k + AA | 2l | 2l + AA | |
| C. albicans | 32–64 | >128 | 32 | >128 | 16 | >128 |
| C. krusei | 4–8 | >32 | 8 | >32 | 4 | >32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerqueira, F.; Maia, M.; Gabriel, C.; Medeiros, R.; Cravo, S.; Ribeiro, A.I.; Dantas, D.; Dias, A.M.; Saraiva, L.; Raimundo, L.; et al. Mechanism of Antifungal Activity by 5-Aminoimidazole-4-Carbohydrazonamide Derivatives against Candida albicans and Candida krusei. Antibiotics 2021, 10, 183. https://doi.org/10.3390/antibiotics10020183
Cerqueira F, Maia M, Gabriel C, Medeiros R, Cravo S, Ribeiro AI, Dantas D, Dias AM, Saraiva L, Raimundo L, et al. Mechanism of Antifungal Activity by 5-Aminoimidazole-4-Carbohydrazonamide Derivatives against Candida albicans and Candida krusei. Antibiotics. 2021; 10(2):183. https://doi.org/10.3390/antibiotics10020183
Chicago/Turabian StyleCerqueira, Fátima, Marta Maia, Carla Gabriel, Rui Medeiros, Sara Cravo, Ana Isabel Ribeiro, Daniela Dantas, Alice Maria Dias, Lucília Saraiva, Liliana Raimundo, and et al. 2021. "Mechanism of Antifungal Activity by 5-Aminoimidazole-4-Carbohydrazonamide Derivatives against Candida albicans and Candida krusei" Antibiotics 10, no. 2: 183. https://doi.org/10.3390/antibiotics10020183
APA StyleCerqueira, F., Maia, M., Gabriel, C., Medeiros, R., Cravo, S., Ribeiro, A. I., Dantas, D., Dias, A. M., Saraiva, L., Raimundo, L., & Pinto, E. (2021). Mechanism of Antifungal Activity by 5-Aminoimidazole-4-Carbohydrazonamide Derivatives against Candida albicans and Candida krusei. Antibiotics, 10(2), 183. https://doi.org/10.3390/antibiotics10020183








