Toward Safe Pharmacotherapy: The Interplay between Meropenem and Parenteral Nutrition Admixtures
Abstract
:1. Introduction
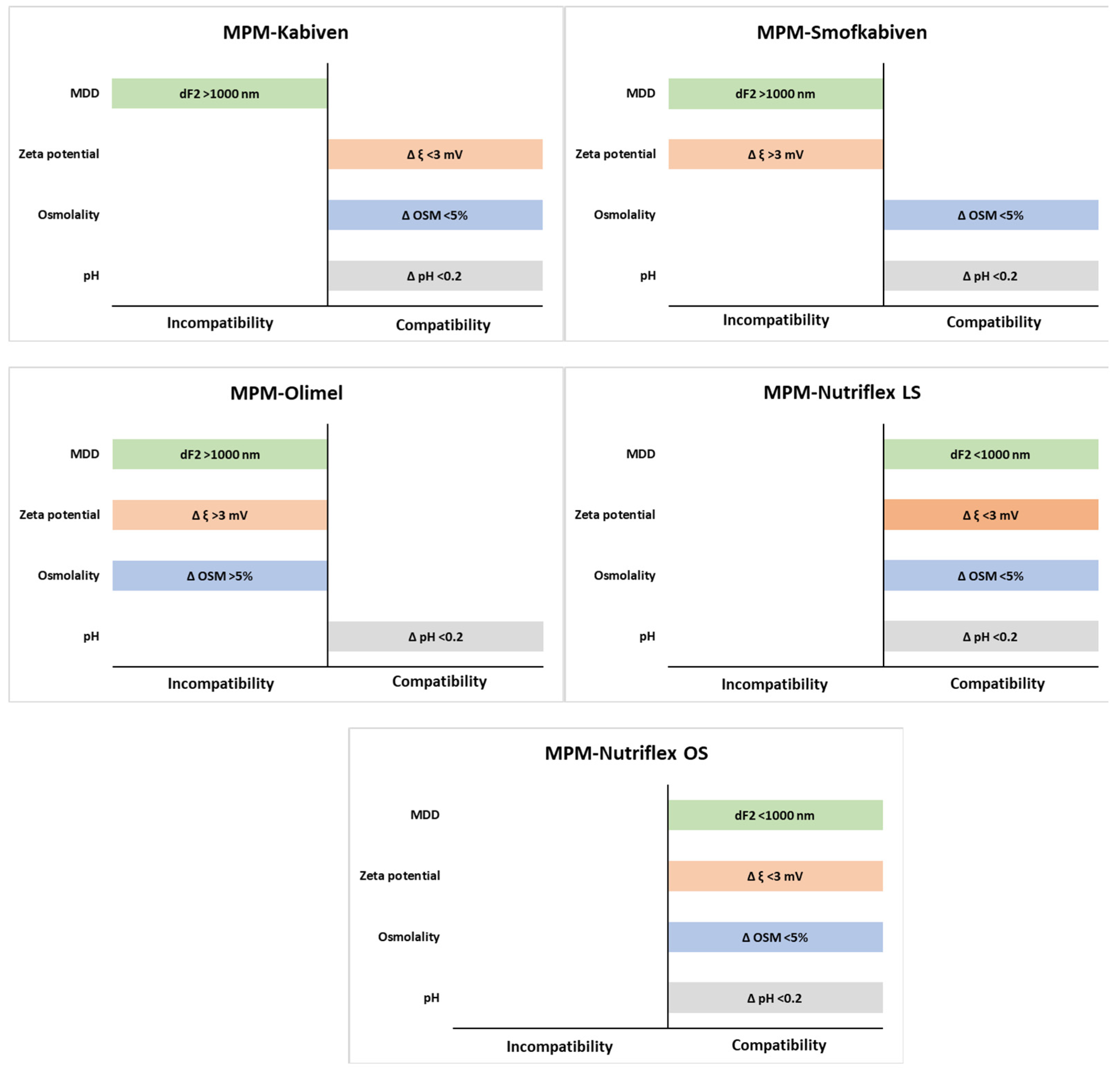
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Physicochemical Stability Assessment
4.3. Acceptance Limits
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statements
Conflicts of Interest
References
- Pinelli, F.; Cecero, E.; Degl’Innocenti, D.; Selmi, V.; Giua, R.; Villa, G.; Chelazzi, C.; Romagnoli, S.; Pittiruti, M. Infection of totally implantable venous access devices: A review of the literature. J. Vasc. Access 2018, 19, 230–242. [Google Scholar] [CrossRef]
- Kanji, S.; Lam, J.; Johanson, C.; Singh, A.; Goddard, R.; Fairbairn, J.; Lloyd, T.; Monsour, D.; Kakal, J. Systematic review of physical and chemical compatibility of commonly used medications administered by continuous infusion in intensive care units. Crit. Care Med. 2010, 38, 1890–1898. [Google Scholar] [CrossRef]
- Taxis, K.; Barber, N. Ethnographic study of incidence and severity of intravenous drug errors. BMJ 2003, 326, 684–687. [Google Scholar] [CrossRef] [Green Version]
- Bouchoud, L.; Fonzo-Christe, C.; Klingmüller, M.; Bonnabry, P. Compatibility of intravenous medications with parenteral nutrition: In vitro evaluation. J. Parenter. Enteral Nutr. 2013, 37, 416–424. [Google Scholar] [CrossRef]
- Staven, V.; Iqbal, H.; Wang, S.; Grønlie, I.; Tho, I. Physical compatibility of total parenteral nutrition and drugs in Y-site administration to children from neonates to adolescents. J. Pharm. Pharmacol. 2017, 69, 448–462. [Google Scholar] [CrossRef]
- Gostyńska, A.; Stawny, M.; Dettlaff, K.; Jelińska, A. The Interactions between Ciprofloxacin and Parenteral Nutrition Admixtures. Pharmaceutics 2020, 12, 27. [Google Scholar] [CrossRef] [Green Version]
- Stawny, M.; Nadolna, M.; Jelińska, A. In vitro compatibility studies of vancomycin with ready-to-use parenteral nutrition admixtures for safer clinical practice. Clin. Nutr. 2020, 39, 2539–2546. [Google Scholar] [CrossRef]
- Garcia, J.; Garg, A.; Song, Y.; Fotios, A.; Andersen, C.; Garg, S. Compatibility of intravenous ibuprofen with lipids and parenteral nutrition, for use as a continuous infusion. PLoS ONE 2018, 13, e0190577. [Google Scholar] [CrossRef] [Green Version]
- Tomczak, S.; Stawny, M.; Dettlaff, K.; Kieliszek, M.; Słomińska, D.; Jelińska, A. Physicochemical compatibility and stability of linezolid with parenteral nutrition. Molecules 2019, 24, 1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aeberhard, C.; Steuer, C.; Saxer, C.; Huber, A.; Stanga, Z.; Mühlebach, S. Physicochemical stability and compatibility testing of levetiracetam in all-in-one parenteral nutrition admixtures in daily practice. Eur. J. Pharm. Sci. 2017, 96, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Stawny, M.; Gostyńska, A.; Dettlaff, K.; Jelińska, A.; Główka, E.; Ogrodowczyk, M. Effect of lipid emulsion on stability of ampicillin in total parenteral nutrition. Nutrients 2019, 11, 559. [Google Scholar] [CrossRef] [Green Version]
- Mediavilla, M.M.; Molina, A.; Navarro, L.; Grau, L.; Pujol, M.D.; Cardenete, J.; Cardona, D.; Riera, P. Physicochemical compatibility of amiodarone with parenteral nutrition. J. Parenter. Enter Nutr. 2018, 43, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.L.; Petrovski, M.; Senarathna, S.G.; Mukadam, N.; Strunk, T.; Batty, K.T. Compatibility of pentoxifylline and parenteral medications. Arch. Dis. Child 2020, 105, 395–397. [Google Scholar]
- Campos-Baeta, Y.; Saavedra-Mitjans, M.; Garin, N.; Cardenete, J.; Cardona, D.; Riera, P. Physicochemical compatibility of dexmedetomidine with parenteral nutrition. Nutr. Clin. Pract. 2020, 35, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.M.; Wilder, A.G.; Foushee, J.A. Physical compatibility of various drugs with neonatal total parenteral nutrient solution during simulated Y-site administration. Am. J. Health Syst. Pharm. 2013, 70, 520–524. [Google Scholar] [CrossRef]
- Stawny, M.; Olijarczyk, R.; Jaroszkiewicz, E.; Jelińska, A. Pharmaceutical point of view on parenteral nutrition. Sci. World J. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trissel, L.A. Everything in a compatibility study is important. Am. J. Health Syst. Pharm. 1996, 53, 2990. [Google Scholar] [CrossRef] [PubMed]
- Husson, E.; Crauste-Manciet, S.; Hadj-Salah, E.; Seguier, J.C.; Brossard, D. Compatibility of parenteral drugs with commercialized total parenteral admixtures during simulated Y-site infusion. Nutr. Clin. Metabol. 2003, 17, 72–79. [Google Scholar] [CrossRef]
- Trissel, L.A.; Gilbert, D.L.; Martinez, J.F.; Baker, M.B.; Walter, W.V.; Mirtallo, J.M. Compatibility of medications with 3-in-1 parenteral nutrition admixtures. J. Parenter. Enter Nutr. 1999, 23, 67–74. [Google Scholar] [CrossRef]
- The United States Pharmacopeia and National Formulary, 38th ed.; United States Pharmacopeial Convention: Rockville, MD, USA, 2015.
- Staven, V.; Wang, S.; Grønlie, I.; Tho, I. Development and evaluation of a test program for Y-site compatibility testing of total parenteral nutrition and intravenous drugs. Nutr. J. 2016, 15, 29. [Google Scholar] [CrossRef] [Green Version]
- Wang, W. Tolerability of hypertonic injectables. Int. J. Pharm. 2015, 490, 308–315. [Google Scholar] [CrossRef] [PubMed]
- European Directorate for Quality in Medicines and Healthcare (EDQM). European Pharmacopoeia 9.0; 2.9.20. Particulate Contamination: Visible Particles; EDQM: Strasburg, France, 2017. [Google Scholar]
| Compatibility Studies | Conclusions | References |
|---|---|---|
| Compatibility studies of Nutriflex Lipid Special and amoxicillin/clavulanic acid, calcium chloride, cefepime, cyclosporine, esomeprazole, fentanyl, fluorouracil, furosemide, magnesium sulfate, meropenem, metoclopramide, metronidazole, midazolam, morphine sulfate, noradrenaline, octreotide, ondansetron, pantoprazole, paracetamol, piperacillin/tazobactam, potassium phosphate, tacrolimus, tropisetron, vancomycin | Albumin, esomeprazole, pantoprazole, tropisetron, and 5-fluorouracil were not compatible with Nutriflex Lipid Special. | Bouchoud et al., 2013 [4] |
| RTU PN admixtures: Olimel N5E and Numeta G16E Drugs: ampicillin, ceftazidime, clindamycin, dexamethasone, fluconazole, fosphenytoin, furosemide, metronidazole, ondansetron, and paracetamol | Ampicillin, fosphenytoin, and furosemide precipitated when mixed with PN. Ceftazidime, clindamycin, dexamethasone, fluconazole, metronidazole, ondansetron and paracetamol were compatible. | Staven et al., 2017 [5] |
| Compatibility studies of ciprofloxacin and eighteen compounded PN admixtures for adults | Compatibility of ciprofloxacin with PN admixtures depended on drug concentration and calcium and magnesium molar ratio. | Gostyńska et al., 2019 [6] |
| Compatibility studies of vancomycin and five PN admixtures: Kabiven, Nutriflex Lipid Special, Olimel N9E, Nutriflex Omega Special, and Smofkabiven | Vancomycin was compatible with Kabiven, Nutriflex Lipid Special, and Nutriflex Omega Special. | Stawny et al., 2020 [7] |
| PN and lipid solutions used in a tertiary neonatal unit included a Starter, Standard Preterm and low carbohydrate PN, and SMOFLipid 20% with Vitalipid N infant and Soluvit N Drug: ibuprofen lysine | Ibuprofen lysine was compatible with tested PN admixtures and lipids. | Garcia et al., 2018 [8] |
| Compatibility and stability studies of linezolid with six compounded PN admixtures for adults | Linezolid was compatible and stable with tested PN admixtures. | Tomczak et al., 2019 [9] |
| Compatibility and stability studies of levetiracetam with two compounded PN admixtures for adults | Levetiracetam was compatible and stable with tested PN admixtures. | Aeberhard et al., 2017 [10] |
| Stability studies of ampicillin with two compounded PN admixtures for adults containing Lipofundin MCT/LCT or Lipidem | Administration of ampicillin with TPN admixture at the tested dose is possible when used ex tempore and with light protection. | Stawny et al., 2019 [11] |
| Compatibility studies of amiodarone with two compounded PN admixtures for adults containing Lipofundin MCT/LCT or Smofilipid | Amiodarone was physicochemically compatible with tested PN admixtures via a Y-site administration. | Mediavilla et al., 2019 [12] |
| Compatibility studies of pentoxifylline with six compounded PN admixtures used in neonatal intensive care | Pentoxifylline was physicochemically compatible with six PN admixtures used in neonatology. | Campbell et al., 2019 [13] |
| Compatibility studies of dexmedetomidine and three compounded PN admixtures for adults | Dexmedetomidine was compatible with tested PN admixtures. | Campos-Baeta et al., 2019 [14] |
| Compatibility studies of amiodarone, caffeine citrate, clindamycin, enalaprilat, epinephrine, fluconazole, fosphenytoin sodium, hydrocortisone, metoclopramide, midazolam, pentobarbital, phenobarbital, and rifampin with neonatal PN admixtures | Caffeine citrate, clindamycin, enalaprilat, epinephrine, fluconazole, fosphenytoin sodium, hydrocortisone, metoclopramide, and midazolam were compatible with tested PN admixtures. Amiodarone, pentobarbital, phenobarbital, and rifampin were not compatible with the neonatal TPN solution and should not be coadministered via Y-site injection. | Fox et al., 2013 [15] |
| Sample | MPM:PN Ratio | pH ± SD | Osmolality ± SD | Zeta Potential ± SD | |||
|---|---|---|---|---|---|---|---|
| (mOsm/kg H2O) | (mV) | ||||||
| 0 h | 4 h * | 0 h | 4 h | 0 h | 4 h | ||
| MPM–Kabiven | 1:1 | 6.70 ± 0.00 | 6.66 ± 0.00 | 672 ± 1 | 685 ± 1 | −19.0 ± 0.8 | −19.2 ± 0.7 |
| 2:1 | 7.07 ± 0.01 | 7.06 ± 0.01 | 528 ± 3 | 541 ± 4 | −25.3 ± 1.1 | −23.0 ± 0.8 | |
| 4:1 | 7.37 ± 0.01 | 7.32 ± 0.00 | 440 ± 3 | 437 ± 4 | −25.6 ± 0.3 | −25.9 ± 0.3 | |
| MPM–Nutriflex LS | 1:1 | 6.47 ± 0.00 | 6.43 ± 0.00 | 977 ± 5 | 947 ± 4 ** | −26.8 ± 1.6 | −27.0 ± 1.2 |
| 2:1 | 6.89 ± 0.00 | 6.86 ± 0.00 | 710 ± 3 | 725 ± 3 ** | −32.8 ± 1.1 | −32.2 ± 0.3 | |
| 4:1 | 7.20 ± 0.00 | 7.17 ± 0.01 | 546 ± 4 | 542 ± 5 | −34.9 ± 1.1 | −32.5 ± 0.7 | |
| MPM–Olimel | 1:1 | 6.98 ± 0.00 | 6.85 ± 0.01 | 805 ± 11 | 892 ± 5 ** | −20.6 ± 1.2 | −32.1 ± 1.6 ** |
| 2:1 | 7.22 ± 0.00 | 7.19 ± 0.00 | 630 ± 6 | 673 ± 4 ** | −20.1 ± 1.4 | −31.8 ± 5.9 ** | |
| 4:1 | 7.42 ± 0.01 | 7.35 ± 0.01 | 476 ± 1 | 516 ± 4 ** | −20.4 ± 0.6 | −32.0 ± 3.6 ** | |
| MPM–Nutriflex OS | 1:1 | 6.44 ± 0.01 | 6.37 ± 0.00 | 957 ± 8 | 986 ± 1 ** | −14.8 ± 0.4 | −14.0 ± 0.3 |
| 2:1 | 6.85 ± 0.00 | 6.8 ± 0.00 | 731 ± 1 | 749 ± 1 ** | −19.2 ± 0.8 | −22.0 ± 0.2 | |
| 4:1 | 7.15 ± 0.01 | 7.12 ± 0.01 | 549 ± 0 | 552 ± 0 | −14.2 ± 0.9 | −12.9 ± 0.2 | |
| MPM–Smofkabiven | 1:1 | 6.41 ± 0.00 | 6.37 ± 0.01 | 901 ± 3 | 902 ± 2 | −21.2 ± 0.6 | −16.3 ± 0.4 ** |
| 2:1 | 6.87 ± 0.01 | 6.85 ± 0.01 | 688 ± 4 | 675 ± 3 ** | −23.5 ± 0.9 | −20.5 ± 1.1 | |
| 4:1 | 7.23 ± 0.01 | 7.16 ± 0.01 | 514 ± 3 | 505 ± 3 | −24.1 ± 1.4 | −20.7 ± 0.8 | |
| Sample | MPM:PN Ratio | PDI ± SD | MDD ± SD | dF1 ± SD | dF2 ± SD | |||
|---|---|---|---|---|---|---|---|---|
| (nm) | ||||||||
| 4 h | 0 h | 4 h | 0 h | 4 h | 0 h | 4 h | ||
| MPM–Kabiven | 1:1 | 0.05 ± 0.02 | 280.2 ± 3.2 | 280.2 ± 7.2 | 319.6 ± 4.2 | 319.6 ± 6.5 | n.d. | n.d. |
| 2:1 | 0.17 ± 0.01 | 275.9 ± 4.5 | 275.9 ± 7.0 | 320.8 ± 3.7 | 320.7 ± 4.3 | n.d. | 4687 ± 7 | |
| 4:1 | 0.18 ± 0.01 | 277.8 ± 6.2 | 277.8 ± 6.3 | 326.9 ± 4.2 | 326.9 ± 6.2 | n.d. | 4972 ± 5 | |
| MPM–Nutriflex LS | 1:1 | 0.06 ± 0.03 | 212.4 ± 2.2 | 212.4 ± 2.3 | 228.6 ± 2.3 | 228.6 ± 2.9 | n.d. | n.d. |
| 2:1 | 0.07 ± 0.02 | 211.1 ± 1.9 | 208.3 ± 2.5 | 228.2 ± 2.1 | 227.8 ± 3.3 | n.d. | n.d. | |
| 4:1 | 0.05 ± 0.02 | 208.7 ± 2.5 | 208.7 ± 2.2 | 221.9 ± 3.4 | 221.9 ± 3.7 | n.d. | n.d. | |
| MPM–Olimel | 1:1 | 0.16 ± 0.04 | 256.6 ± 1.4 | 266.8 ± 7.6 ** | 281.9 ± 5.8 | 296.2 ± 5.4 | n.d. | 5064 ± 9 |
| 2:1 | 0.18 ± 0.01 | 254.4 ± 2.8 | 266.1 ± 4.6 | 281.4 ± 2.0 | 296.2 ± 4.7 | n.d. | 5190 ± 8 | |
| 4:1 | 0.21 ± 0.01 | 253.0 ± 2.6 | 262.8 ± 7.2 | 283.2 ± 8.1 | 282.4 ± 6.2 | 5170 ± 9 | 5389 ± 12 | |
| MPM–Nutriflex OS | 1:1 | 0.07 ± 0.01 | 223.8 ± 1.7 | 220.9 ± 2.5 | 247.2 ± 1.3 | 238.4 ± 6.1 | n.d. | n.d. |
| 2:1 | 0.08 ± 0.02 | 215.1 ± 1.3 | 216.4 ± 2.4 | 234.6 ± 4.3 | 238.2 ± 7.3 | n.d. | n.d. | |
| 4:1 | 0.06 ± 0.03 | 218.6 ± 1.2 | 219.5 ± 3.9 | 236.2 ± 7.6 | 239.7 ± 4.5 | n.d. | n.d. | |
| MPM–Smofkabiven | 1:1 | 0.06 ± 0.01 | 237.1 ± 2.4 | 236.4 ± 2.1 | 272.8 ± 7.2 | 263.2 ± 3.0 | n.d. | n.d. |
| 2:1 | 0.15 ± 0.01 | 234.5 ± 1.3 | 233.9 ± 0.4 | 258.1 ± 3.7 | 257.4 ± 4.6 | n.d. | 4996 ± 8 | |
| 4:1 | 0.16 ±0.01 | 234.0 ± 2.5 | 232.0 ± 0.2 | 265.4 ± 3.5 | 255.0 ± 4.3 | n.d. | 5151 ± 10 | |
| Kabiven | Nutriflex LS | Olimel | Nutriflex OS | SmofKabiven | |
|---|---|---|---|---|---|
| g/1000 mL | |||||
| Alanine | 4.7 | 6.8 | 8.3 | 6.8 | 7.1 |
| Arginine | 3.3 | 3.8 | 5.6 | 3.8 | 6.1 |
| Aspartic acid | 1.0 | 2.1 | 1.7 | 2.1 | - |
| Glutamic acid | 1.6 | 4.9 | 2.9 | 4.9 | - |
| Glicyne | 2.3 | 2.3 | 3.9 | 2.3 | 5.6 |
| Histidine | 2.0 | 1.8 | 3.4 | 1.8 | 1.5 |
| Izoleucine | 1.6 | 3.3 | 2.9 | 3.3 | 2.6 |
| Leucine | 2.3 | 4.4 | 3.9 | 4.4 | 3.8 |
| Lisyne | 2.7 | 3.2 | 4.5 | 3.2 | 3.4 |
| Methionine | 1.6 | 2.7 | 2.9 | 2.7 | 2.2 |
| Phenylalanine | 2.3 | 4.9 | 3.9 | 4.9 | 2.6 |
| Proline | 2.0 | 4.7 | 3.4 | 4.7 | 5.7 |
| Serine | 1.3 | 4.2 | 2.3 | 4.2 | 3.3 |
| Taurine | - | - | - | - | 0.54 |
| Threonine | 1.6 | 2.6 | 2.9 | 2.6 | 2.2 |
| Tryptophan | 0.6 | 0.8 | 0.9 | 0.8 | 1.0 |
| Tyrosine | 0.1 | - | 0.1 | 0.2 | |
| Valine | 2.1 | 3.6 | 3.7 | 3.6 | 3.1 |
| Total amino acids | 33.1 | 56.1 | 56.9 | 56.1 | 50.8 |
| Nitrogen | 5.3 | 8.0 | 9.0 | 8.0 | 8.1 |
| Glucose | 97.4 | 144.0 | 110.0 | 144.0 | 126.6 |
| LCT | 39.0 | 20.0 | 8.0 | 20.0 | 11.4 |
| MCT | - | 20.0 | - | 16.0 | 11.4 |
| Olive oil | - | - | 32.0 | - | 9.5 |
| Omega-3 acids | - | - | - | 4.0 | 5.7 |
| Total lipids | 39.0 | 40.0 | 40.0 | 40.0 | 38.1 |
| mmol/1000 mL | |||||
| Sodium | 31.2 | 53.6 | 35.0 | 53.6 | 40.6 |
| Potassium | 23.4 | 37.6 | 30.0 | 37.6 | 30.5 |
| Magnesium | 3.9 | 4.2 | 4.0 | 4.2 | 5.1 |
| Calcium | 1.9 | 4.2 | 3.5 | 4.2 | 2.6 |
| Zinc | - | 0.032 | - | 0.032 | 0.041 |
| Chlorides | 45.5 | 48.0 | 45.3 | 48.0 | 35.2 |
| Phosphates | 37.7 | 16.0 | 15.0 | 16.0 | 12.9 |
| Acetates | 9.7 | 48.0 | 53.3 | 48.0 | 106.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gostyńska, A.; Piwowarczyk, L.; Nadolna, M.; Jelińska, A.; Dettlaff, K.; Ogrodowczyk, M.; Popielarz-Brzezińska, M.; Stawny, M. Toward Safe Pharmacotherapy: The Interplay between Meropenem and Parenteral Nutrition Admixtures. Antibiotics 2021, 10, 217. https://doi.org/10.3390/antibiotics10020217
Gostyńska A, Piwowarczyk L, Nadolna M, Jelińska A, Dettlaff K, Ogrodowczyk M, Popielarz-Brzezińska M, Stawny M. Toward Safe Pharmacotherapy: The Interplay between Meropenem and Parenteral Nutrition Admixtures. Antibiotics. 2021; 10(2):217. https://doi.org/10.3390/antibiotics10020217
Chicago/Turabian StyleGostyńska, Aleksandra, Ludwika Piwowarczyk, Malwina Nadolna, Anna Jelińska, Katarzyna Dettlaff, Magdalena Ogrodowczyk, Maria Popielarz-Brzezińska, and Maciej Stawny. 2021. "Toward Safe Pharmacotherapy: The Interplay between Meropenem and Parenteral Nutrition Admixtures" Antibiotics 10, no. 2: 217. https://doi.org/10.3390/antibiotics10020217
APA StyleGostyńska, A., Piwowarczyk, L., Nadolna, M., Jelińska, A., Dettlaff, K., Ogrodowczyk, M., Popielarz-Brzezińska, M., & Stawny, M. (2021). Toward Safe Pharmacotherapy: The Interplay between Meropenem and Parenteral Nutrition Admixtures. Antibiotics, 10(2), 217. https://doi.org/10.3390/antibiotics10020217









