Association between Antimicrobial Peptide Histatin 5 Levels and Prevalence of Candida in Saliva of Patients with Down Syndrome
Abstract
1. Introduction
2. Results
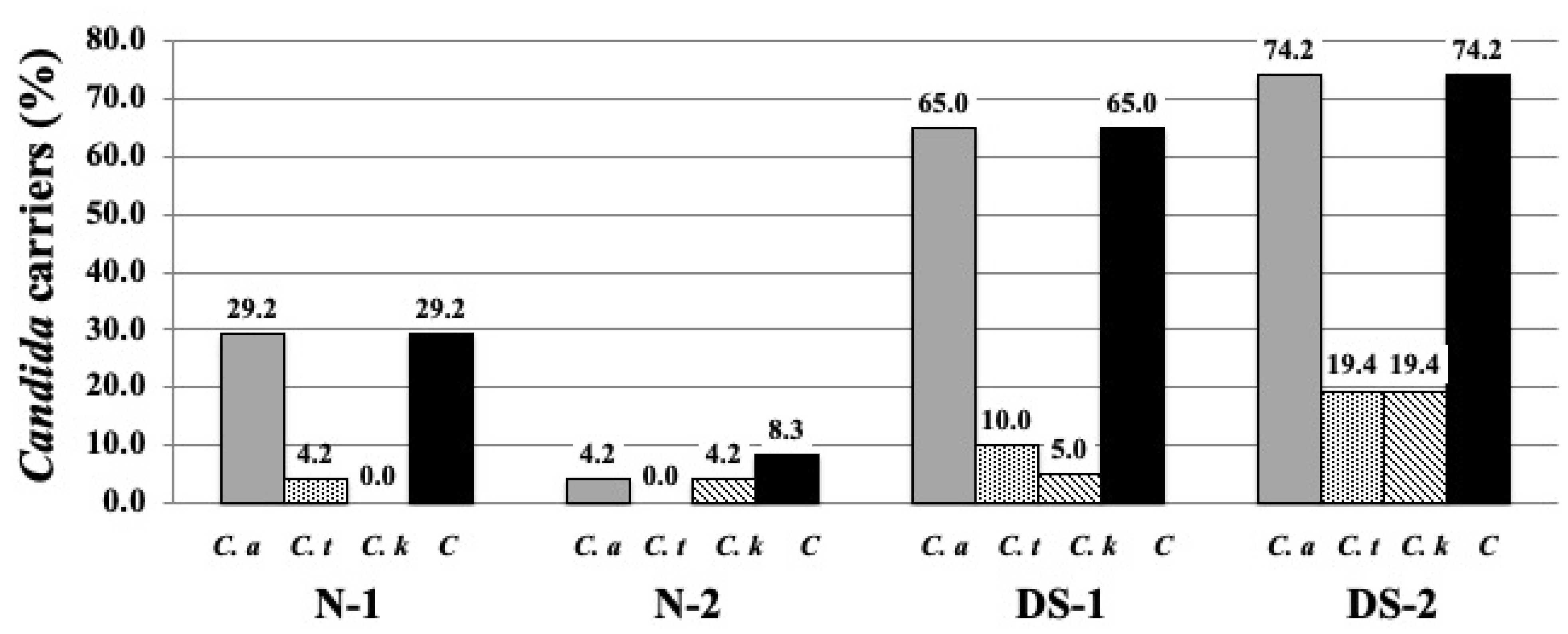
2.1. Prevalence of Candida Species Colonization on the DS and N Groups
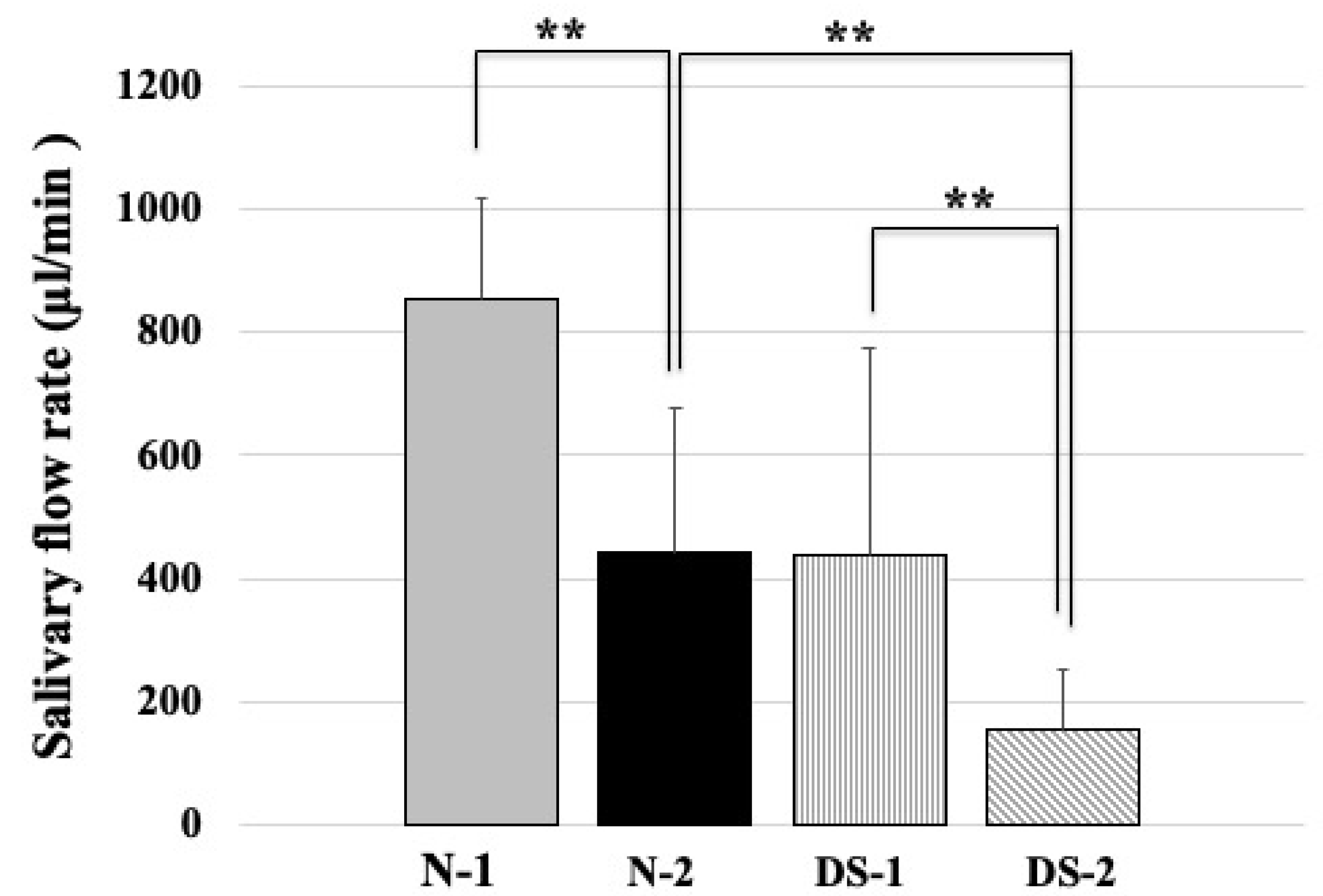
2.2. Comparison of Salivary Flow Rates between the DS and N Groups
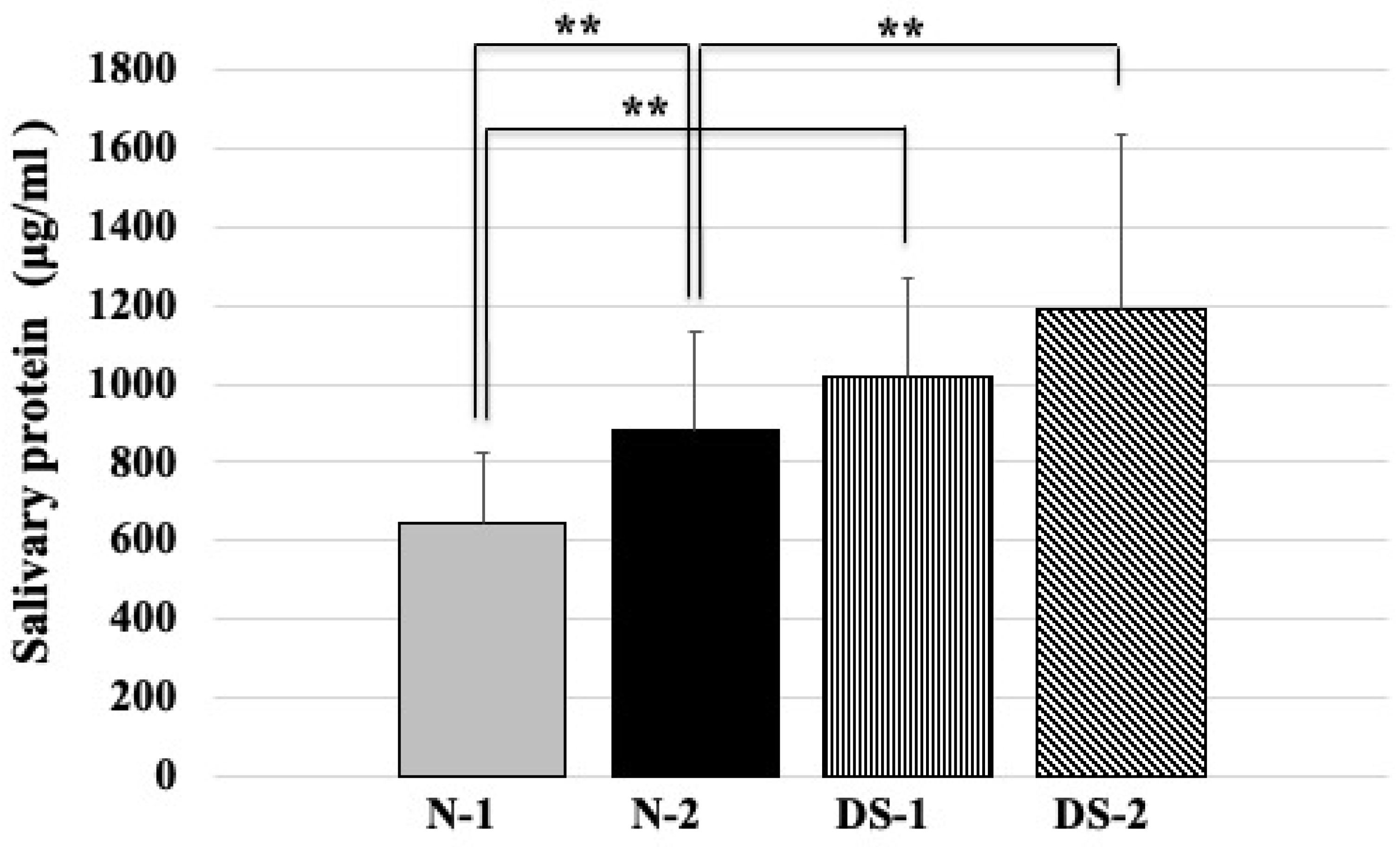
2.3. Comparison of Total Salivary Protein Concentrations between the DS and N Groups
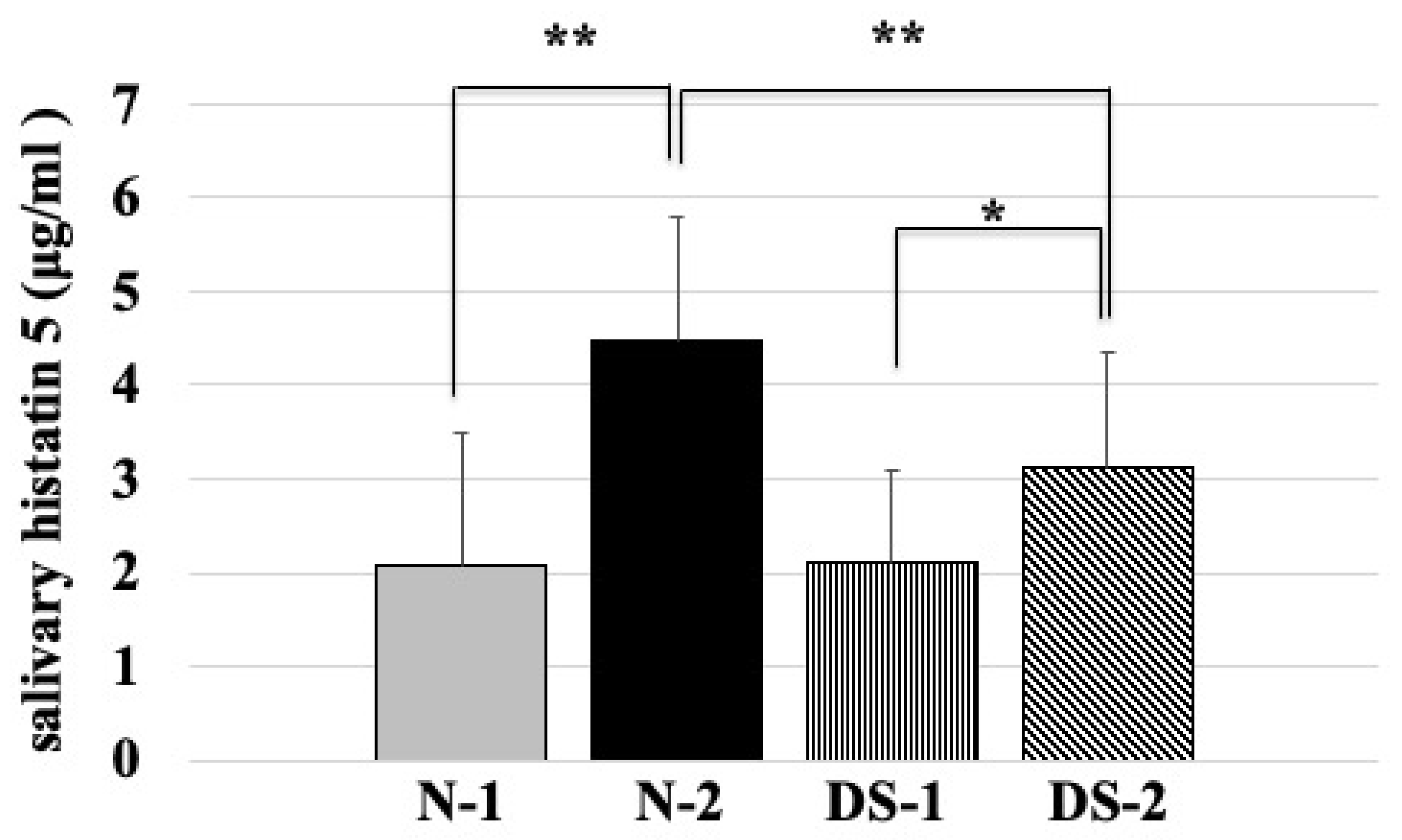
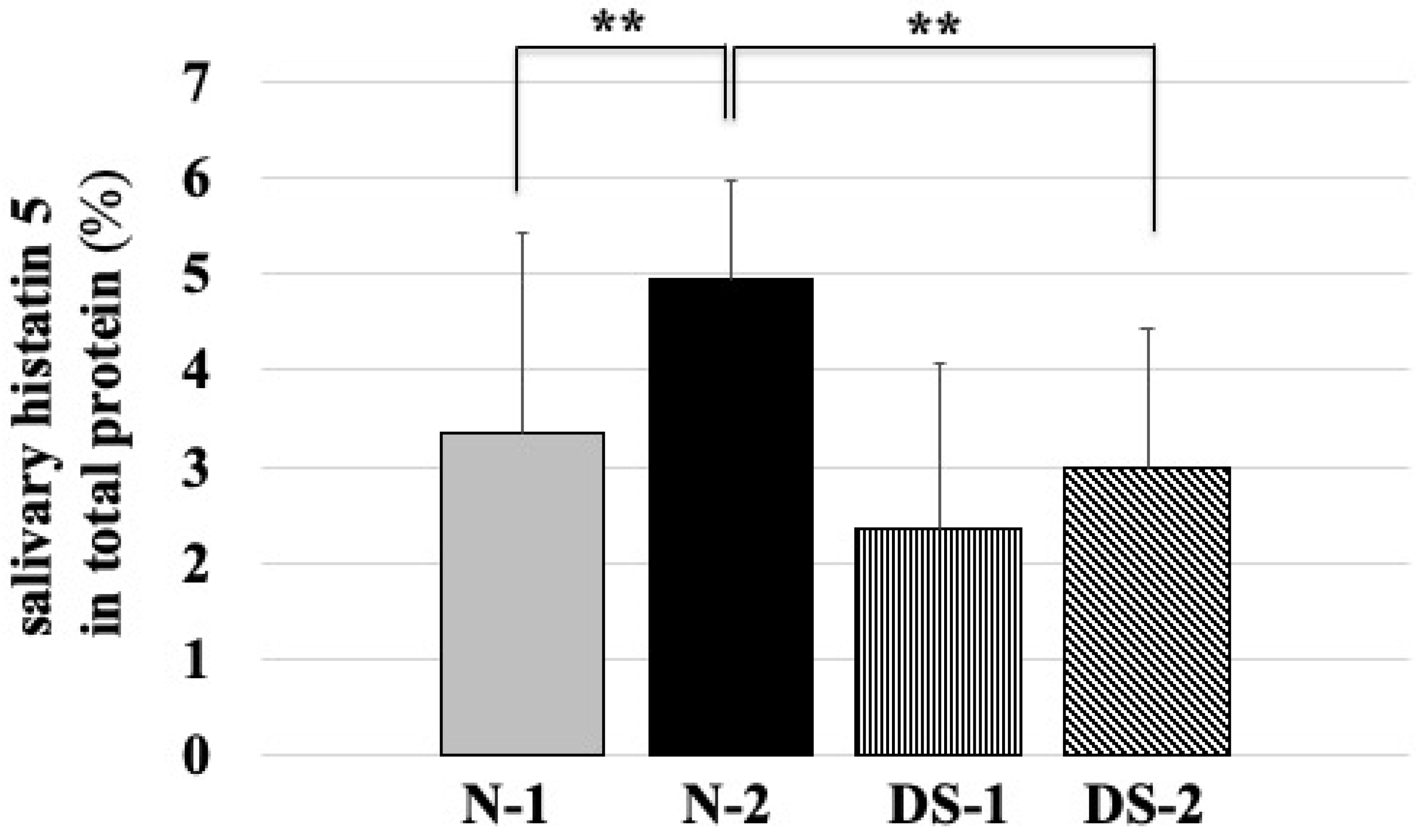
2.4. Comparison of Salivary Histatin 5 Concentrations between the DS and N Groups
2.5. Correlation with Histatin 5 and Total Salivary Protein Concentration
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Saliva Collection and Measurement of the Salivary Flow Rate
4.3. Measurement of Salivary Antimicrobial Peptide
4.4. Isolation and Identification of Candida Species
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lejeune, J.; Turpin, R.; Gautier, M. Mongolism; a chromosomal disease (trisomy). Bull. Acad. Natl. Med. 1959, 143, 256–265. [Google Scholar]
- Modeer, T.; Barr, M.; Dahllof, G. Periodontal disease in children with down’s syndrome. Scand. J. Dent. Res. 1990, 98, 228–234. [Google Scholar] [CrossRef]
- Pueschel, S.M.; Anneren, G.; Durlach, R.; Flores, J.; Sustrova, M.; Verma, I.C. Guidelines for optimal medical care of persons with down syndrome. International league of societies for persons with mental handicap (ilsmh). Acta Paediatr. 1995, 84, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Burgio, G.R.; Ugazio, A.G.; Nespoli, L.; Marcioni, A.F.; Bottelli, A.M.; Pasquali, F. Derangements of immunoglobulin levels, phytohemagglutinin responsiveness and t and b cell markers in down’s syndrome at different ages. Eur. J. Immunol. 1975, 5, 600–603. [Google Scholar] [CrossRef]
- Akpan, A.; Morgan, R. Oral candidiasis. Postgrad. Med. J. 2002, 78, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Berdicevsky, I.; Ben-Aryeh, H.; Szargel, R.; Gutman, D. Oral candida of asymptomatic denture wearers. Int. J. Oral Surg. 1980, 9, 113–115. [Google Scholar] [CrossRef]
- Arendorf, T.M.; Walker, D.M. The prevalence and intra-oral distribution of candida albicans in man. Arch. Oral Biol. 1980, 25, 1–10. [Google Scholar] [CrossRef]
- Marcotte, H.; Lavoie, M.C. Oral microbial ecology and the role of salivary immunoglobulin a. Microbiol. Mol. Biol. Rev. 1998, 62, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, F.G.; Xu, T.; McMillian, F.M.; Levitz, S.M.; Diamond, R.D.; Offner, G.D.; Troxler, R.F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on candida albicans. J. Biol. Chem. 1988, 263, 7472–7477. [Google Scholar] [CrossRef]
- Helmerhorst, E.J.; Reijnders, I.M.; van’t Hof, W.; Simoons-Smit, I.; Veerman, E.C.; Amerongen, A.V. Amphotericin b- and fluconazole-resistant candida spp., aspergillus fumigatus, and other newly emerging pathogenic fungi are susceptible to basic antifungal peptides. Antimicrob. Agents Chemother. 1999, 43, 702–704. [Google Scholar] [CrossRef]
- Carlstedt, K.; Krekmanova, L.; Dahllof, G.; Ericsson, B.; Braathen, G.; Modeer, T. Oral carriage of candida species in children and adolescents with down’s syndrome. Int. J. Paediatr. Dent. 1996, 6, 95–100. [Google Scholar] [CrossRef]
- Sonesson, M.; Eliasson, L.; Matsson, L. Minor salivary gland secretion in children and adults. Arch. Oral Biol. 2003, 48, 535–539. [Google Scholar] [CrossRef]
- Mohiddin, G.; Narayanaswamy, A.B.; Masthan, K.M.; Nagarajan, A.; Panda, A.; Behura, S.S. Oral candidal and streptococcal carriage in down syndrome patients. J. Nat. Sci. Biol. Med. 2015, 6, 300–305. [Google Scholar]
- Dudko, A.; Kurnatowska, A.J.; Kurnatowski, P. Prevalence of fungi in cases of geographical and fissured tongue. Ann. Parasitol. 2013, 59, 113–117. [Google Scholar]
- Makinen, K.K. Studies on oral enzymes. I. Fractionation and characterization of aminopeptidases of human saliva. Acta Odontol. Scand. 1966, 24, 579–604. [Google Scholar] [CrossRef]
- Faria Carrada, C.; Almeida Ribeiro Scalioni, F.; Evangelista Cesar, D.; Lopes Devito, K.; Ribeiro, L.C.; Almeida Ribeiro, R. Salivary periodontopathic bacteria in children and adolescents with down syndrome. PLoS ONE 2016, 11, e0162988. [Google Scholar] [CrossRef]
- Helmerhorst, E.J.; Oppenheim, F.G. Saliva: A dynamic proteome. J. Dent. Res. 2007, 86, 680–693. [Google Scholar] [CrossRef]
- Cabras, T.; Pisano, E.; Montaldo, C.; Giuca, M.R.; Iavarone, F.; Zampino, G.; Castagnola, M.; Messana, I. Significant modifications of the salivary proteome potentially associated with complications of down syndrome revealed by top-down proteomics. Mol. Cell Proteom. 2013, 12, 1844–1852. [Google Scholar] [CrossRef]
- Odeh, M.; Hershkovits, M.; Bornstein, J.; Loberant, N.; Blumenthal, M.; Ophir, E. Congenital absence of salivary glands in down syndrome. Arch. Dis. Child. 2013, 98, 781–783. [Google Scholar] [CrossRef]
- Khan, S.A.; Fidel, P.L., Jr.; Thunayyan, A.A.; Varlotta, S.; Meiller, T.F.; Jabra-Rizk, M.A. Impaired histatin-5 levels and salivary antimicrobial activity against c. Albicans in hiv infected individuals. J. AIDS Clin. Res. 2013, 4. [Google Scholar] [CrossRef]
- Chaushu, S.; Chaushu, G.; Zigmond, M.; Yefenof, E.; Stabholz, A.; Shapira, J.; Merrick, J.; Bachrach, G. Age-dependent deficiency in saliva and salivary antibodies secretion in down’s syndrome. Arch. Oral Biol. 2007, 52, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Chaushu, S.; Yefenof, E.; Becker, A.; Shapira, J.; Chaushu, G. Severe impairment of secretory ig production in parotid saliva of down syndrome individuals. J. Dent. Res. 2002, 81, 308–312. [Google Scholar] [CrossRef]
- Campese, M.; Sun, X.; Bosch, J.A.; Oppenheim, F.G.; Helmerhorst, E.J. Concentration and fate of histatins and acidic proline-rich proteins in the oral environment. Arch. Oral Biol. 2009, 54, 345–353. [Google Scholar] [CrossRef]
- Davidovich, E.; Aframian, D.J.; Shapira, J.; Peretz, B. A comparison of the sialochemistry, oral ph, and oral health status of down syndrome children to healthy children. Int. J. Paediatr. Dent. 2010, 20, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Vylkova, S.; Li, X.S.; Edgerton, M. Calcium blocks fungicidal activity of human salivary histatin 5 through disruption of binding with candida albicans. J. Dent. Res. 2003, 82, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.T.; Ferreira, M.C.; Mendes, F.M.; de Oliveira Guare, R. Assessing salivary osmolality as a caries risk indicator in cerebral palsy children. Int. J. Paediatr. Dent. 2014, 24, 84–89. [Google Scholar] [CrossRef]
- Schiff, E.; Mogilner, J.G.; Sella, E.; Doweck, I.; Hershko, O.; Ben-Arye, E.; Yarom, N. Hypnosis for postradiation xerostomia in head and neck cancer patients: A pilot study. J. Pain Symptom Manag. 2009, 37, 1086–1092 e1081. [Google Scholar] [CrossRef]
- Gornowicz, A.; Tokajuk, G.; Bielawska, A.; Maciorkowska, E.; Jablonski, R.; Wojcicka, A.; Bielawski, K. The assessment of siga, histatin-5, and lactoperoxidase levels in saliva of adolescents with dental caries. Med. Sci. Monit. 2014, 20, 1095–1100. [Google Scholar]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Cary, S.G.; Blair, E.B. New transport medium for shipment of clinical specimens. I. Fecal specimens. J. Bacteriol. 1964, 88, 96–98. [Google Scholar] [CrossRef]
- Tan, G.L.; Peterson, E.M. Chromagar candida medium for direct susceptibility testing of yeast from blood cultures. J. Clin. Microbiol. 2005, 43, 1727–1731. [Google Scholar] [CrossRef] [PubMed]

| Under 20 Years Old | Over 40 Years Old | |||
|---|---|---|---|---|
| N-1 | DS-1 | N-2 | DS-2 | |
| Male | 12 | 12 | 7 | 22 |
| Female | 12 | 8 | 17 | 9 |
| Total | 24 | 20 | 24 | 31 |
| Average of age (SD) | 8.5 ± 2.0 | 11.3 ± 4.2 | 47.1 ± 4.9 | 48.9 ± 6.5 |
| Study Group | Correlation Coefficient |
|---|---|
| N-1 | 0.08134 |
| N-2 | 0.77589 ** |
| DS-1 | 0.40228 |
| DS-2 | 0.14843 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komatsu, T.; Watanabe, K.; Hamada, N.; Helmerhorst, E.; Oppenheim, F.; Lee, M.C.-i. Association between Antimicrobial Peptide Histatin 5 Levels and Prevalence of Candida in Saliva of Patients with Down Syndrome. Antibiotics 2021, 10, 494. https://doi.org/10.3390/antibiotics10050494
Komatsu T, Watanabe K, Hamada N, Helmerhorst E, Oppenheim F, Lee MC-i. Association between Antimicrobial Peptide Histatin 5 Levels and Prevalence of Candida in Saliva of Patients with Down Syndrome. Antibiotics. 2021; 10(5):494. https://doi.org/10.3390/antibiotics10050494
Chicago/Turabian StyleKomatsu, Tomoko, Kiyoko Watanabe, Nobushiro Hamada, Eva Helmerhorst, Frank Oppenheim, and Masaichi Chang-il Lee. 2021. "Association between Antimicrobial Peptide Histatin 5 Levels and Prevalence of Candida in Saliva of Patients with Down Syndrome" Antibiotics 10, no. 5: 494. https://doi.org/10.3390/antibiotics10050494
APA StyleKomatsu, T., Watanabe, K., Hamada, N., Helmerhorst, E., Oppenheim, F., & Lee, M. C.-i. (2021). Association between Antimicrobial Peptide Histatin 5 Levels and Prevalence of Candida in Saliva of Patients with Down Syndrome. Antibiotics, 10(5), 494. https://doi.org/10.3390/antibiotics10050494








