Indole Derivatives Obtained from Egyptian Enterobacter sp. Soil Isolates Exhibit Antivirulence Activities against Uropathogenic Proteus mirabilis
Abstract
:1. Introduction
2. Results and Discussion
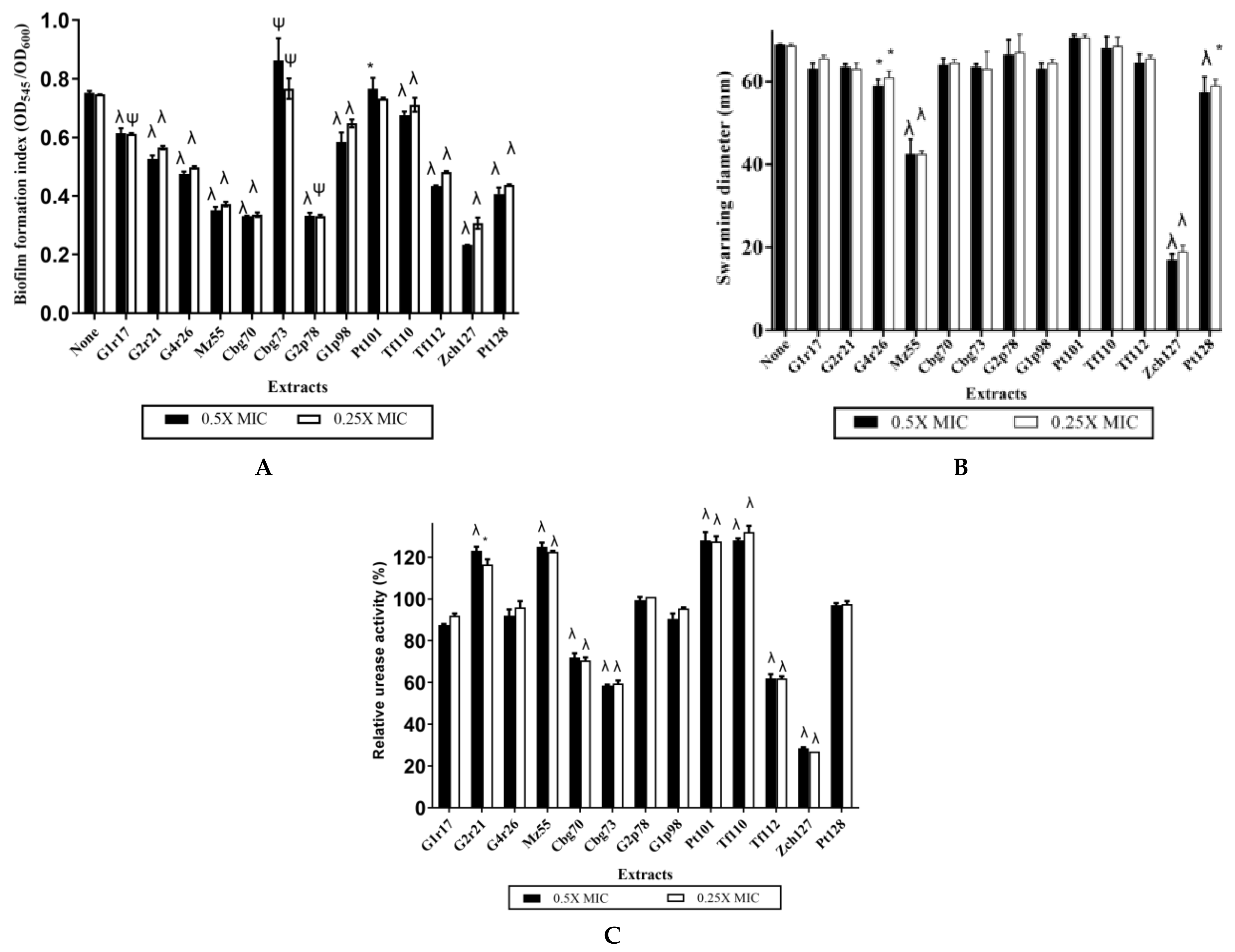
2.1. Some Rhizobacteria Enterobacter sp. Isolates Exhibit High Indole Production Coupled with Potential Antivirulence Activity against P. mirabilis
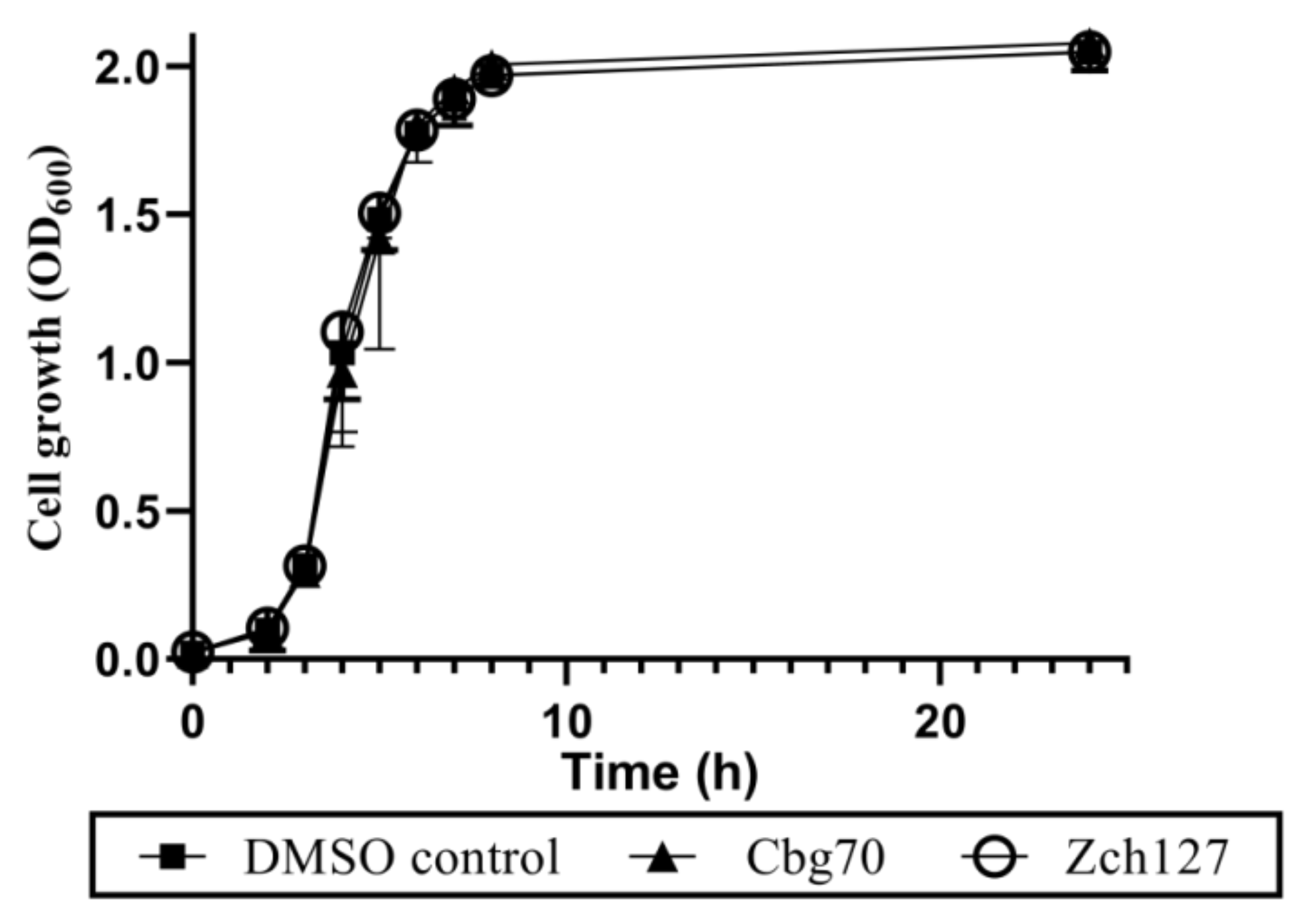
2.2. Half MICs of Cbg70 and Zch127 Have no Inhibitory Effect on Growth of P. mirabilis
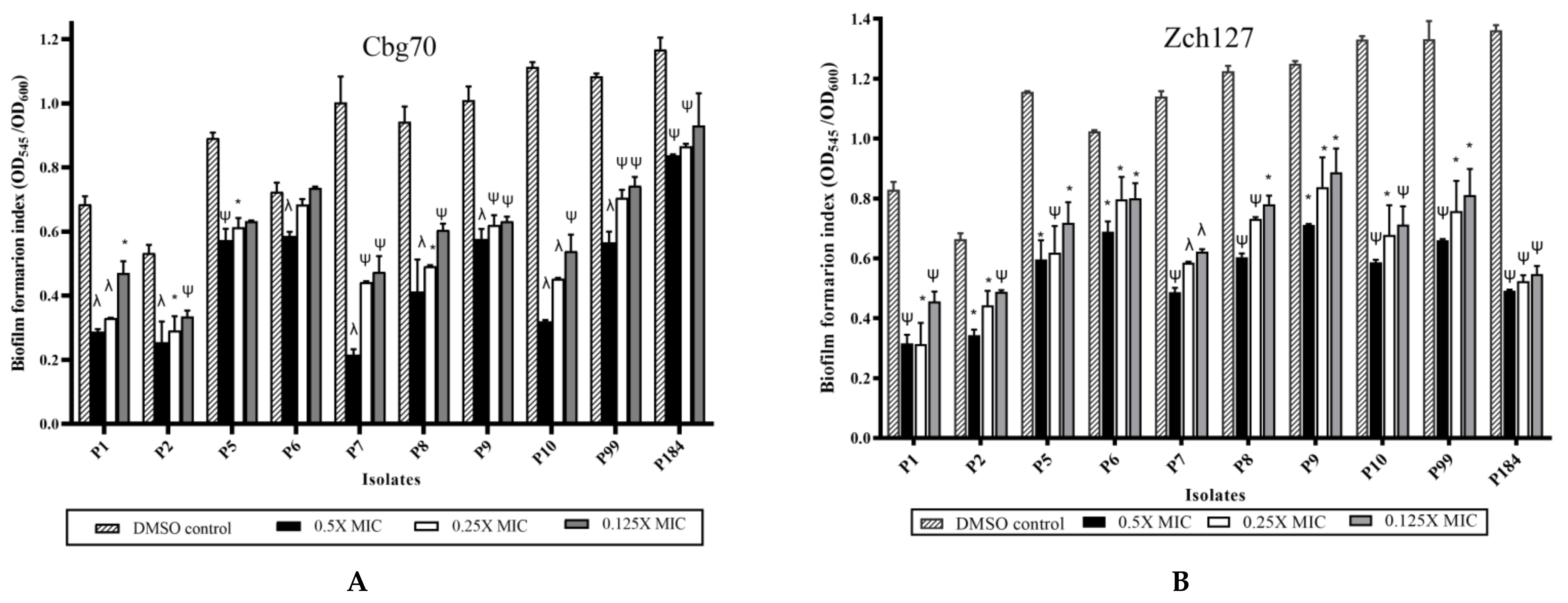
2.3. Sub MIC Concentrations of Cbg70 and Zch127 Extracts Significantly Inhibit P. mirabilis Biofilm Formation
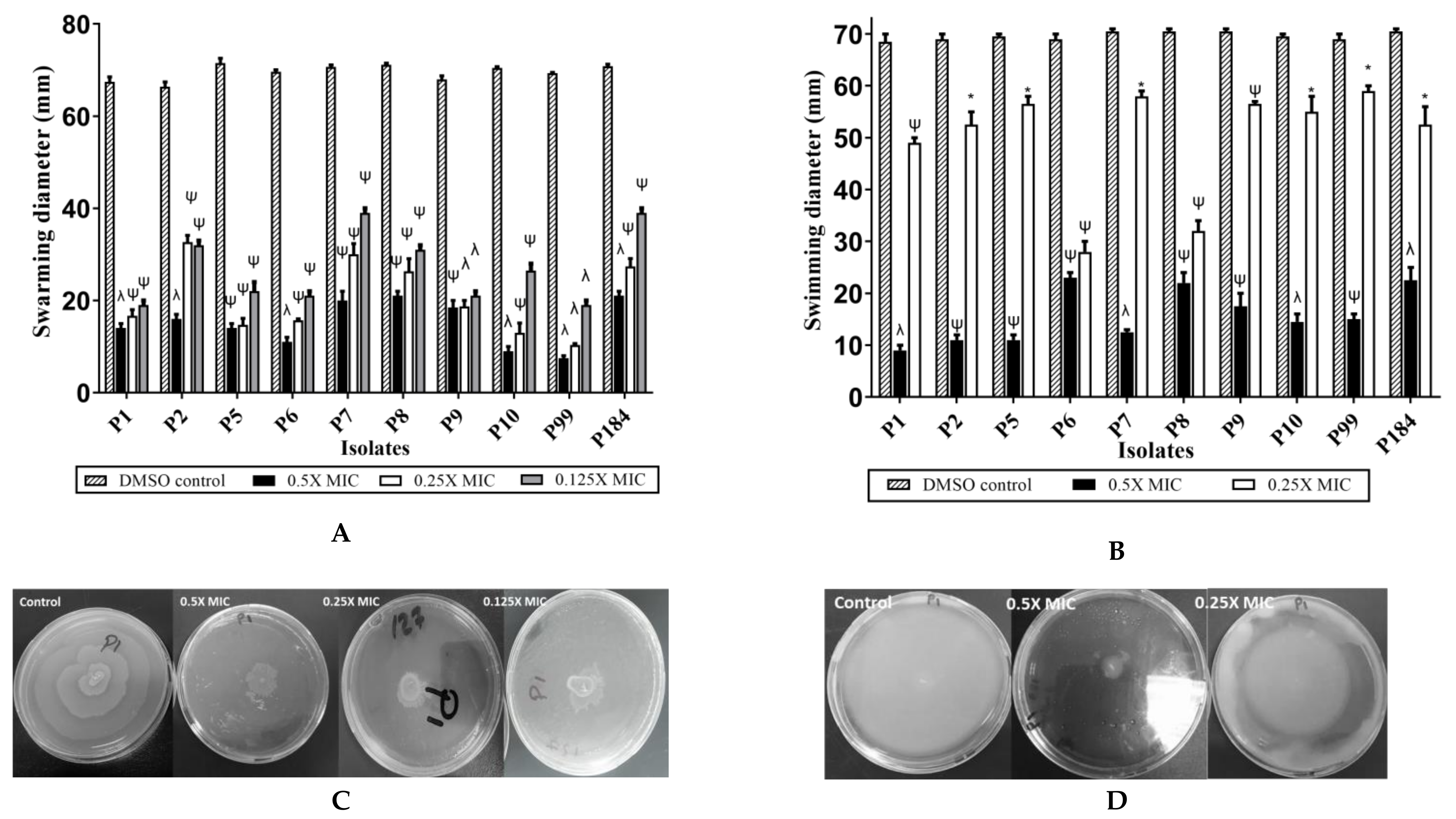
2.4. Indole Extract Zch 127 Inhibited the Swimming and Swarming Motility of P. mirabilis
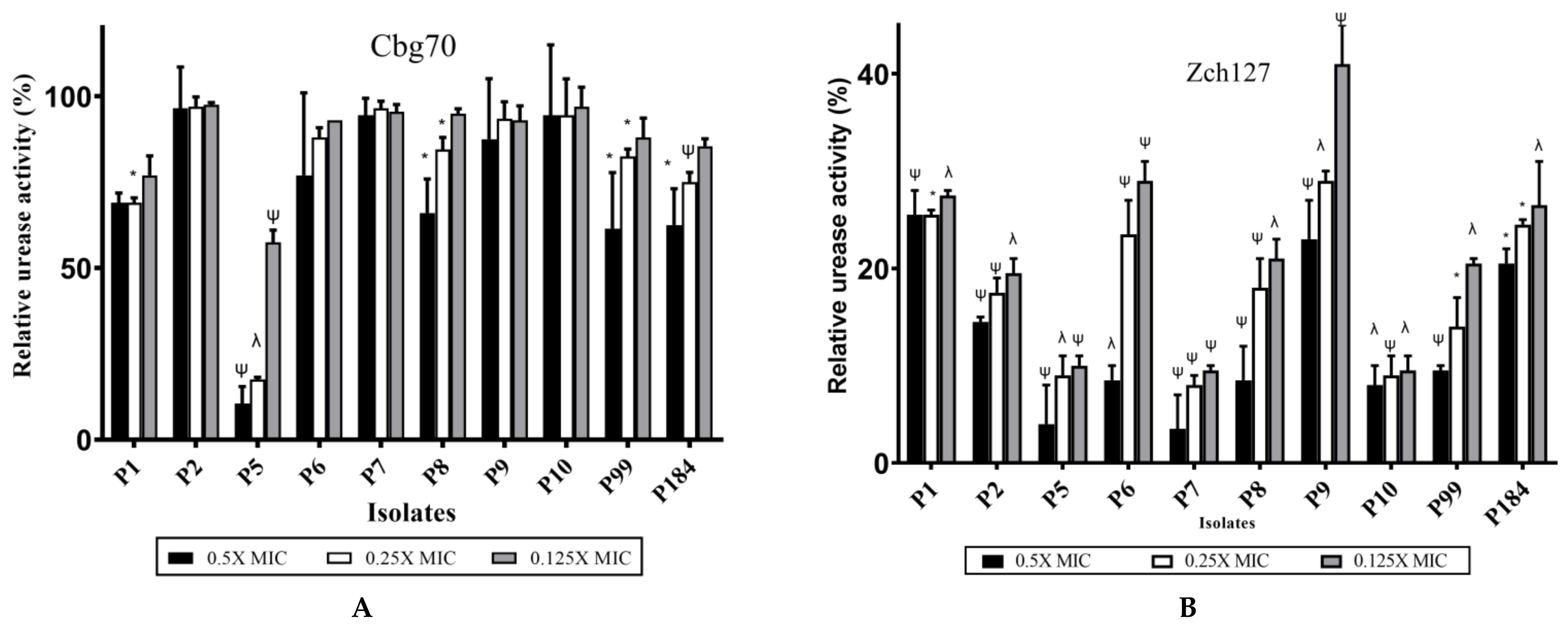
2.5. Inhibitory Effect of Zch127 Crude Extract on the Urease Activity of P. mirabilis
2.6. The Effect of Cbg70 and Zch127 Crude Extracts on the Antibiotic Sensitivity of P. mirabilis
2.7. Identification of Indole Derivatives in the Crude Extracts by LC-MS Analysis
2.8. Identification and Verification of Indole Compounds in the Extracts Using HPLC
2.9. Effect of Synthetic IAA and IAN on P. mirabilis Virulence Traits
3. Materials and Methods
3.1. Reagents and Standards
3.2. Clinical Strains and Growth Conditions
3.3. Isolation of Bacteria from Soil, Identification, and Screening for Indole Production
3.4. Preparation of Crude Extracts from Enterobacter sp. Isolates
3.5. Determination of Minimum Inhibitory Concentration (MIC) of Crude Extract against P. mirabilis Isolates
3.6. Assessment of the Antivirulence Potential of Crude Extract against P. mirabilis
3.6.1. Effect on Biofilm Formation
3.6.2. Effect on the Swarming and Swimming Behavior
3.6.3. Effect on Urease Production
3.6.4. Effect on the Minimum Inhibitory Concentration (MIC) of Antibiotics
3.7. HPLC and LC-MS/MS Analysis of the Microbial Extract
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- E Nicolle, L. Catheter associated urinary tract infections. Antimicrob. Resist. Infect. Control. 2014, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- Ranjbar-Omid, M.; Arzanlou, M.; Amani, M.; Al-Hashem, S.K.S.; Mozafari, N.A.; Doghaheh, H.P. Allicin from garlic inhibits the biofilm formation and urease activity of Proteus mirabilis in vitro. FEMS Microbiol. Lett. 2015, 362, 9. [Google Scholar] [CrossRef] [Green Version]
- Wasfi, R.; Hamed, S.M.; Amer, M.A.; Fahmy, L.I. Proteus mirabilis Biofilm: Development and Therapeutic Strategies. Front. Cell. Infect. Microbiol. 2020, 10, 414. [Google Scholar] [CrossRef]
- Dandrea, M.M.; Literacka, E.; Zioga, A.; Giani, T.; Baraniak, A.; Fiett, J.; Sadowy, E.; Tassios, P.T.; Rossolini, G.M.; Gniadkowski, M.; et al. Evolution and Spread of a Multidrug-Resistant Proteus mirabilis Clone with Chromosomal AmpC-Type Cephalosporinases in Europe. Antimicrob. Agents Chemother. 2011, 55, 2735–2742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasfi, R.; Abdellatif, G.R.; Elshishtawy, H.M.; Ashour, H.M. First-time characterization of viable but non-culturable Proteus mirabilis: Induction and resuscitation. J. Cell. Mol. Med. 2020, 24, 2791–2801. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Kim, Y.-G.; Cho, M.H.; Kim, J.-A.; Lee, J. 7-fluoroindole as an antivirulence compound against Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2012, 329, 36–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-H.; Wood, T.K.; Lee, J. Roles of Indole as an Interspecies and Interkingdom Signaling Molecule. Trends Microbiol. 2015, 23, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, J. Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev. 2010, 34, 426–444. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Cho, M.H.; Lee, J. 3-Indolylacetonitrile DecreasesEscherichia coliO157:H7 Biofilm Formation andPseudomonas aeruginosaVirulence. Environ. Microbiol. 2010, 13, 62–73. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-G.; Kim, C.-J.; Lee, J.-C.; Cho, M.H.; Lee, J. Indole-3-acetaldehyde from Rhodococcus sp. BFI 332 inhibits Escherichia coli O157:H7 biofilm formation. Appl. Microbiol. Biotechnol. 2012, 96, 1071–1078. [Google Scholar] [CrossRef]
- Nikaido, E.; Giraud, E.; Baucheron, S.; Yamasaki, S.; Wiedemann, A.; Okamoto, K.; Takagi, T.; Yamaguchi, A.; Cloeckaert, A.; Nishino, K. Effects of indole on drug resistance and virulence of Salmonella enterica serovar Typhimurium revealed by genome-wide analyses. Gut Pathog. 2012, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Cho, H.S.; Kim, Y.; Kim, J.-A.; Banskota, S.; Cho, M.H.; Lee, J. Indole and 7-benzyloxyindole attenuate the virulence of Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2013, 97, 4543–4552. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, Y.; Oh, S.; Chun, T.; Kim, S. Inhibitory effect of skatole (3-methylindole) on enterohemorrhagic Escherichia coli O157:H7 ATCC 43894 biofilm formation mediated by elevated endogenous oxidative stress. Lett. Appl. Microbiol. 2014, 58, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Palaniyandi, S.A.; Yang, S.H.; Zhang, L.; Suh, J.-W. Effects of actinobacteria on plant disease suppression and growth promotion. Appl. Microbiol. Biotechnol. 2013, 97, 9621–9636. [Google Scholar] [CrossRef] [PubMed]
- Kutáček, M.; Procházka, Ž.; Grünberger, D. Biogenesis of Ascorbigen, 3-Indolylacetonitrile and Indole-3-carboxylic Acid from d,l-Tryptophan-3-14C in Brassica oleracea L. Nat. Cell Biol. 1960, 187, 61–62. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Delage, B.; Williams, D.E.; Dashwood, R.H. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res. 2007, 55, 224–236. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.; Meng, Q.; Saha, T.; Sarkar, F.H.; Rosen, E.M. Low Concentrations of Diindolylmethane, a Metabolite of Indole-3-Carbinol, Protect against Oxidative Stress in a BRCA1-Dependent Manner. Cancer Res. 2009, 69, 6083–6091. [Google Scholar] [CrossRef] [Green Version]
- Ladha, J.K.; Barraquio, W.L.; Watanabe, I. Isolation and identification of nitrogen-fixing Enterobacter cloacae and Klebsiella planticola associated with rice plants. Can. J. Microbiol. 1983, 29, 1301–1308. [Google Scholar] [CrossRef]
- Tsuda, K.; Kosaka, Y.; Tsuge, S.; Kubo, Y.; Horino, O. Evaluation of the Endophyte Enterobacter cloacae SM10 Isolated from Spinach Roots for Biological Control against Fusarium Wilt of Spinach. J. Gen. Plant Pathol. 2001, 67, 78–84. [Google Scholar] [CrossRef]
- Panigrahi, S.; Mohanty, S.; Rath, C. Characterization of endophytic bacteria Enterobacter cloacae MG00145 isolated from Ocimum sanctum with Indole Acetic Acid (IAA) production and plant growth promoting capabilities against selected crops. S. Afr. J. Bot. 2020, 134, 17–26. [Google Scholar] [CrossRef]
- Pratt, L.A.; Kolter, R. Genetic analysis ofEscherichia colibiofilm formation: Roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 1998, 30, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Packiavathy, I.A.S.V.; Priya, S.; Pandian, S.K.; Ravi, A.V. Inhibition of biofilm development of uropathogens by curcumin—An anti-quorum sensing agent from Curcuma longa. Food Chem. 2014, 148, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Son, J.-S.; Lee, S.-J.; Jun, S.Y.; Yoon, S.J.; Kang, S.H.; Paik, H.R.; Kang, J.O.; Choi, Y.-J. Antibacterial and biofilm removal activity of a podoviridae Staphylococcus aureus bacteriophage SAP-2 and a derived recombinant cell-wall-degrading enzyme. Appl. Microbiol. Biotechnol. 2010, 86, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Bosch, A.; Bettiol, M.; González, D.L.N.; Erben, M.F.; Lamberti, Y. Novel Guanidine Compound against Multidrug-Resistant Cystic Fibrosis-Associated Bacterial Species. Molecules 2018, 23, 1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Attila, C.; Cirillo, S.L.G.; Cirillo, J.D.; Wood, T.K. Indole and 7-hydroxyindole diminish Pseudomonas aeruginosa virulence. Microb. Biotechnol. 2008, 2, 75–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, D.N.; Edwards, L.; Kornienko, A.; Frolova, L.V.; Rogelj, S. Synergistic action of substituted indole derivatives and clinically used antibiotics against drug-resistant bacteria. Futur. Microbiol. 2020, 15, 579–590. [Google Scholar] [CrossRef]
- Yaikhan, T.; Chuerboon, M.; Tippayatham, N.; Atimuttikul, N.; Nuidate, T.; Yingkajorn, M.; Tun, A.W.; Buncherd, H.; Tansila, N. Indole and Derivatives Modulate Biofilm Formation and Antibiotic Tolerance of Klebsiella pneumoniae. Indian J. Microbiol. 2019, 59, 460–467. [Google Scholar] [CrossRef]
- CLSI. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-First Informational Supplement; CLSI Document M100-S21; The Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Mujahid Sasikala, C.; Ramana, C.V. Production of indole-3-acetic acid and related indole derivatives from L-tryptophan by Rubrivivax benzoatilyticus JA2. Appl. Microbiol. Biotechnol. 2011, 89, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.-H.; Chang, C.-Y.; Lin, H.-R. Systematic profiling of indole-3-acetic acid biosynthesis in bacteria using LC–MS/MS. J. Chromatogr. B 2015, 988, 53–58. [Google Scholar] [CrossRef]
- Qiu, Y.-Q. KEGG Pathway Database. Encycl. Syst. Biol. 2013, 1068–1069. [Google Scholar] [CrossRef]
- Jasim, B.; John, C.J.; Shimil, V.; Jyothis, M.; Radhakrishnan, E. Studies on the factors modulating indole-3-acetic acid production in endophytic bacterial isolates from Piper nigrum and molecular analysis of ipdc gene. J. Appl. Microbiol. 2014, 117, 786–799. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.M.; Berrué, F.; Kerr, R.; Patten, C.L. Metabolomic analysis of indolepyruvate decarboxylase pathway derivatives in the rhizobacterium Enterobacter cloacae. Rhizosphere 2018, 6, 98–111. [Google Scholar] [CrossRef]
- Bruto, M.; Prigent-Combaret, C.; Muller, D.; Moënne-Loccoz, Y. Analysis of genes contributing to plant-beneficial functions in plant growth-promoting rhizobacteria and related Proteobacteria. Sci. Rep. 2014, 4, srep06261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, M.; Suzuki, T.; Fujita, T.; Masuda, M.; Shimizu, S. Occurrence of enzymes involved in biosynthesis of indole-3-acetic acid from indole-3-acetonitrile in plant-associated bacteria, Agrobacterium and Rhizobium. Proc. Natl. Acad. Sci. USA 1995, 92, 714–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogunmwonyi, I.N.; Igbinosa, E.; Aiyegoro, O.; Odjadjare, E. Microbial analysis of different top soil samples of selected site in Obafemi Awolowo University, Nigeria. Sci. Res. Essays 2008, 3, 120–124. [Google Scholar]
- Lwin, K.M.; Myint, M.M.; Tar, T.; Aung, W.Z.M. Isolation of Plant Hormone (Indole-3-Acetic Acid—IAA) Producing Rhizobacteria and Study on Their Effects on Maize Seedling. Eng. J. 2012, 16, 137–144. [Google Scholar] [CrossRef]
- Bric, J.M.; Bostock, R.M.; Silverstone, S.E. Rapid In Situ Assay for Indoleacetic Acid Production by Bacteria Immobilized on a Nitrocellulose Membrane. Appl. Environ. Microbiol. 1991, 57, 535–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, K.; Nawaz, M.; Nazir, J.; Anjum, A.; Yaqub, T.; Ahmad, M.-u.-D.; Rehman, M.U.; Aziz, G.; Khan, M. Isolation, characterization and effect of auxin producing bacteria on growth of Triticum aestivum. J. Anim. Plant Sci. 2015, 25, 1003–1007. [Google Scholar]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Simultaneous detection and quantification of indole-3-acetic acid (IAA) and indole-3-butyric acid (IBA) produced by rhizobacteria from l-tryptophan (Trp) using HPTLC. J. Microbiol. Methods 2015, 110, 7–14. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. J. Vis. Exp. 2011, 2437, e2437. [Google Scholar] [CrossRef] [PubMed]
- Naves, P.; Del Prado, G.; Huelves, L.; Gracia, M.; Ruiz, V.; Blanco, J.; Rodríguez-Cerrato, V.; Ponte, M.; Soriano, F. Measurement of biofilm formation by clinical isolates ofEscherichia coliis method-dependent. J. Appl. Microbiol. 2008, 105, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Labrecque, J.; Bodet, C.; Chandad, F.; Grenier, D. Effects of a high-molecular-weight cranberry fraction on growth, biofilm formation and adherence of Porphyromonas gingivalis. J. Antimicrob. Chemother. 2006, 58, 439–443. [Google Scholar] [CrossRef] [Green Version]
- Aygül, A.; Kibar, F.; Çıragil, P. Quercetin and Cinnamaldehyde Show Antipathogenic Activity Against Proteus mirabilis Isolates: Inhibition of Swarming Motility and Urease Activity. Flora J. Infect. Dis. Clin. Microbiol. 2020, 25, 76–83. [Google Scholar] [CrossRef]
- Abdel-Baky, R.M.; Ali, M.A.; Abuo-Rahma, G.E.-D.A.A.; Abdelaziz, N. Inhibition of Urease Enzyme Production and some Other Virulence Factors Expression in Proteus mirabilis by N-Acetyl Cysteine and Dipropyl Disulphide. Adv. Exp. Med. Biol. 2017, 973, 99–113. [Google Scholar] [CrossRef]
- Nicolosi, D.; Tempera, G.; Genovese, C.; Furneri, P.M. Anti-Adhesion Activity of A2-type Proanthocyanidins (a Cranberry Major Component) on Uropathogenic E. coli and P. mirabilis Strains. Antibiotics 2014, 3, 143–154. [Google Scholar] [CrossRef]
- Chung, K.-R.; Shilts, T.; Ertã¼Rk ÃœMran; Timmer, L.W.; Ueng, P.P. Indole derivatives produced by the fungusColletotrichum acutatumcausing lime anthracnose and postbloom fruit drop of citrus. FEMS Microbiol. Lett. 2003, 226, 23–30. [Google Scholar] [CrossRef] [Green Version]



| Antibiotic | Isolate No. | MIC | MIC with Extract Cbg70 a | Fold Change in MIC with Cbg70 | MIC with Extract Zch127 b | Fold Change in MIC with Zch127 |
|---|---|---|---|---|---|---|
| Ceftriaxone | P1 | 64(R) | 32(R) | −2X | 16(R) | −4X |
| P2 | 16(R) | 16(R) | − | 4(R) | −4X | |
| P5 | 16(R) | 16(R) | − | 8(R) | −2X | |
| P6 | 32(R) | 32(R) | − | 32(R) | − | |
| P7 | 4(R) | 8(R) | 2X | 4(R) | − | |
| P8 | 128(R) | 128(R) | − | 64(R) | −2X | |
| P9 | 64(R) | 64(R) | − | 64(R) | − | |
| P10 | 8(R) | 8(R) | − | 8(R) | − | |
| P99 | 2(I) | 1(S) | −2X | 1(S) | −2X | |
| P184 | 16(R) | 16(R) | − | 8(R) | −2X | |
| Ciprofloxacin | P1 | 16(R) | 16(R) | − | 16(R) | − |
| P2 | 16(R) | 32(R) | 2X | 64(R) | 4X | |
| P5 | 32(R) | 64(R) | 2X | 64(R) | 2X | |
| P6 | 32(R) | 64(R) | 2X | 32(R) | − | |
| P7 | 4(R) | 8(R) | 2X | 8(R) | 2X | |
| P8 | 64(R) | 64(R) | − | 64(R) | − | |
| P9 | 64(R) | 64(R) | − | 64(R) | − | |
| P10 | 16(R) | 8(R) | −2X | 32 (R) | 2X | |
| P99 | 8(R) | 8(R) | − | 8(R) | − | |
| P184 | 64(R) | 32(R) | −2X | 64(R) | − | |
| Amikacin | P1 | 4(S) | 4(S) | − | 0.5(S) | −8X |
| P2 | 8(S) | 8(S) | − | 2(S) | −4X | |
| P5 | 8(S) | 16(S) | 2X | 1(S) | −8X | |
| P6 | 8(S) | 16(S) | 2X | 2(S) | −4X | |
| P7 | 4(S) | 8(S) | 2X | 0.5(S) | −8X | |
| P8 | 8(S) | 32(I) | 4X | 0.5(S) | −16X | |
| P9 | 16(S) | 16(S) | − | 0.5(S) | −32X | |
| P10 | 4(S) | 4(S) | − | 4(S) | − | |
| P99 | 4(S) | 16(S) | 4X | 1(S) | −4X | |
| P184 | 8(S) | 32(S) | 4X | 0.5(S) | −16X |
| Cbg70 | |||
| * Rt (min) | Peak No. | Mass (m/z) Fragmentation | Indole Derivative |
| 2.87 | 13 | 205[M+H] + (205,159,144,143,130,117,115) | Tryptophan |
| 8.01 | 30 | 176[M+H] + (176,130, 103) | Indole-3-acetic acid (IAA) |
| 8.01 | 33 | 162[M+H] + (162,144,143,117,115) | Indole-3- ethanol (TOL) |
| 8.3 | 33 | 146[M+H] + (146,118,117,91) | Indole-3-aldehyde |
| 11.2 | 45 | 205[M+2H] + (205,142, 139,117) | Indole-3-pyruvic acid |
| 11.88 | 48 | 198[M+K] + (198,133,118) | Indole-3-acetaldehyde (IAld) |
| 1.07 | 3 | 188[M-H]− (188,128, 59) | Indole-3-propionic acid |
| 4.62 | 17 | 158[M-H]− (158,130, 117) | Indole-3- acetaldehyde |
| 6.76 | 28 | 174[M-H]− (174,130, 128) | Indole-3-acetic acid (IAA) |
| 7.43 | 31 | 160[M-H]− (160,130) | Indole-3-ethanol (TOL) |
| 7.77 | 31 | 173[M-H]− (174,131, 130, 111) | Indole-3-acetamide |
| Zch127 | |||
| Rt (min) | Peak No. | Mass (m/z) Fragmentation | Indole Derivative |
| 1.31 | 10 | 132[M+H] + (132,86, 69) | 3-methyl indole (skatole) |
| 1.98 | 7 | 162[M+H] + (162,144,120) | Indole-3-carboxylic acid (I3CA) |
| 6.66 | 19 | 176[M+H] + (176,130, 118, 103, 77) | Indole-3-acetic acid (IAA) |
| 6.93 | 19 | 245[M+K] + (245,174, 173, 130) | Indole-3-lactic acid (ILA) |
| 8.01 | 26 | 162[M+H] + (162,144,143,117,115) | Indole-3-ethanol (TOL) |
| 9.04 | 37 | 205[M+2H] + (205,158,130,87,72) | Indole-3-pyruvic acid |
| 13.1 | 76 | 199[M+H+K] + (199,133,117) | Indole-3- acetaldehyde |
| 14.46 | 86 | 196[M+H+K] + (196,131,117, 79) | Indole-3-acetonitrile |
| 2.84 | 9 | 203[M-H]− (203, 158, 146, 142, 132,118, 116) | Tryptophan |
| 6.27 | 18 | 173[M-H]− (173, 143, 130) | Indole-3-acetamide (IAM) |
| 8.72 | 50 | 202[M-H]- (202, 158, 130) | Indole-3-pyruvic acid |
| 16.21 | 97 | 130[M-H]- (130, 115.4) | 3-methyl indole (skatole) |
| Compound | Rt | Cbg70 2 days (µM) | Cbg70 4 days (µM) | Zch127 2 days (µM) | Zch127 4 days (µM) |
|---|---|---|---|---|---|
| Tryptophan | 4.040 | 44.226 | 36.648 | 40.798 | 9.866 |
| IAM | 8.221 | 22.800 | 25.120 | 19.120 | 22.880 |
| ILA | 11.984 | 21.465 | 24.148 | 24.045 | 10.939 |
| IAA | 18.005 | 99.915 | 199.680 | 117.241 | 321.278 |
| IAN | 24.830 | 0 | 0 | 4.407 | 19.729 |
| IPA | 36.380 | 19.428 | 24.390 | 24.978 | 7.232 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amer, M.A.; Wasfi, R.; Attia, A.S.; Ramadan, M.A. Indole Derivatives Obtained from Egyptian Enterobacter sp. Soil Isolates Exhibit Antivirulence Activities against Uropathogenic Proteus mirabilis. Antibiotics 2021, 10, 363. https://doi.org/10.3390/antibiotics10040363
Amer MA, Wasfi R, Attia AS, Ramadan MA. Indole Derivatives Obtained from Egyptian Enterobacter sp. Soil Isolates Exhibit Antivirulence Activities against Uropathogenic Proteus mirabilis. Antibiotics. 2021; 10(4):363. https://doi.org/10.3390/antibiotics10040363
Chicago/Turabian StyleAmer, Mai A., Reham Wasfi, Ahmed S. Attia, and Mohamed A. Ramadan. 2021. "Indole Derivatives Obtained from Egyptian Enterobacter sp. Soil Isolates Exhibit Antivirulence Activities against Uropathogenic Proteus mirabilis" Antibiotics 10, no. 4: 363. https://doi.org/10.3390/antibiotics10040363
APA StyleAmer, M. A., Wasfi, R., Attia, A. S., & Ramadan, M. A. (2021). Indole Derivatives Obtained from Egyptian Enterobacter sp. Soil Isolates Exhibit Antivirulence Activities against Uropathogenic Proteus mirabilis. Antibiotics, 10(4), 363. https://doi.org/10.3390/antibiotics10040363







