Antibiotics or No Antibiotics, That Is the Question: An Update on Efficient and Effective Use of Antibiotics in Dental Practice
Abstract
1. Introduction
2. Types of Antibiotics and Administration Protocols
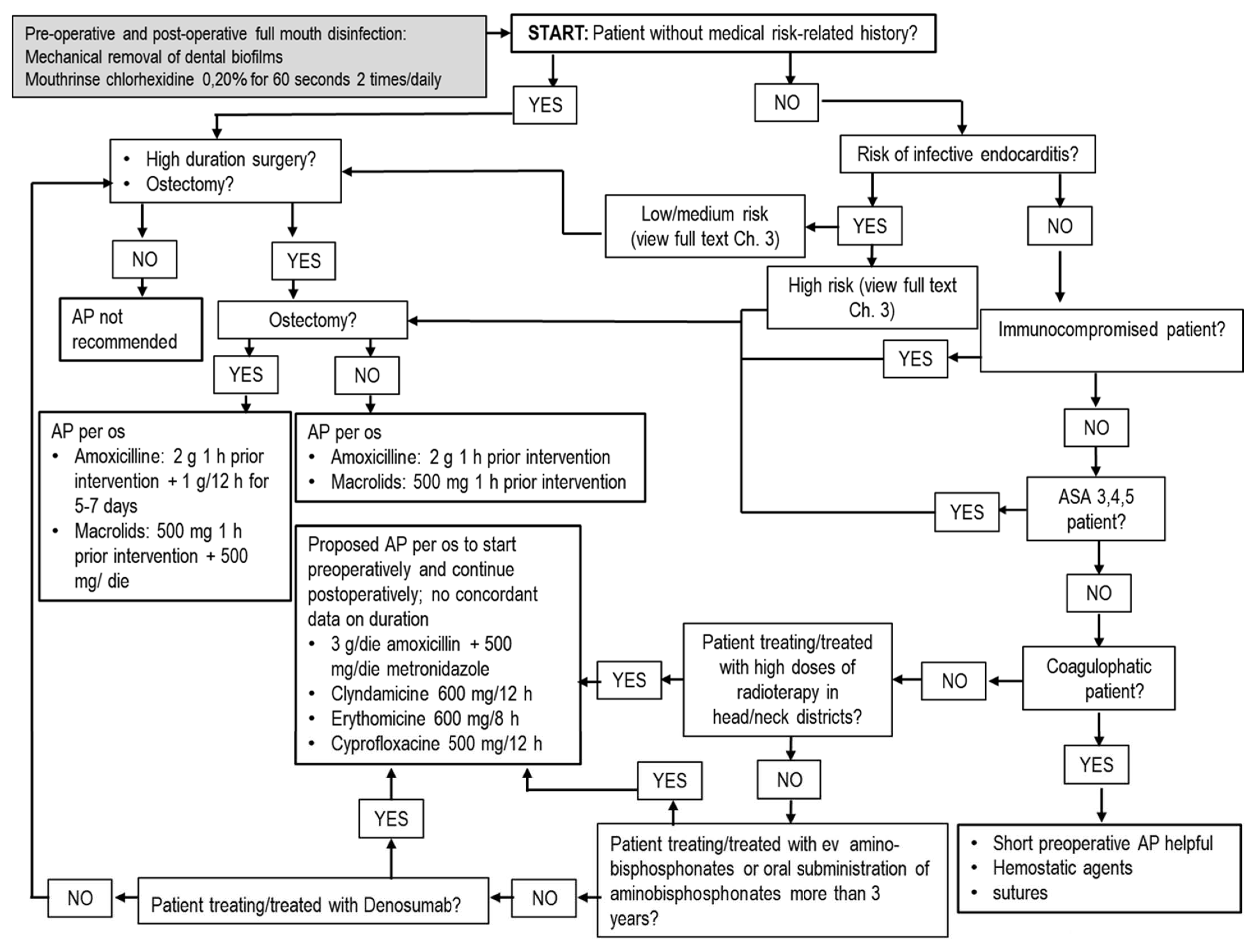
3. Antibiotic Prophylaxis for Dental Procedures in Patients at Risk for Infection
4. Chlorhexidine
5. Use of Antibiotics in Dentistry
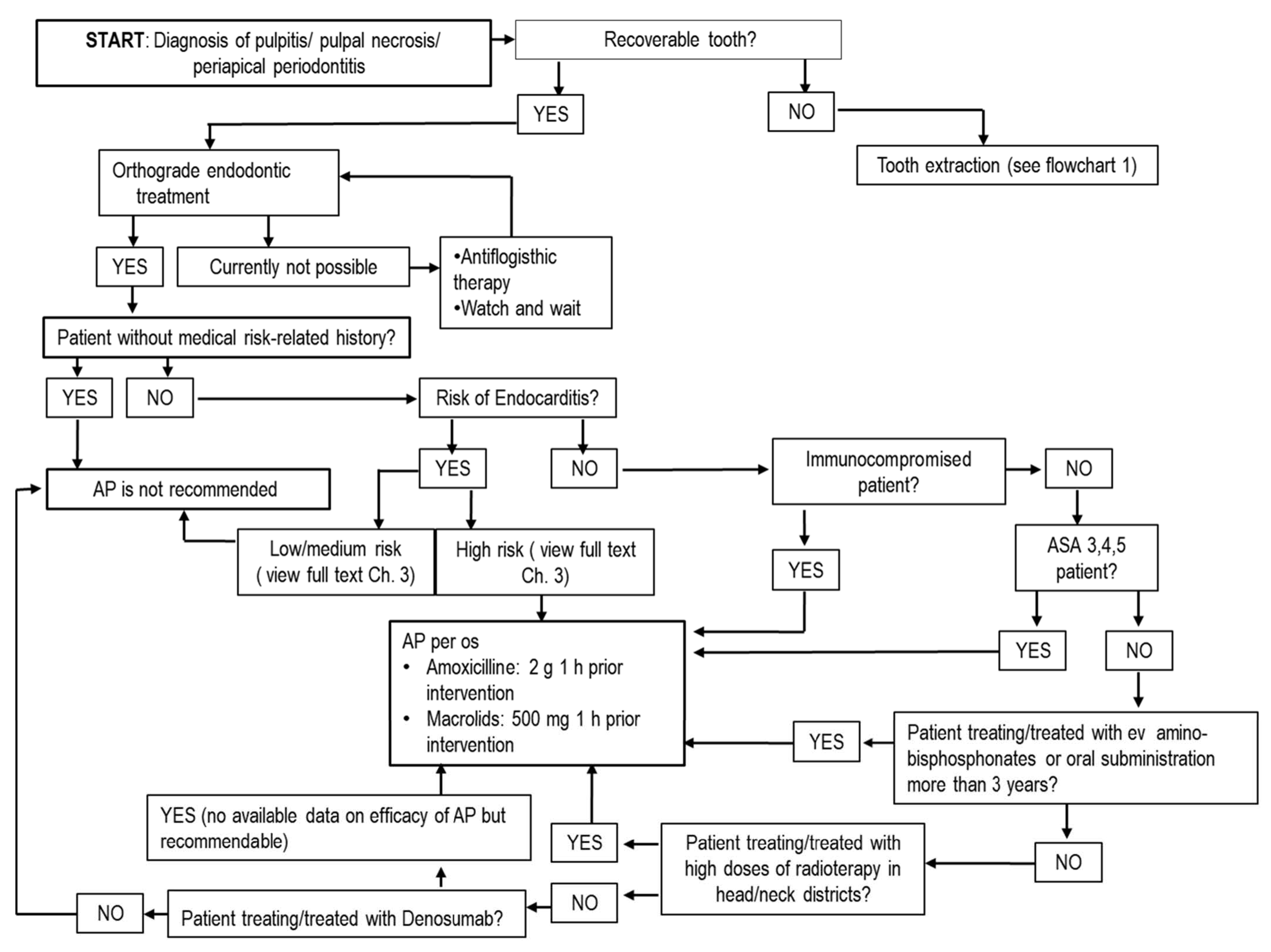
5.1. Antibiotics in Endodontics
5.2. Antibiotics in Periodontology
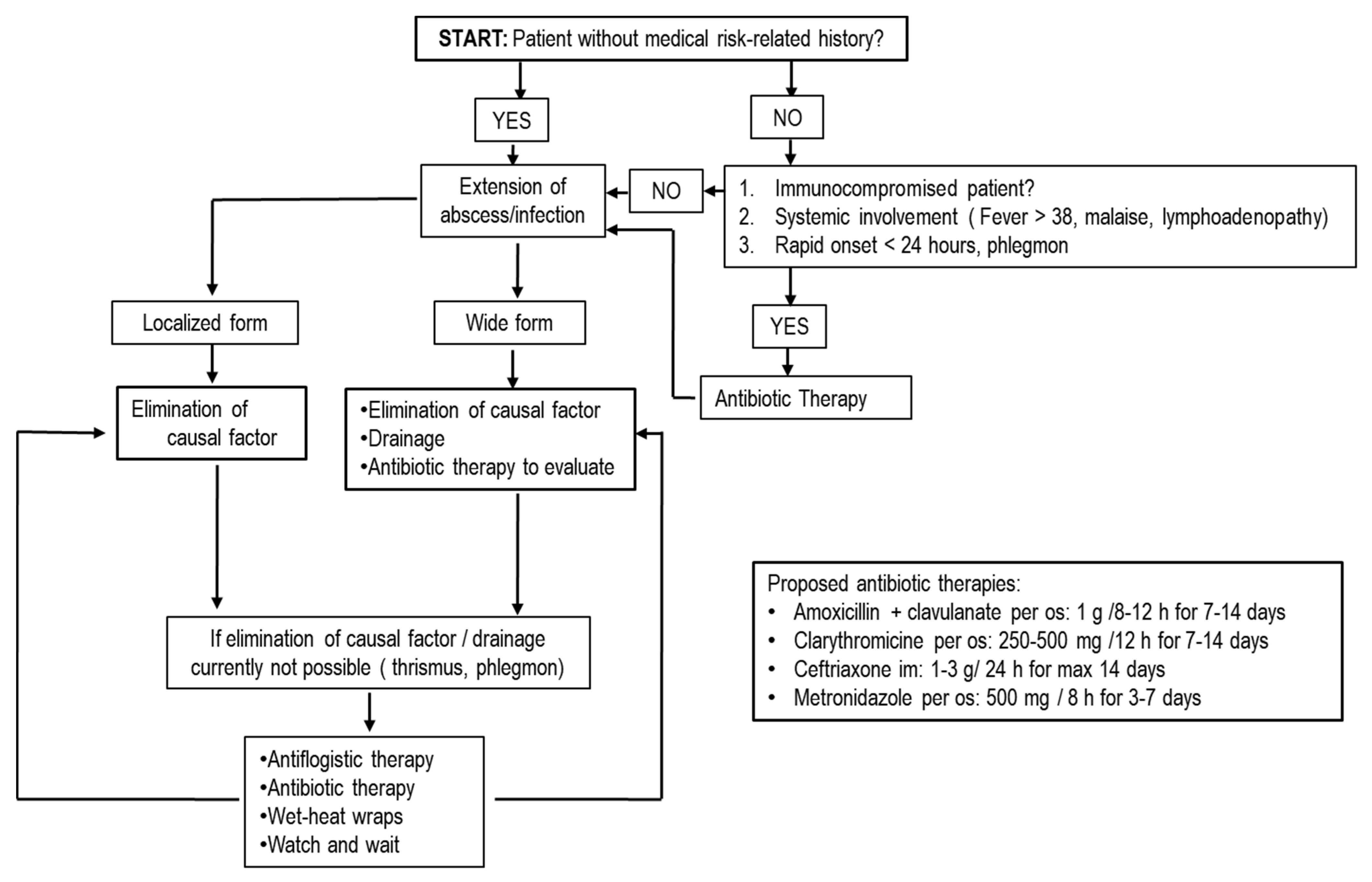
5.3. Antibiotics in Extractive Oral Surgery
5.4. Antibiotics in Implantology and Regenerative Techniques
6. Conclusions
7. Highlights
- ➢
- Antibiotics in dental practice can only be used in selected situations, for specific patients and using appropriate techniques, generally with short preoperative schemes. Postoperative schemes can be adopted for long-duration surgeries with osteotomy or for antibiotic therapies in complicated abscesses.
- ➢
- Antibiotics must be considered as pharmacological adjuvants that cannot cover or replace medical intervention.
- ➢
- Correct management of oral bacterial load/contamination with elimination of infective foci, dental biofilms and good periodontal health, along with atraumatic surgical techniques, are the main factors influencing the success rates of interventions, rather than antibiotic administration.
- ➢
- Antibiotics are not able to reduce clinical symptoms such as pain and swelling.
- ➢
- Routine tooth extractions in healthy patient can be executed without antibiotics, with the same incidence of complications.
- ➢
- Implant insertion can be managed with short preoperative AP to reduce risk of failure, whilst no beneficial effects are obtained with postoperative regimen schemes.
- ➢
- Chlorhexidine must be used properly and for short periods due to the possibility of inducing cross-resistance to antibiotics.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sidana, S.; Mistry, Y.; Gandevivala, A.; Motwani, N. Evaluation of the Need for Antibiotic Prophylaxis During Routine Intra-alveolar Dental Extractions in Healthy Patients: A Randomized Double-Blind Controlled Trial. J. Evid. Based Dent. Pract. 2017, 17, 184–189. [Google Scholar] [CrossRef]
- Ma, Z.; Lee, S.; Jeong, K.C. Mitigating Antibiotic Resistance at the Livestock-Environment Interface:A Review. J. Microbiol. Biotechnol. 2019, 29, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Moore, L.S.P.; Castro-Sanchez, E.; Charani, E.; Davies, F.; Satta, G.; Ellington, M.J.; Holmes, A.H. COVID-19 and the potential long-term impact on antimicrobial resistance. J. Antimicrob. Chemother. 2020, 75, 1681–1684. [Google Scholar] [CrossRef]
- Lodi, G.; Figini, L.; Sardella, A.; Carrassi, A.; Del Fabbro, M.; Furness, S. Antibiotics to prevent complications following tooth extractions. Cochrane Database Syst. Rev. 2012, 11, CD003811. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.; Tonkin-Crine, S.; Pavitt, S.H.; McEachan, R.R.C.; Douglas, G.V.A.; Aggarwal, V.R.; Sandoe, J.A.T. Factors associated with antibiotic prescribing for adults with acute conditions: An umbrella review across primary care and a sys-tematic review focusing on primary dental care. J. Antimicrob. Chemother. 2019, 74, 2139–2152. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W. Oral Sepsis as a Cause of Disease. BMJ 1900, 2, 215–216. [Google Scholar] [CrossRef]
- Niederman, M.S. Appropriate use of antimicrobial agents: Challenges and strategies for improvement. Crit. Care Med. 2003, 31, 608–616. [Google Scholar] [CrossRef]
- Vila, P.M.; Zenga, J.; Fowler, S.; Jackson, R.S. Antibiotic Prophylaxis in Clean-Contaminated Head and Neck Surgery: A Systematic Review and Meta-analysis. Otolaryngol. Neck Surg. 2017, 157, 580–588. [Google Scholar] [CrossRef]
- Garner, J.S. CDC Guideline for Prevention of Surgical Wound Infections, 1985. Infect. Control. 1986, 7, 193–200. [Google Scholar] [CrossRef]
- American Society of Health-System Pharmacists (ASHP). Therapeutic Guidelines on Antimicrobial Prophylaxis in Surgery. Am. J. Health Syst. Pharm. 1999, 56, 1839–1888. [Google Scholar] [CrossRef]
- Payer, M.; Tan, W.C.; Han, J.; Ivanovski, S.; Mattheos, N.; Pjetursson, B.E.; Zhuang, L.; Fokas, G.; Wong, M.C.M.; Acham, S.; et al. The effect of systemic antibiotics on clinical and patient-reported outcome measures of oral implant therapy with simultaneous guided bone regeneration. Clin. Oral Implant. Res. 2020, 31, 442–451. [Google Scholar] [CrossRef]
- Fluent, M.T.; Jacobsen, P.L.; Hicks, L.A.; Osap, T.S.D.V. Considerations for responsible antibiotic use in dentistry. J. Am. Dent. Assoc. 2016, 147, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.E.; Salmasian, H.; Li, J.; Liu, J.; Zachariah, P.; Wright, J.D.; Freedberg, D.E. Surgical Antibiotic Prophylaxis and Risk for Postoperative Antibiotic-Resistant Infections. J. Am. Coll. Surg. 2017, 225, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Błochowiak, K.J. Dental treatment and recommended management in patients at risk of infective endocarditis. Pol. J. Cardio-Thorac. Surg. 2019, 16, 37–41. [Google Scholar] [CrossRef]
- Segura-Egea, J.J.; Gould, K.; Sen, B.H.; Jonasson, P.; Cotti, E.; Mazzoni, A.; Sunay, H.; Tjaderhane, L.; Dummer, P.M.H. Eu-ropean Society of Endodontology position statement: The use of antibiotics in endodontics. Int. Endod. J. 2018, 51, 20–25. [Google Scholar] [CrossRef]
- Krishnan, K.; Chen, T.; Paster, B.J. A practical guide to the oral microbiome and its relation to health and disease. Oral Dis. 2017, 23, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.R.; Chagas, O.L., Jr.; Velasques, B.D.; Bobrowski, Â.N.; Correa, M.B.; Torriani, M.A. The Use of Antibiotics in Odontogenic Infections: What Is the Best Choice? A Systematic Review. J. Oral Maxillofac. Surg. 2017, 75, 2606.e1–2606.e11. [Google Scholar] [CrossRef] [PubMed]
- Huttner, A.; Bielicki, J.; Clements, M.; Frimodt-Møller, N.; Muller, A.; Paccaud, J.-P.; Mouton, J. Oral amoxicillin and amoxicillin–clavulanic acid: Properties, indications and usage. Clin. Microbiol. Infect. 2020, 26, 871–879. [Google Scholar] [CrossRef]
- Kuriyama, T.; Williams, D.W.; Yanagisawa, M.; Iwahara, K.; Shimizu, C.; Nakagawa, K.; Yamamoto, E.; Karasawa, T. Anti-microbial susceptibility of 800 anaerobic isolates from patients with dentoalveolar infection to 13 oral antibiotics. Oral Micro-biol. Immunol. 2007, 22, 285–288. [Google Scholar] [CrossRef]
- Moore, P.A. Dental therapeutic indications for the newer long-acting macrolide antibiotics. J. Am. Dent. Assoc. 1999, 130, 1341–1343. [Google Scholar] [CrossRef]
- Lodi, G.; Sardella, A.; Salis, A.; Demarosi, F.; Tarozzi, M.; Carrassi, A. Tooth Extraction in Patients Taking Intravenous Bisphosphonates: A Preventive Protocol and Case Series. J. Oral Maxillofac. Surg. 2010, 68, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, P.B.; Brennan, M.T.; Sasser, H.C.; Fox, P.C.; Paster, B.J.; Bahrani-Mougeot, F.K. Bacteremia associated with tooth-brushing and dental extraction. Circulation 2008, 117, 3118–3125. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, P.B.; Brennan, M.T.; Thornhill, M.; Michalowicz, B.S.; Noll, J.; Bahrani-Mougeot, F.K.; Sasser, H.C. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J. Am. Dent. Assoc. 2009, 140, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, P.B.; Loven, B.; Brennan, M.T.; Fox, P.C. The evidence base for the efficacy of antibiotic prophylaxis in dental practice. J. Am. Dent. Assoc. 2007, 138, 458–474. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e1159–e1195. [Google Scholar] [CrossRef]
- Østergaard, L.; Valeur, N.; Ihlemann, N.; Bundgaard, H.; Gislason, G.; Torp-Pedersen, C.; Bruun, N.E.; Søndergaard, L.; Køber, L.; Fosbøl, E.L. Incidence of infective endocarditis among patients considered at high risk. Eur. Heart J. 2017, 39, 623–629. [Google Scholar] [CrossRef]
- Robinson, A.N.; Tambyah, P.A. Infective endocarditis—An update for dental surgeons. Singap. Dent. J. 2017, 38, 2–7. [Google Scholar] [CrossRef]
- Quan, T.P.; Muller-Pebody, B.; Fawcett, N.; Young, B.C.; Minaji, M.; Sandoe, J.; Hopkins, S.; Crook, D.; Peto, T.; Johnson, A.P.; et al. Investigation of the impact of the NICE guidelines regarding antibiotic prophylaxis during invasive dental pro-cedures on the incidence of infective endocarditis in England: An electronic health records study. BMC Med. 2020, 18, 84. [Google Scholar] [CrossRef]
- Cahill, T.J.; Harrison, J.L.; Jewell, P.; Onakpoya, I.; Chambers, J.B.; Dayer, M.; Lockhart, P.; Roberts, N.; Shanson, D.; Thornhill, M.; et al. Antibiotic prophylaxis for infective endocarditis: A systematic review and me-ta-analysis. Heart 2017, 103, 937–944. [Google Scholar] [CrossRef]
- Goff, D.; E Mangino, J.; Glassman, A.H.; Goff, D.; Larsen, P.; Scheetz, R. Review of Guidelines for Dental Antibiotic Prophylaxis for Prevention of Endocarditis and Prosthetic Joint Infections and Need for Dental Stewardship. Clin. Infect. Dis. 2019, 71, 455–462. [Google Scholar] [CrossRef]
- Patel, H.; Kumar, S.; Ko, N.L.K.; Catania, J.; Javaid, A. Ineffective Antibiotic Prophylaxis: An Unusual Presentation of Infective Endocarditis with Insights into the Appropriateness of Prophylaxis. Cureus 2019, 11, e4860. [Google Scholar] [CrossRef] [PubMed]
- Prophylaxis Against Infective Endocarditis: Antimicrobial Prophylaxis Against Infective Endocarditis in Adults and Children Undergoing Interventional Procedures; Centre for Clinical Practice at NICE: London, UK, 2008.
- Segura-Egea, J.J.; Gould, K.; Sen, B.H.; Jonasson, P.; Cotti, E.; Mazzoni, A.; Sunay, H.; Tjaderhane, L.; Dummer, P.M.H. Anti-biotics in Endodontics: A review. Int. Endod. J. 2017, 50, 1169–1184. [Google Scholar] [CrossRef]
- Kannan, T. ASA Grading: A Step Forward. J. Perioper. Pract. 2017, 27, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Limones, A.; Sáez-Alcaide, L.-M.; Díaz-Parreño, S.-A.; Helm, A.; Bornstein, M.-M.; Molinero-Mourelle, P. Medication-related osteonecrosis of the jaws (MRONJ) in cancer patients treated with denosumab VS. zoledronic acid: A systematic review and meta-analysis. Med. Oral Patol. Oral Cir. Bucal 2020, 25, 326. [Google Scholar] [CrossRef] [PubMed]
- Aarup-Kristensen, S.; Hansen, C.R.; Forner, L.; Brink, C.; Eriksen, J.G.; Johansen, J. Osteoradionecrosis of the mandible after radiotherapy for head and neck cancer: Risk factors and dose-volume correlations. Acta Oncol. 2019, 58, 1373–1377. [Google Scholar] [CrossRef]
- Goldvaser, H.; Amir, E. Role of Bisphosphonates in Breast Cancer Therapy. Curr. Treat. Options Oncol. 2019, 20, 26. [Google Scholar] [CrossRef]
- D’Agostino, S.; Maida, S.; Besharat, K.; Dolci, M. ONJ Update 2018 Congress Abstracts Osteonecrosi delle ossa mascellari (ONJ) da bifosfonati e altri farmaci: Prevenzione, diagnosi, farmacovigilanza, trattamento. Alessandria, 5 maggio 2018. Minerva Stomatol. 2018, 67, 1–45. [Google Scholar]
- Rademacher, W.M.H.; Walenkamp, G.H.I.M.; Moojen, D.J.F.; E Hendriks, J.G.; A Goedendorp, T.; Rozema, F.R. Antibiotic prophylaxis is not indicated prior to dental procedures for prevention of periprosthetic joint infections. Acta Orthop. 2017, 88, 568–574. [Google Scholar] [CrossRef]
- Haraji, A.; Rakhshan, V. Single-Dose Intra-Alveolar Chlorhexidine Gel Application, Easier Surgeries, and Younger Ages Are Associated With Reduced Dry Socket Risk. J. Oral Maxillofac. Surg. 2014, 72, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance Toward Chlorhexidine in Oral Bacteria—Is There Cause for Concern? Front. Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef]
- Saleem, H.G.M.; Seers, C.A.; Sabri, A.N.; Reynolds, E.C. Dental plaque bacteria with reduced susceptibility to chlorhexidine are multidrug resistant. BMC Microbiol. 2016, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.; Addy, M.; Wade, W.; Newcombe, R.G. The magnitude and duration of the effects of some mouthrinse products on salivary bacterial counts. J. Clin. Periodontol. 1994, 21, 397–401. [Google Scholar] [CrossRef]
- Hita-Iglesias, P.; Torres-Lagares, D.; Flores-Ruiz, R.; Magallanes-Abad, N.; Basallote-Gonzalez, M.; Gutierrez-Perez, J.L. Ef-fectiveness of chlorhexidine gel versus chlorhexidine rinse in reducing alveolar osteitis in mandibular third molar surgery. J. Oral Maxillofac. Surg. 2008, 66, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Wassenaar, T.M.; Ussery, D.; Nielsen, L.N.; Ingmer, H. Review and phylogenetic analysis of qac genes that reduce suscep-tibility to quaternary ammonium compounds in Staphylococcus species. Eur. J. Microbiol. Immunol. 2015, 5, 44–61. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Tomida, J.; Kawamura, Y. Responses of Pseudomonas aeruginosa to antimicrobials. Front. Microbiol. 2014, 4, 422. [Google Scholar] [CrossRef]
- Venter, H.; Henningsen, M.L.; Begg, S.L. Antimicrobial resistance in healthcare, agriculture and the environment: The bi-ochemistry behind the headlines. Essays Biochem. 2017, 61, 1–10. [Google Scholar] [CrossRef]
- Kampf, G. Biocidal Agents Used for Disinfection Can Enhance Antibiotic Resistance in Gram-Negative Species. Antibiotics 2018, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Cervinkova, D.; Babak, V.; Marosevic, D.; Kubikova, I.; Jaglic, Z. The role of the qacA gene in mediating resistance to qua-ternary ammonium compounds. Microb. Drug Resist 2013, 19, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Cope, A.L.; Francis, N.; Wood, F.; Chestnutt, I.G. Systemic antibiotics for symptomatic apical periodontitis and acute apical abscess in adults. Cochrane Database Syst. Rev. 2018, 9, CD010136. [Google Scholar] [CrossRef]
- Haapasalo, M.; Shen, Y.; Wang, Z.; Gao, Y. Irrigation in endodontics. Br. Dent. J. 2014, 216, 299–303. [Google Scholar] [CrossRef]
- Kawashima, N.; Wadachi, R.; Suda, H.; Yeng, T.; Parashos, P. Root canal medicaments. Int. Dent. J. 2009, 59, 5–11. [Google Scholar] [PubMed]
- Gandolfi, M.G.; Siboni, F.; Botero, T.; Bossù, M.; Riccitiello, F.; Prati, C. Calcium silicate and calcium hydroxide materials for pulp capping: Biointeractivity, porosity, solubility and bioactivity of current formulations. J. Appl. Biomater. Funct. Mater. 2015, 13, 43–60. [Google Scholar] [CrossRef]
- Fouad, A.F.; Abbott, P.V.; Tsilingaridis, G.; Cohenca, N.; Lauridsen, E.; Bourguignon, C.; O’Connell, A.; Flores, M.T.; Day, P.F.; Hicks, L.; et al. International Association of Dental Traumatology guidelines for the management of traumatic dental injuries: Avulsion of permanent teeth. Dent. Traumatol. 2020, 36, 331–342. [Google Scholar] [CrossRef]
- Desimone, D.C.; Tleyjeh, I.M.; de Sa, D.D.C.; Anavekar, N.S.; Lahr, B.D.; Sohail, M.R.; Steckelberg, J.M.; Wilson, W.R.; Baddour, L.M.; Mayo, G. Incidence of infective endocarditis caused by viridans group strep-tococci before and after publication of the 2007 American Heart Association’s endocarditis prevention guidelines. Circulation 2012, 126, 60–64. [Google Scholar] [CrossRef]
- Gill, A.S.; Morrissey, H.; Rahman, A. A Systematic Review and Meta-Analysis Evaluating Antibiotic Prophylaxis in Dental Implants and Extraction Procedures. Medicina 2018, 54, 95. [Google Scholar] [CrossRef]
- Dodson, T.B.; Susarla, S.M. Impacted wisdom teeth. BMJ Clin. Evid. 2014, 2014, 1302. [Google Scholar] [PubMed]
- Galvao, E.L.; da Silveira, E.M.; de Oliveira, E.S.; da Cruz, T.M.M.; Flecha, O.D.; Falci, S.G.M.; Goncalves, P.F. Association between mandibular third molar position and the occurrence of pericoronitis: A systematic review and meta-analysis. Arch. Oral Biol. 2019, 107, 104486. [Google Scholar] [CrossRef] [PubMed]
- Troeltzsch, M.; Lohse, N.; Moser, N.; Kauffmann, P.; Cordesmeyer, R.; Aung, T.; Brodine, B.; Troeltzsch, M. A review of pathogenesis, diagnosis, treatment options, and differential diagnosis of odontogenic infections: A rather mundane patholo-gy? Quintessence Int. 2015, 46, 351–361. [Google Scholar] [PubMed]
- Van Winkelhoff, A.J.; Rams, T.E.; Slots, J. Systemic antibiotic therapy in periodontics. Periodontology 2000 1996, 10, 45–78. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.; Griffiths, G.S.; Nibali, L.; Suvan, J.; Moles, D.R.; Laurell, L.; Tonetti, M.S. Adjunctive benefits of systemic amox-icillin and metronidazole in non-surgical treatment of generalized aggressive periodontitis: A randomized placebo-controlled clinical trial. J. Clin. Periodontol. 2005, 32, 1096–1107. [Google Scholar] [CrossRef]
- Rabelo, C.C.; Feres, M.; Gonçalves, C.; Figueiredo, L.C.; Faveri, M.; Tu, Y.-K.; Chambrone, L. Systemic antibiotics in the treatment of aggressive periodontitis. A systematic review and a Bayesian Network meta-analysis. J. Clin. Periodontol. 2015, 42, 647–657. [Google Scholar] [CrossRef]
- Rovai, E.S.; Souto, M.L.S.; Ganhito, J.A.; Holzhausen, M.; Chambrone, L.; Pannuti, C.M. Efficacy of Local Antimicrobials in the Non-Surgical Treatment of Patients With Periodontitis and Diabetes: A Systematic Review. J. Periodontol. 2016, 87, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Golub, L.M.; Lee, H.-M. Periodontal therapeutics: Current host-modulation agents and future directions. Periodontology 2000 2020, 82, 186–204. [Google Scholar] [CrossRef] [PubMed]
- Eickholz, P.; Kim, T.-S.; Bürklin, T.; Schacher, B.; Renggli, H.H.; Schaecken, M.T.; Holle, R.; Kübler, A.; Ratka-Krüger, P. Non-surgical periodontal therapy with adjunctive topical doxycycline: A double-blind randomized controlled multicenter study. J. Clin. Periodontol. 2002, 29, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Matesanz-Pérez, P.; García-Gargallo, M.; Figuero, E.; Bascones-Martínez, A.; Sanz, M.; Herrera, D. A systematic review on the effects of local antimicrobials as adjuncts to subgingival debridement, compared with subgingival debridement alone, in the treatment of chronic periodontitis. J. Clin. Periodontol. 2013, 40, 227–241. [Google Scholar] [CrossRef]
- Taberner-Vallverdu, M.; Sanchez-Garces, M.A.; Gay-Escoda, C. Efficacy of different methods used for dry socket preven-tion and risk factor analysis: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e750–e758. [Google Scholar]
- Braimah, R.O.; Ndukwe, K.C.; Owotade, J.F.; Aregbesola, S.B. Comparative efficacy of amoxicillin/clavulanic acid and levofloxacin in the reduction of postsurgical sequelae after third molar surgery: A randomized, double-blind, clinical trial in a Nigerian university teaching hospital. Niger. J. Surg. 2016, 22, 70–76. [Google Scholar] [CrossRef]
- Cervino, G.; Cicciù, M.; Biondi, A.; Bocchieri, S.; Herford, A.S.; Laino, L.; Fiorillo, L. Antibiotic Prophylaxis on Third Molar Extraction: Systematic Review of Recent Data. Antibiotics 2019, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Taberner-Vallverdu, M.; Nazir, M.; Sanchez-Garces, M.; Gay-Escoda, C. Efficacy of different methods used for dry socket management: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e633–e639. [Google Scholar] [CrossRef]
- Hasheminia, D.; Moaddabi, A.; Moradi, S.; Soltani, P.; Moannaei, M.; Issazadeh, M. The efficacy of 1% Betadine mouthwash on the incidence of dry socket after mandibular third molar surgery. J. Clin. Exp. Dent. 2018, 10, e445–e449. [Google Scholar] [CrossRef] [PubMed]
- Requena-Calla, S.; Funes-Rumiche, I. Effectiveness of intra-alveolar chlorhexidine gel in reducing dry socket following surgical extraction of lower third molars. A pilot study. J. Clin. Exp. Dent. 2016, 8, e160–e163. [Google Scholar] [CrossRef]
- Costerton, J.; Montanaro, L.; Arciola, C. Biofilm in Implant Infections: Its Production and Regulation. Int. J. Artif. Organs 2005, 28, 1062–1068. [Google Scholar] [CrossRef]
- Thomas, M.; Puleo, D. Infection, Inflammation, and Bone Regeneration: A Paradoxical Relationship. J. Dent. Res. 2011, 90, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Lalla, E.; Lamster, I.B.; Feit, M.; Huang, L.; Spessot, A.; Qu, W.; Kislinger, T.; Lu, Y.; Stern, D.M.; Schmidt, A.M. Blockade of RAGE suppresses periodontitis-associated bone loss in diabetic mice. J. Clin. Investig. 2000, 105, 1117–1124. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef]
- Cucchi, A.; Chierico, A.; Fontana, F.; Mazzocco, F.; Cinquegrana, C.; Belleggia, F.; Rossetti, P.; Soardi, C.M.; Todisco, M.; Luongo, R.; et al. Statements and Recommendations for Guided Bone Regeneration. Implant. Dent. 2019, 28, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, F.R.; Andrés, C.R.; Arteagoitia, I. Which antibiotic regimen prevents implant failure or infection after dental implant surgery? A systematic review and meta-analysis. J. Cranio-Maxillofac. Surg. 2018, 46, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Arduino, P.G.; Tirone, F.; Schiorlin, E.; Esposito, M. Single preoperative dose of prophylactic amoxicillin versus a 2-day postoperative course in dental implant surgery: A two-centre randomised controlled trial. Eur. J. Oral Implant. 2015, 8, 143–149. [Google Scholar]
- Lund, B.; Hultin, M.; Tranaeus, S.; Naimi-Akbar, A.; Klinge, B. Complex systematic review—Perioperative antibiotics in conjunction with dental implant placement. Clin. Oral Implant. Res. 2015, 26, 1–14. [Google Scholar] [CrossRef]
- Park, J.S.; Tennant, M.; Walsh, L.J.; Kruger, E. Is there a consensus on antibiotic usage for dental implant placement in healthy patients? Aust. Dent. J. 2018, 63, 25–33. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Worthington, H.V. Interventions for replacing missing teeth: Antibiotics at dental implant placement to prevent complications. Cochrane Database Syst. Rev. 2013, 2013, CD004152. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Aguirre, J.J.; Gorosabel, A.; Barrio, P.; Errazquin, J.M.; Roman, P.; Pla, R.; Carrete, J.; de Petro, J.; Orive, G. A mul-ticentre placebo-controlled randomised clinical trial of antibiotic prophylaxis for placement of single dental implants. Eur. J. Oral Implantol. 2009, 2, 283–292. [Google Scholar] [PubMed]
| First Choice |
|---|
| Amoxicillin = 1 g with oral administration (per os)/8 h for 7 days or more |
| Amoxicillin + clavunalate = 1 g per os/8–12 h for 7 days or more |
| Amoxicillin (500 mg per os/8 h) + metronidazole (500 mg per os/ 8 h) = AP for patients at risk of bisphosphonate-related osteonecrosis of the jaws (BRONJ) or medication-related osteonecrosis of the jaws (MRONJ); AT in combination with nonsurgical treatment for aggressive periodontitis |
| Patients Allergic to Betalactams |
| Claritromicine= 250–500 mg per os/12 h for 7–14 days |
| Azitromycine= 500 mg per os/24 h for 3 days or more Clindamycin= 300 mg/6 h for 7–14 days |
| Posological Adjustments in Children (Weight < 20 Kg and/or Age < 10 Years) |
| Amoxicillin = 12.5–25 mg/Kg/8 h |
| Macrolids = 15 mg/Kg/24 h Clindamycin = 5 mg/Kg/6 h |
| First Choice |
|---|
| Amoxicillin = 2 g with oral administration (per os)/1 h before procedure |
| Amoxicillin + clavunalate = 2 g per os/1 h before procedure |
| Patients Allergic to Betalactams |
| Macrolides = 500 mg per os/1 h before procedure |
| Clindamycin = 600 mg per os/1 h before procedure |
| Posology Adjustments in Children (Weight < 20 Kg and/or Age < 10 Years) |
| Amoxicillin = 50 mg/Kg |
| Macrolides = 15 mg/Kg Clindamycin = 20 mg/Kg |
| ● Patients at high risk of infective endocarditis |
| ● Immunocompromised patients with leukopenia <3.500 u/mm3 or seral levels of immunoglobulins <2 g/L |
| ● Patients ASA 3,4,5 |
| ● Patients undergoing to high dose irradiation on jawbones, or to assumption of amino-bisphosphonates/denosumab |
| ● Patients with joint prosthesis with high risk of adverse outcomes |
| ● Patients undergoing prolonged and extensive surgical interventions |
| ● Patients undergoing surgery in infected sites |
| ● Patients undergoing insertion of fixtures and/or biomaterials |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonavoglia, A.; Leone, P.; Solimando, A.G.; Fasano, R.; Malerba, E.; Prete, M.; Corrente, M.; Prati, C.; Vacca, A.; Racanelli, V. Antibiotics or No Antibiotics, That Is the Question: An Update on Efficient and Effective Use of Antibiotics in Dental Practice. Antibiotics 2021, 10, 550. https://doi.org/10.3390/antibiotics10050550
Buonavoglia A, Leone P, Solimando AG, Fasano R, Malerba E, Prete M, Corrente M, Prati C, Vacca A, Racanelli V. Antibiotics or No Antibiotics, That Is the Question: An Update on Efficient and Effective Use of Antibiotics in Dental Practice. Antibiotics. 2021; 10(5):550. https://doi.org/10.3390/antibiotics10050550
Chicago/Turabian StyleBuonavoglia, Alessio, Patrizia Leone, Antonio Giovanni Solimando, Rossella Fasano, Eleonora Malerba, Marcella Prete, Marialaura Corrente, Carlo Prati, Angelo Vacca, and Vito Racanelli. 2021. "Antibiotics or No Antibiotics, That Is the Question: An Update on Efficient and Effective Use of Antibiotics in Dental Practice" Antibiotics 10, no. 5: 550. https://doi.org/10.3390/antibiotics10050550
APA StyleBuonavoglia, A., Leone, P., Solimando, A. G., Fasano, R., Malerba, E., Prete, M., Corrente, M., Prati, C., Vacca, A., & Racanelli, V. (2021). Antibiotics or No Antibiotics, That Is the Question: An Update on Efficient and Effective Use of Antibiotics in Dental Practice. Antibiotics, 10(5), 550. https://doi.org/10.3390/antibiotics10050550









