Antibiotic Resistance and Virulence Gene Patterns Associated with Avian Pathogenic Escherichia coli (APEC) from Broiler Chickens in Qatar
Abstract
:1. Introduction
2. Results
2.1. Sample Isolation
2.2. Detection of APEC Associated Virulence Genes in Non-Healthy and Healthy Chicken Samples
2.3. Sequence Analysis of Virulence Genes Detected in Isolated APEC Strains
2.4. Clustering Analysis Based on Genetic Profile of E. coli Isolated from Non-Healthy and Healthy Chickens
2.5. Restriction Fragment Length Polymorphism (RLFP) Analysis
2.6. Phenotypic Resistance Profile of E. coli Isolated from Non-Healthy and Healthy Chickens
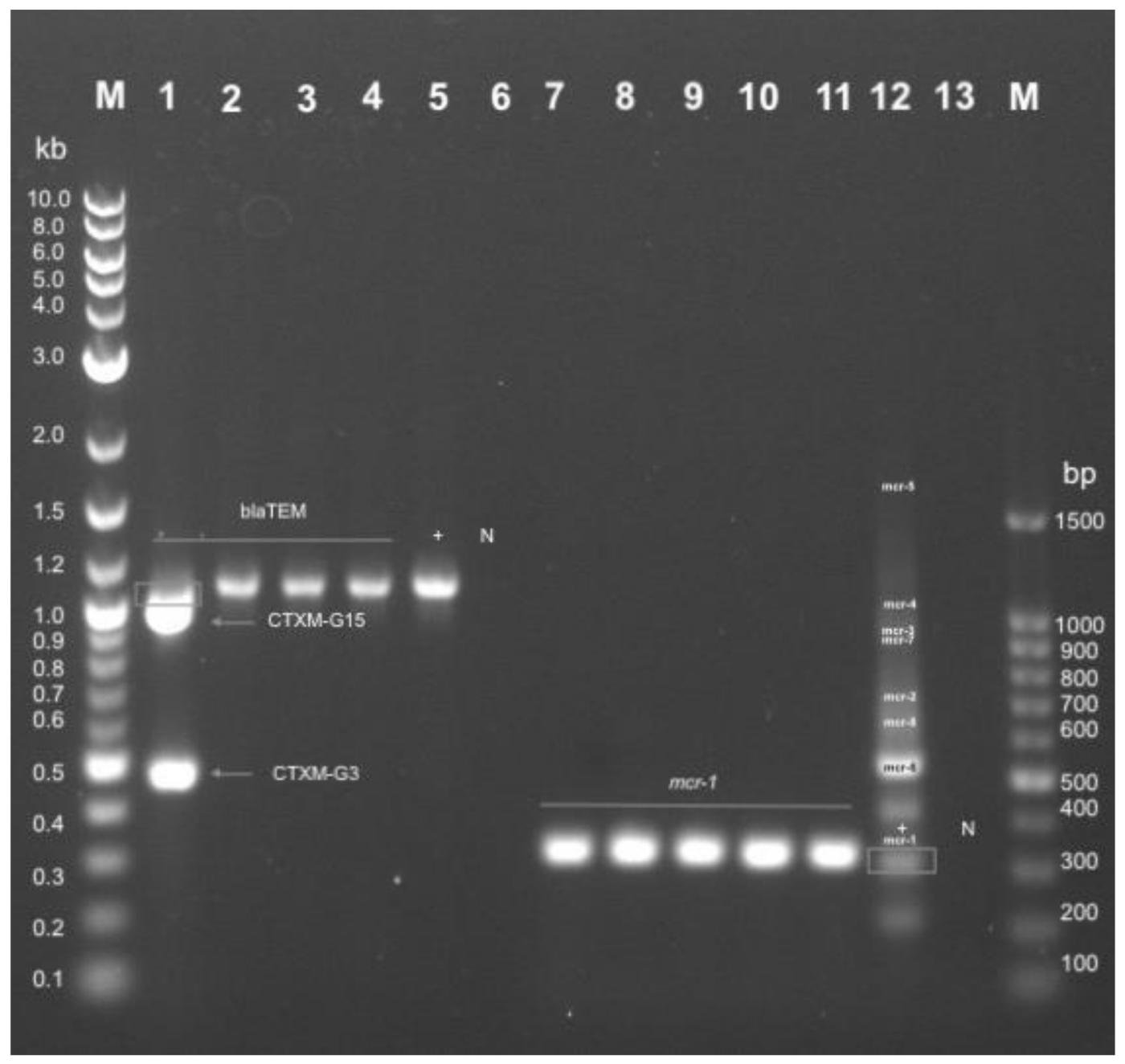
2.7. Genotypic Resistance Profile of E. coli Isolated from Non-Healthy and Healthy Chickens
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Bacterial Isolation
4.3. Identification of Avian Pathogenic E. coli (APEC) Strains
4.4. Clustering of Virulence Genes
4.5. Sequencing and Sequence Analysis
4.6. Phenotypic Antibiotic Susceptibility Testing
4.7. Extended Spectrum Beta Lactomase (ESBL) Phenotype Confirmation
4.8. Genotypic Characterization of Colistin and ESBL Resistant Isolates
4.9. Restriction Fragment Length Polymorphism (RFLP) Analysis
4.10. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassen, T.B.; Bilali, H.E.; Al-Maadeed, M. Agri-food markets in Qatar: Drivers, trends, and policy responses. Sustainability 2020, 12, 3643. [Google Scholar] [CrossRef]
- Gomes, T.A.; Elias, W.P.; Scaletsky, I.C.; Guth, B.E.; Rodrigues, J.F.; Piazza, R.M.; Ferreira, L.C.; Martinez, M.B. Diarrheagenic Escherichia coli. Braz. J. Microbiol. 2016, 47, 3–30. [Google Scholar] [CrossRef] [Green Version]
- Bozcal, E. Distribution and virulence properties of extra-intestinal pathogenic Escherichia coli in Turkey. Microbiol. Med. 2016, 31. [Google Scholar] [CrossRef] [Green Version]
- Subedi, M.; Luitel, H.; Devkota, B.; Bhattarai, R.K.; Phuyal, S.; Panthi, P.; Shrestha, A.; Chaudhary, D.K. Antibiotic resistance pattern and virulence genes content in avian pathogenic Escherichia coli (APEC) from broiler chickens in Chitwan, Nepal. BMC Vet. Res. 2018, 14, 113. [Google Scholar] [CrossRef] [PubMed]
- De Carli, S.; Ikuta, N.; Lehmann, F.K.M.; da Silveira, V.P.; de Melo Predebon, G.; Fonseca, A.S.K.; Lunge, V.R. Virulence gene content in Escherichia coli isolates from poultry flocks with clinical signs of colibacillosis in Brazil. Poult. Sci. 2015, 94, 2635–2640. [Google Scholar] [CrossRef]
- Ewers, C.; Li, G.; Wilking, H.; Kieβling, S.; Alt, K.; Antáo, E.-M.; Laturnus, C.; Diehl, I.; Glodde, S.; Homeier, T. Avian pathogenic, uropathogenic, and newborn meningitis-causing Escherichia coli: How closely related are they? Int. J. Med. Microbiol. 2007, 297, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Maurer, J.J.; Hubert, S.; De Villena, J.F.; McDermott, P.F.; Meng, J.; Ayers, S.; English, L.; White, D.G. Antimicrobial susceptibility and molecular characterization of avian pathogenic Escherichia coli isolates. Vet. Microbiol. 2005, 107, 215–224. [Google Scholar] [CrossRef]
- Guetiya Wadoum, R.; Zambou, N.; Anyangwe, F.; Njimou, J.; Coman, M.; Verdenelli, M.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Cresci, A. Abusive use of antibiotics in poultry farming in Cameroon and the public health implications. Br. Poult. Sci. 2016, 57, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Sanchez, S.; Moscoso, S.; Solis de los Santos, F.; Andino, A.; Hanning, I. Antibiotic use in poultry: A driving force for organic poultry production. Food Prot. Trends 2015, 35, 440–447. [Google Scholar]
- Xu, J.; Sangthong, R.; McNeil, E.; Tang, R.; Chongsuvivatwong, V. Antibiotic use in chicken farms in northwestern China. Antimicrob. Resist. Infect. Control 2020, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Vaish, R.; Pradeep, M.; Setty, C.; Kandi, V. Evaluation of virulence factors and antibiotic sensitivity pattern of Escherichia coli isolated from extraintestinal infections. Cureus 2016, 8, 1–13. [Google Scholar]
- Matin, M.A.; Islam, M.A.; Khatun, M.M. Prevalence of colibacillosis in chickens in greater Mymensingh district of Bangladesh. Vet. World 2017, 10, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eltai, N.O.; Yassine, H.M.; El-Obeid, T.; Al-Hadidi, S.H.; Al Thani, A.A.; Alali, W.Q. Prevalence of Antibiotic-Resistant Escherichia coli Isolates from Local and Imported Retail Chicken Carcasses. J. Food Prot. 2020, 83, 2200–2208. [Google Scholar] [CrossRef] [PubMed]
- Apata, D. Antibiotic resistance in poultry. Int. J. Poult. Sci. 2009, 8, 404–408. [Google Scholar] [CrossRef] [Green Version]
- WHO. Joint External Evaluation Qatar: Mission Report; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Johnson, T.J.; Kariyawasam, S.; Wannemuehler, Y.; Mangiamele, P.; Johnson, S.J.; Doetkott, C.; Skyberg, J.A.; Lynne, A.M.; Johnson, J.R.; Nolan, L.K. The genome sequence of avian pathogenic Escherichia coli strain O1: K1: H7 shares strong similarities with human extraintestinal pathogenic E. coli genomes. J. Bacteriol. 2007, 189, 3228–3236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eltai, N.O.; Yassine, H.M.; Al Thani, A.A.; Madi, M.A.A.; Ismail, A.; Ibrahim, E.; Alali, W.Q. Prevalence of antibiotic resistant Escherichia coli isolates from fecal samples of food handlers in Qatar. Antimicrob. Resist. Infect. Control 2018, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Eltai, N.O.; Abdfarag, E.A.; Al-Romaihi, H.; Wehedy, E.; Mahmoud, M.H.; Alawad, O.K.; Al-Hajri, M.M.; Al Thani, A.A.; Yassine, H.M. Antibiotic resistance profile of commensal Escherichia coli isolated from broiler chickens in Qatar. J. Food Prot. 2018, 81, 302–307. [Google Scholar] [CrossRef]
- Johnson, T.J.; Wannemuehler, Y.; Doetkott, C.; Johnson, S.J.; Rosenberger, S.C.; Nolan, L.K. Identification of minimal predictors of avian pathogenic Escherichia coli virulence for use as a rapid diagnostic tool. J. Clin. Microbiol. 2008, 46, 3987–3996. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.-G.; Cha, S.-Y.; Choi, E.-J.; Kim, B.; Song, H.-J.; Jang, H.-K. Epidemiological prevalence of avian pathogenic Escherichia coli differentiated by multiplex PCR from commercial chickens and hatchery in Korea. J. Bacteriol. Virol. 2008, 38, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Sarker, M.S.; Mannan, M.S.; Ali, M.Y.; Bayzid, M.; Ahad, A.; Bupasha, Z.B. Antibiotic resistance of Escherichia coli isolated from broilers sold at live bird markets in Chattogram, Bangladesh. J. Adv. Vet. Anim. Res. 2019, 6, 272. [Google Scholar] [CrossRef]
- Moawad, A.A.; Hotzel, H.; Awad, O.; Tomaso, H.; Neubauer, H.; Hafez, H.M.; El-Adawy, H. Occurrence of Salmonella enterica and Escherichia coli in raw chicken and beef meat in northern Egypt and dissemination of their antibiotic resistance markers. Gut Pathog. 2017, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Altalhi, A.D.; Gherbawy, Y.A.; Hassan, S.A. Antibiotic resistance in Escherichia coli isolated from retail raw chicken meat in Taif, Saudi Arabia. Foodborne Pathog. Dis. 2010, 7, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Laarem, M.; Barguigua, A.; Nayme, K.; Akila, A.; Zerouali, K.; El Mdaghri, N.; Timinouni, M. Occurrence of plasmid-mediated quinolone resistance and virulence genes in avian Escherichia coli isolates from Algeria. J. Infect. Dev. Ctries. 2017, 11, 143–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Lai, H.; Zou, L.; Yin, S.; Wang, C.; Han, X.; Xia, X.; Hu, K.; He, L.; Zhou, K. Antimicrobial resistance and resistance genes in Salmonella strains isolated from broiler chickens along the slaughtering process in China. Int. J. Food Microbiol. 2017, 259, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Romero-Barrios, P.; Deckert, A.; Parmley, E.J.; Leclair, D. Antimicrobial Resistance Profiles of Escherichia coli and Salmonella Isolates in Canadian Broiler Chickens and Their Products. Foodborne Pathog. Dis. 2020, 17, 672–678. [Google Scholar] [CrossRef]
- Overdevest, I.; Willemsen, I.; Rijnsburger, M.; Eustace, A.; Xu, L.; Hawkey, P.; Heck, M.; Savelkoul, P.; Vandenbroucke-Grauls, C.; van der Zwaluw, K. Extended-spectrum β-lactamase genes of Escherichia coli in chicken meat and humans, The Netherlands. Emerg. Infect. Dis. 2011, 17, 1216. [Google Scholar] [CrossRef]
- Roth, N.; Käsbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Domig, K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult. Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef]
- Garcia Ovando, H.; Gorla, N.; Luders, C.; Poloni, G.; Errecalde, C.; Prieto, G.; Puelles, I. Comparative pharmacokinetics of enrofloxacin and ciprofloxacin in chickens. J. Vet. Pharmacol. Ther. 1999, 22, 209–212. [Google Scholar] [CrossRef]
- Ewers, C.; Janßen, T.; Kießling, S.; Philipp, H.-C.; Wieler, L.H. Rapid detection of virulence-associated genes in avian pathogenic Escherichia coli by multiplex polymerase chain reaction. Avian Dis. 2005, 49, 269–273. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, CLSI Supplement M100, 30th ed.; CLSI: Annapolis, MD, USA, 2020. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, CLSI Supplement M100, 26th ed.; CLSI: Annapolis, MD, USA, 2016. [Google Scholar]
- Rebelo, A.R.; Bortolaia, V.; Kjeldgaard, J.S.; Pedersen, S.K.; Leekitcharoenphon, P.; Hansen, I.M.; Guerra, B.; Malorny, B.; Borowiak, M.; Hammerl, J.A. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Eurosurveillance 2018, 23, 17–00672. [Google Scholar] [CrossRef]
- Woodford, N.; Fagan, E.J.; Ellington, M.J. Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum β-lactamases. J. Antimicrob. Chemother. 2006, 57, 154–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bora, A.; Hazarika, N.K.; Shukla, S.K.; Prasad, K.N.; Sarma, J.B.; Ahmed, G. Prevalence of blaTEM, blaSHV and blaCTX-M genes in clinical isolates of Escherichia coli and Klebsiella pneumoniae from Northeast India. Indian J. Pathol. Microbiol. 2014, 57, 249. [Google Scholar]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, R.; Van Heerden, E.; Piater, L. Using current molecular techniques for rapid differentiation of Salmonella Typhi and Salmonella Typhimurium. Afr. J. Biotechnol. 2009, 8, 1–4. [Google Scholar]



| Resistance Phenotype 1 | Multi-Drug Resistance (MDR) 2 | Frequency | Percentage (%) | ||
|---|---|---|---|---|---|
| E. coli (NH) 3 | E. coli (H) 4 | E. coli (NH) 3 | E. coli (H) 4 | ||
| AMP, K.F., CIP, T.E., FOS, C | + | 20.0 | 1.0 | 14.8 | 5.9 |
| AMP, AUG, K.F., CIP, T.E., FOS, C | + | 17.0 | 1.0 | 12.6 | 5.9 |
| AMP, K.F., CIP, TE., C.S., FOS, C | + | 9.0 | 0.0 | 6.7 | 0.0 |
| AMP, AUG, K.F., CIP, T.E., C.S., FOS, C | + | 8.0 | 0.0 | 5.9 | 0.0 |
| AMP, AUG, K.F., CIP, T.E., SXT, FOS, C | + | 6.0 | 0.0 | 4.4 | 0.0 |
| AMP, K.F., CIP, FOS, C | + | 5.0 | 0.0 | 3.7 | 0.0 |
| AMP, K.F., CIP, T.E., SXT, FOS, C | + | 4.0 | 2.0 | 3.0 | 11.8 |
| AMP, K.F., CIP, T.E., C.S., C | + | 4.0 | 0.0 | 3.0 | 0.0 |
| AMP, K.F., CIP, T.E., C.S., FOS | + | 4.0 | 0.0 | 3.0 | 0.0 |
| AMP, K.F., CIP, T.E., SXT, FOS | + | 3.0 | 4.0 | 2.2 | 23.5 |
| AMP, AUG, K.F., CIP, FOS, C | + | 3.0 | 0.0 | 2.2 | 0.0 |
| AMP, K.F., CIP, T.E., SXT, C | + | 2.0 | 2.0 | 1.5 | 11.8 |
| AMP, AUG, K.F., CIP, T.E., CSC.S., SXT, FOS | + | 2.0 | 0.0 | 1.5 | 0.0 |
| AMP, AUG, K.F., CIP, T.E., FOS | + | 2.0 | 0.0 | 1.5 | 0.0 |
| AMP, AUG, K.F., CIP, T.E., SXT, C | + | 2.0 | 0.0 | 1.5 | 0.0 |
| AMP, AUG, K.F., T.E., C.S., FOS, C | + | 2.0 | 0.0 | 1.5 | 0.0 |
| AMP, K.F., CIP, C.S., FOS, C | + | 2.0 | 0.0 | 1.5 | 0.0 |
| AMP, K.F., CIP, C.S., SXT, FOS, C | + | 2.0 | 0.0 | 1.5 | 0.0 |
| AMP, K.F., CIP, SXT, FOS, C | + | 2.0 | 0.0 | 1.5 | 0.0 |
| AMP, K.F., CIP, T.E., FOS | + | 2.0 | 0.0 | 1.5 | 0.0 |
| MP, K.F., CXM, CRO, CIP, T.E., C.N., SXT, FOS, C | + | 2.0 | 0.0 | 1.5 | 0.0 |
| AMP, AUG, KFKF, CIP, T.E., C.N., FOS | + | 1.0 | 1.0 | 0.7 | 5.9 |
| AMP, KF, CIP, TE, CN, SXT, FOS, C | + | 1.0 | 1.0 | 0.7 | 5.9 |
| AMP, K.F., CIP, T.E., SXT | + | 1.0 | 1.0 | 0.7 | 5.9 |
| AMP, AUG, K.F., CIP, SXT, FOS, C, TZP | + | 1.0 | 0.0 | 0.7 | 0.0 |
| AMP, AUG, K.F., CIP, C | + | 1.0 | 0.0 | 0.7 | 0.0 |
| AMP, AUG, K.F., CIP, FOS, C | + | 1.0 | 0.0 | 0.7 | 0.0 |
| AMP, AUG, K.F., CIP, SXT, C | + | 1.0 | 0.0 | 0.7 | 0.0 |
| AMP, AUG, K.F., CIP, SXT, FOS | + | 1.0 | 0.0 | 0.7 | 0.0 |
| AMP, AUG, K.F., CIP, T.E., C | + | 1.0 | 0.0 | 0.7 | 0.0 |
| AMP, AUG, K.F., CIP, T.E., CSC.S., C.N., FOS, C | + | 1.0 | 0.0 | 0.7 | 0.0 |
| AMP, AUG, K.F., CIP, T.E., CSC.S., C.N., FOS, C, TZP | + | 1.0 | 0.0 | 0.7 | 0.0 |
| AMP, AUG, K.F., CIP, T.E., C.N., SXT, C | + | 1.0 | 0.0 | 0.7 | 0.0 |
| AMP, AUG, K.F., CIP, T.E., FOS | + | 1.0 | 0.0 | 0.7 | 0.0 |
| 5 AMP, AUG, K.F., CXM, CRO, CIP, T.E., C.N., SXT, C | + | 1.0 | 0.0 | 0.7 | 0.0 |
| 5 AMP, AUG, K.F., CXM, CRO, CIP, T.E., C.S., FOS, C | + | 1.0 | 0.0 | 0.7 | 0.0 |
| AMP, K.F., CIP | + | 1.0 | 0.0 | 0.7 | 0.0 |
| AMP, K.F., CIP, C.S., C | + | 1.0 | 0.0 | 0.7 | 0.0 |
| AMP, K.F., CIP, FOS | + | 1.0 | 0.0 | 0.7 | 0.0 |
| AMP, K.F., CIP, SXT | + | 1.0 | 0.0 | 0.7 | 0.0 |
| AMP, K.F., CIP, SXT, FOS | + | 1.0 | 0.0 | 0.7 | 0.0 |
| AMP, K.F., CIP, T.E., C | + | 1.0 | 0.0 | 0.7 | 0.0 |
| AMP, KF, CIP, TE, CN, SXT, FOS | + | 1.0 | 0.0 | 0.7 | 0.0 |
| AMP, K.F., CIP, T.E., C.S., C.N., C | + | 1.0 | 0.0 | 0.7 | 0.0 |
| AMP, K.F., CIP, T.E., C.S., C.N., SXT, FOS, C | + | 1.0 | 0.0 | 0.7 | 0.0 |
| AMP, K.F., CIP, T.E., C.S., SXT, FOS, C | + | 1.0 | 0.0 | 0.7 | 0.0 |
| 5 AMP, K.F., CXM, CRO, CIP, T.E. | + | 1.0 | 0.0 | 0.7 | 0.0 |
| 5 AMP, K.F., CXM, CRO, CIP, T.E., C.S., FOS, C | + | 1.0 | 0.0 | 0.7 | 0.0 |
| AMP, K.F., T.E., SXT, FOS | + | 1.0 | 0.0 | 0.7 | 0.0 |
| AUG, K.F., CIP, T.E., C.S., FOS, C | + | 1.0 | 0.0 | 0.7 | 0.0 |
| K.F., CIP, C.N. | + | 1.0 | 0.0 | 0.7 | 0.0 |
| K.F., CIP, C.N., FOS | + | 1.0 | 0.0 | 0.7 | 0.0 |
| K.F., CIP, C.N., FOS, C | + | 1.0 | 0.0 | 0.7 | 0.0 |
| K.F., CIP, FOS | + | 0.0 | 2.0 | 0.0 | 11.8 |
| AMP, AUG, K.F., CIP, SXT, FOS, C | + | 0.0 | 1.0 | 0.0 | 5.9 |
| 6 CIP, T.E. | - | 0.0 | 1.0 | 0.0 | 5.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johar, A.; Al-Thani, N.; Al-Hadidi, S.H.; Dlissi, E.; Mahmoud, M.H.; Eltai, N.O. Antibiotic Resistance and Virulence Gene Patterns Associated with Avian Pathogenic Escherichia coli (APEC) from Broiler Chickens in Qatar. Antibiotics 2021, 10, 564. https://doi.org/10.3390/antibiotics10050564
Johar A, Al-Thani N, Al-Hadidi SH, Dlissi E, Mahmoud MH, Eltai NO. Antibiotic Resistance and Virulence Gene Patterns Associated with Avian Pathogenic Escherichia coli (APEC) from Broiler Chickens in Qatar. Antibiotics. 2021; 10(5):564. https://doi.org/10.3390/antibiotics10050564
Chicago/Turabian StyleJohar, Alreem, Najlaa Al-Thani, Sara H. Al-Hadidi, Elyes Dlissi, Mahmoud H. Mahmoud, and Nahla O. Eltai. 2021. "Antibiotic Resistance and Virulence Gene Patterns Associated with Avian Pathogenic Escherichia coli (APEC) from Broiler Chickens in Qatar" Antibiotics 10, no. 5: 564. https://doi.org/10.3390/antibiotics10050564
APA StyleJohar, A., Al-Thani, N., Al-Hadidi, S. H., Dlissi, E., Mahmoud, M. H., & Eltai, N. O. (2021). Antibiotic Resistance and Virulence Gene Patterns Associated with Avian Pathogenic Escherichia coli (APEC) from Broiler Chickens in Qatar. Antibiotics, 10(5), 564. https://doi.org/10.3390/antibiotics10050564







