The Use of Colistin in Food-Producing Animals in Estonia—Vaccination as an Effective Alternative to Consumption of Critically Important Antimicrobials in Pigs
Abstract
:1. Introduction
2. Results
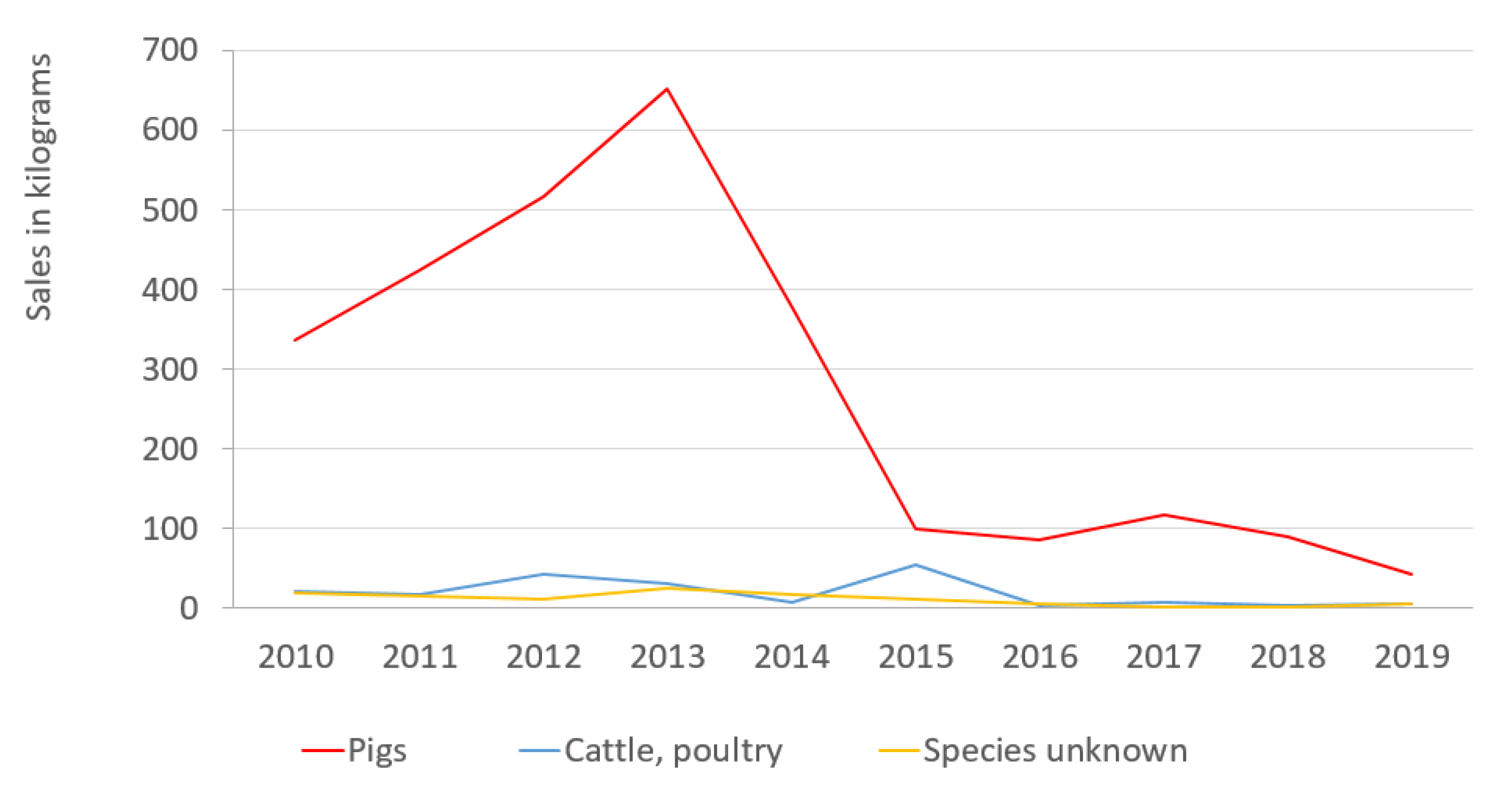
2.1. The Sales of Colistin by Animal Species in 2010–2019
2.2. Association between Vaccination of Piglets Against E. coli and Consumption of Colistin in Pigs
3. Discussion
4. Materials and Methods
4.1. Collection of Sales Data
4.2. Collection of Data Allowing Differentiation of Target Species
4.3. Calculation of Colistin Consumption Data
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scott, H.M.; Acuff, G.; Bergeron, G.; Bourassa, M.W.; Gill, J.; Graham, D.W.; Kahn, L.H.; Morley, P.S.; Salois, M.J.; Simjee, S.; et al. Critically important antibiotics: Criteria and approaches for measuring and reducing their use in food animal agriculture. Ann. N. Y. Acad. Sci. 2019, 1441, 8–16. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Critically Important Antimicrobials for Human Medicine. 2018. Available online: https://apps.who.int/iris/bitstream/handle/10665/312266/9789241515528-eng.pdf (accessed on 3 August 2020).
- European Medicines Agency, Antimicrobial Advice ad hoc Expert Group (EMA/AMEG). Categorisation of Antibiotics Used in Animals Promotes Responsible Use to Protect Public and Animal Health. (EMA/688114/2020). Available online: https://www.ema.europa.eu/en/documents/report/categorisation-antibiotics-european-union-answer-request-european-commission-updating-scientific_en.pdf (accessed on 15 February 2020).
- Koyama, Y.; Kurosasa, A.; Tsuchiya, A.; Takakuta, K. A new antibiotic colist∈ produced by spore-forming soil bacteria. J. Antibiot. 1950, 3, 457–458. [Google Scholar]
- Biswas, S.; Brunel, J.-M.; Dubus, J.-C.; Reynaud-Gaubert, M.; Rolain, J.-M. Colistin: An update on the antibiotic of the 21st century. Expert Rev. Anti-Infect. Ther. 2012, 10, 917–934. [Google Scholar] [CrossRef]
- Catry, B.; Cavaleri, M.; Baptiste, K.; Grave, K.; Grein, K.; Holm, A.; Jukes, H.; Liebana, E.; Navas, A.L.; Mackay, D.; et al. Use of colistin-containing products within the European Union and European Economic Area (EU/EEA): Development of resistance in animals and possible impact on human and animal health. Int. J. Antimicrob. Agents 2015, 46, 297–306. [Google Scholar] [CrossRef]
- Callens, B.; Persoons, D.; Maes, D.; Laanen, M.; Postma, M.; Boyen, F.; Haesebrouck, F.; Butaye, P.; Catry, B.; Dewulf, J. Prophylactic and metaphylactic antimicrobial use in Belgian fattening pig herds. Prev. Veter. Med. 2012, 106, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Rhouma, M.; Beaudry, F.; Thériault, W.; Letellier, A. Colistin in Pig Production: Chemistry, Mechanism of Antibacterial Action, Microbial Resistance Emergence, and One Health Perspectives. Front. Microbiol. 2016, 7, 1789. [Google Scholar] [CrossRef]
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Veter. Scand. 2017, 59, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmerman, T.; Dewulf, J.; Catry, B.; Feyen, B.; Opsomer, G.; de Kruif, A.; Maes, D. Quantification and evaluation of antimicrobial drug use in group treatments for fattening pigs in Belgium. Prev. Veter.-Med. 2006, 74, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Rhouma, M.; Beaudry, F.; Thériault, W.; Bergeron, N.; Beauchamp, G.; Laurent-Lewandowski, S.; Fairbrother, J.M.; Letellier, A. In vivo therapeutic efficacy and pharmacokinetics of colistin sulfate in an experimental model of enterotoxigenic Escherichia coli infection in weaned pigs. Veter. Res. 2016, 47, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fairbrother, J.M.; Nadeau, É.; Gyles, C.L. Escherichia coliin postweaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 2005, 6, 17–39. [Google Scholar] [CrossRef] [Green Version]
- Koch-Weser, J.; Sidel, V.W.; Federman, E.B.; Kanarek, P.; Finer, D.C.; Eaton, A.E. Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy. Ann. Int. Med. 1970, 72, 857–868. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Rhouma, M.; Beaudry, F.; Letellier, A. Resistance to colistin: What is the fate for this antibiotic in pig production? Int. J. Antimicrob. Agents 2016, 48, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Van Dorp, L.; Shaw, L.P.; Bradley, P.; Wang, Q.; Wang, X.; Jin, L.; Zhang, Q.; Liu, Y.; Rieux, A.; et al. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brauer, A.; Telling, K.; Laht, M.; Kalmus, P.; Lutsar, I.; Remm, M.; Kisand, V.; Tenson, T. Plasmid with Colistin Resistance Genemcr-1in Extended-Spectrum-β-Lactamase-Producing Escherichia coli Strains Isolated from Pig Slurry in Estonia. Antimicrob. Agents Chemother. 2016, 60, 6933–6936. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency (EMA). Updated Advice on the Use of Colistin Products in Animals within the European Union: Development of Resistance and Possible Impact on Human and Animal Health. (EMA/CVMP/CHMP/231573/2016). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/updated-advice-use-colistin-products-animals-within-european-union-development-resistance-possible_en-0.pdf (accessed on 15 May 2020).
- Li, J.; Nation, R.L.; Turnidge, J.D.; Milne, R.W.; Coulthard, K.; Rayner, C.R.; Paterson, D.L. Colistin: The re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 2006, 6, 589–601. [Google Scholar] [CrossRef]
- Andrade, F.F.; Silva, D.; Rodrigues, A.; Pina-Vaz, C. Colistin Update on Its Mechanism of Action and Resistance, Present and Future Challenges. Microorganisms 2020, 8, 1716. [Google Scholar] [CrossRef] [PubMed]
- Dorado-García, A.; Mevius, D.J.; Jacobs, J.J.H.; Van Geijlswijk, I.M.; Mouton, J.W.; Wagenaar, J.A.; Heederik, D.J. Quantitative assessment of antimicrobial resistance in livestock during the course of a nationwide antimicrobial use reduction in the Netherlands. J. Antimicrob. Chemother. 2016, 71, 3607–3619. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xu, C.; Zhang, R.; Chen, Y.; Shen, Y.; Hu, F.; Liu, D.; Lu, J.; Guo, Y.; Xia, X.; et al. Changes in colistin resistance and mcr-1 abundance in Escherichia coli of animal and human origins following the ban of colistin-positive additives in China: An epidemiological comparative study. Lancet Infect. Dis. 2020, 20, 1161–1171. [Google Scholar] [CrossRef]
- EUR-Lex. Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition. Available online: http://data.europa.eu/eli/reg/2003/1831/oj (accessed on 25 February 2020).
- Peterson, L. Squeezing the antibiotic balloon: The impact of antimicrobial classes on emerging resistance. Clin. Microbiol. Infect. 2005, 11, 4–16. [Google Scholar] [CrossRef] [Green Version]
- Jensen, L.M. The ‘Balloon Effect’—Intervention Triggers Shift between Antimicrobial Classes. How Interventions on One Substance Effect the Use of Other Substances—For Gastrointestinal Disorders in Weaner Pigs. Presented at the Acting Second International Conference „Quantification, Benchmarking and Stewardship of Veterinary Antimicrobial Usage“, Bern, Switzerland, July 2019; Available online: https://aacting.org/swfiles/files/Jensen_AACTING_Bern_60.pdf (accessed on 15 December 2020).
- Allen, H.K.; Levine, U.Y.; Looft, T.; Bandrick, M.; Casey, T.A. Treatment, promotion, commotion: Antibiotic alternatives in food-producing animals. Trends Microbiol. 2013, 21, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Postma, M.; Stärk, K.D.; Sjölund, M.; Backhans, A.; Beilage, E.G.; Lösken, S.; Belloc, C.; Collineau, L.; Iten, D.; Visschers, V.; et al. Alternatives to the use of antimicrobial agents in pig production: A multi-country expert-ranking of perceived effectiveness, feasibility and return on investment. Prev. Veter. Med. 2015, 118, 457–466. [Google Scholar] [CrossRef]
- Kruse, A.B.; Nielsen, L.R.; Alban, L. Vaccination against Actinobacillus pleuropneumoniae as an alternative strategy to antimicrobial use in Danish pig herds. In Proceedings of the Safepork 2015 Proceedings, Epidemiology and Control of Hazards in Pork Production Chain—SAFEPORK, One Health Approach under a Concept of Farm to Fork, Porto, Portugal, 7–10 September 2015; pp. 357–359. [Google Scholar] [CrossRef]
- Bak, H.; Rathkjen, P.H. Reduced use of antimicrobials after vaccination of pigs against porcine proliferative enteropathy in a Danish SPF herd. Acta Veter. Scand. 2009, 51, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Van Dommelen, I.; Wertenbroek, N. Reduction of antibiotics after implementing PCV2 vaccination on 460 sow Dutch pigfarm. In Proceedings of the 114th International Conference on the Epidemiology and Control of Biological, Chemical and Physical Hazards in Pigs and Pork, Maastricht, The Netherlands, 19–22 June 2011; pp. 336–338. [Google Scholar] [CrossRef]
- Raith, J.; Trauffler, M.; Firth, C.L.; Lebl, K.; Schleicher, C.; Köfer, J. Influence of porcine circovirus type 2 vaccination on the level of antimicrobial consumption on 65 Austrian pig farms. Veter. Rec. 2016, 178, 504. [Google Scholar] [CrossRef] [PubMed]
- Van Looveren, F.; De Jonghe, E.; Maass, P.; De Backer, P. Reduction of antibiotic use after implementation of Ingelvac® PRRS MLV piglet vaccination in a Belgian wean to finish farm. In Proceedings of the Safepork 2015 Proceedings, Epidemiology and Control of Hazards in Pork Production Chain—SAFEPORK, One Health Approach under a Concept of Farm to Fork, Porto, Portugal, 7–10 September 2015; pp. 353–355. [Google Scholar] [CrossRef]
- Raasch, S.; on the behalf of the MINAPIG Consortium; Collineau, L.; Postma, M.; Backhans, A.; Sjölund, M.; Belloc, C.; Emanuelson, U.; Beilage, E.G.; Stärk, K.; et al. Effectiveness of alternative measures to reduce antimicrobial usage in pig production in four European countries. Porc. Health Manag. 2020, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aasmäe, B.; Kalmus, P.; Onoper, A.; Lehtla, A.; Häkkinen, L.; Birkenfeldt, M. Soovitused antibiootikumide mõistlikuks kasutamiseks eri loomaliikide bakteriaalsete infektsioonide ravis. Eesti Loomaarstlik Ringvaade 2012, 3, 18–24. [Google Scholar]
- Ministry of Rural Affairs. Juhend Antibiootikumide Kasutamiseks Põllumajandusloomadel. Clinical Guideline. 2020. Available online: https://www.agri.ee/sites/default/files/content/valjaanded/juhend-2020-antibiootikumiravi-loomad.pdf (accessed on 15 November 2020).
- Statistics Estonia. PM1720: Births of Calves and Piglets Per Year. Available online: https://andmed.stat.ee/en/stat/majandus__pellumajandus__pellumajandussaaduste-tootmine__loomakasvatussaaduste-tootmine/PM1720 (accessed on 17 December 2020).
- European Surveillance of Veterinary Antimicrobial Consumption (ESVAC). Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2018 (EMA/24309/2020). Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2018-trends-2010-2018-tenth-esvac-report_en.pdf (accessed on 25 October 2020).
- Stege, H.; Bager, F.; Jacobsen, E.; Thougaard, A. VETSTAT—the Danish system for surveillance of the veterinary use of drugs for production animals. Prev. Veter. Med. 2003, 57, 105–115. [Google Scholar] [CrossRef]
- Dupont, N.; Diness, L.H.; Fertner, M.; Kristensen, C.S.; Stege, H. Antimicrobial reduction measures applied in Danish pig herds following the introduction of the “Yellow Card” antimicrobial scheme. Prev. Veter. Med. 2017, 138, 9–16. [Google Scholar] [CrossRef] [PubMed]
- European Surveillance of Veterinary Antimicrobial Consumption (ESVAC). Trends by Country. Country Reports on Sales Trends 2010–2018. Available online: https://www.ema.europa.eu/en/veterinary-regulatory/overview/antimicrobial-resistance/european-surveillance-veterinary-antimicrobial-consumption-esvac#trends-by-country-section (accessed on 25 October 2020).
- Temtem, C.; Kruse, A.B.; Nielsen, L.R.; Pedersen, K.S.; Alban, L. Comparison of the antimicrobial consumption in weaning pigs in Danish sow herds with different vaccine purchase patterns during 2013. Porc. Health Manag. 2016, 2, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurmoja, I.; Petrov, A.; Breidenstein, C.; Zani, L.; Forth, J.H.; Beer, M.; Kristian, M.; Viltrop, A.; Blome, S. Biological characterization of African swine fever virus genotype II strains from north-eastern Estonia in European wild boar. Transbound. Emerg. Dis. 2017, 64, 2034–2041. [Google Scholar] [CrossRef]
- Agricultural Registers and Information Board. Available online: https://www.pria.ee/en/registers (accessed on 10 January 2021).
- Collineau, L.; Rojo-Gimeno, C.; Léger, A.; Backhans, A.; Loesken, S.; Nielsen, E.; Postma, M.; Emanuelson, U.; Beilage, E.; Sjölund, M.; et al. Herd-specific interventions to reduce antimicrobial usage in pig production without jeopardising technical and economic performance. Prev. Veter. Med. 2017, 144, 167–178. [Google Scholar] [CrossRef]
- Dyar, O.J.; Huttner, B.; Schouten, J.; Pulcini, C.; ESGAP (ESCMID Study Group for Antimicrobial stewardship). What is antimicrobial stewardship? Clin. Microbiol. Infect. 2017, 23, 793–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, P.; Vanderhaeghen, W.; Fertner, M.; Fuchs, K.; Obritzhauser, W.; Agunos, A.; Carson, C.; Høg, B.B.; Andersen, V.D.; Chauvin, C.; et al. Monitoring of Farm-Level Antimicrobial Use to Guide Stewardship: Overview of Existing Systems and Analysis of Key Components and Processes. Front. Veter.-Sci. 2020, 7, 540. [Google Scholar] [CrossRef]
- Sjölund, M.; Postma, M.; Collineau, L.; Lösken, S.; Backhans, A.; Belloc, C.; Emanuelson, U.; Beilage, E.; Stärk, K.; Dewulf, J. Quantitative and qualitative antimicrobial usage patterns in farrow-to-finish pig herds in Belgium, France, Germany and Sweden. Prev. Veter. Med. 2016, 130, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, S.; Joosten, P.; Van Gompel, L.; Luiken, R.E.C.; Mevius, D.J.; Wagenaar, J.A.; Heederik, D.J.J.; Dewulf, J.; Wagenaar, J.; Graveland, H.; et al. Quantitative and qualitative analysis of antimicrobial usage patterns in 180 selected farrow-to-finish pig farms from nine European countries based on single batch and purchase data. J. Antimicrob. Chemother. 2019, 74, 807–816. [Google Scholar] [CrossRef]
- Van Rennings, L.; Von Münchhausen, C.; Ottilie, H.; Hartmann, M.; Merle, R.; Honscha, W.; Käsbohrer, A.; Kreienbrock, L. Cross-Sectional Study on Antibiotic Usage in Pigs in Germany. PLoS ONE 2015, 10, e0119114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Centre for Disease Prevention and Control (ECDC); European Food Safety Authority (EFSA); European Medicines Agency (EMA). ECDC, EFSA and EMA Joint Scientific Opinion on a List of Outcome Indicators as Regards Surveillance of Antimicrobial Resistance and Antimicrobial Consumption in Humans and Food-Producing Animals. EFSA J. 2017, 15, e05017. [Google Scholar]
- State Agency of Medicines. Statistics on Veterinary Medicines. Available online: https://ravimiamet.ee/en/statistics-veterinary-medicines (accessed on 14 June 2020).
- WHO Collaborating Centre for Drug Statistics Methodology. ATCvet Classification. Available online: https://www.whocc.no/atcvet/atcvet_index/ (accessed on 25 October 2020).
- European Surveillance of Veterinary Antimicrobial Consumption (ESVAC). Trends in the Sales of Veterinary Antimicrobial Agents in Nine European Countries 2005–2009. (EMA/238630/2011). Available online: https://www.ema.europa.eu/en/documents/report/trends-sales-veterinary-antimicrobial-agents-nine-european-countries_en.pdf (accessed on 25 October 2020).

| Sold Colistin | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|---|---|---|---|
| Total sales, kg | 378 | 458 | 570 | 709 | 403 | 164 | 95 | 127 | 95 | 53 |
| % of total AM | 4.6 | 5.5 | 7.3 | 8.1 | 4.1 | 1.7 | 1.1 | 1.7 | 1.3 | 0.7 |
| Total sales, mg/PCU | 3.51 | 4.31 | 4.88 | 5.75 | 3.12 | 1.33 | 0.73 | 1.14 | 0.83 | n/a |
| For pigs, kg | 336 | 425 | 517 | 651 | 377 | 99 | 86 | 118 | 89 | 42 |
| % of total colistin | 89% | 93% | 91% | 92% | 93% | 60% | 91% | 93% | 93% | 79% |
| For cattle and poultry, kg | 22 | 18 | 42 | 32 | 8 | 54 | 4 | 7 | 4 | 6 |
| % of total colistin | 6% | 4% | 7% | 5% | 2% | 33% | 4% | 5% | 5% | 12% |
| For species unknown, kg | 20 | 15 | 11 | 26 | 18 | 11 | 5 | 2 | 2 | 5 |
| % of total colistin | 5% | 3% | 2% | 4% | 5% | 7% | 6% | 2% | 2% | 9% |
| Characteristic | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|---|---|---|---|
| PCUpigs (in 1000 tonnes) | 35 | 34 | 43 | 47 | 49 | 45 | 38 | 37 | 40 | 42 |
| Colistin for pigs (in mg/PCU) | 9.63 | 12.47 | 12.14 | 13.88 | 7.77 | 2.20 | 2.27 | 3.15 | 2.23 | 1.00 |
| Produced piglets (in 1000 heads) | 752 | 782 | 775 | 750 | 774 | 744 | 592 | 624 | 621 | n/a |
| Escherichia (in 1000 doses) | 0.8 | 1.7 | 0.8 | 2 | 220 | 325 | 209 | 301 | 349 | 362 |
| Escherichia + Clostridium (in 1000 doses) | 42 | 75 | 50 | 83 | 83 | 72 | 77 | 67 | 73 | 65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sammul, M.; Mõtus, K.; Kalmus, P. The Use of Colistin in Food-Producing Animals in Estonia—Vaccination as an Effective Alternative to Consumption of Critically Important Antimicrobials in Pigs. Antibiotics 2021, 10, 499. https://doi.org/10.3390/antibiotics10050499
Sammul M, Mõtus K, Kalmus P. The Use of Colistin in Food-Producing Animals in Estonia—Vaccination as an Effective Alternative to Consumption of Critically Important Antimicrobials in Pigs. Antibiotics. 2021; 10(5):499. https://doi.org/10.3390/antibiotics10050499
Chicago/Turabian StyleSammul, Marju, Kerli Mõtus, and Piret Kalmus. 2021. "The Use of Colistin in Food-Producing Animals in Estonia—Vaccination as an Effective Alternative to Consumption of Critically Important Antimicrobials in Pigs" Antibiotics 10, no. 5: 499. https://doi.org/10.3390/antibiotics10050499






