The Influence of Cellulose-Type Formulants on Anti-Candida Activity of the Tyrocidines
Abstract
1. Introduction
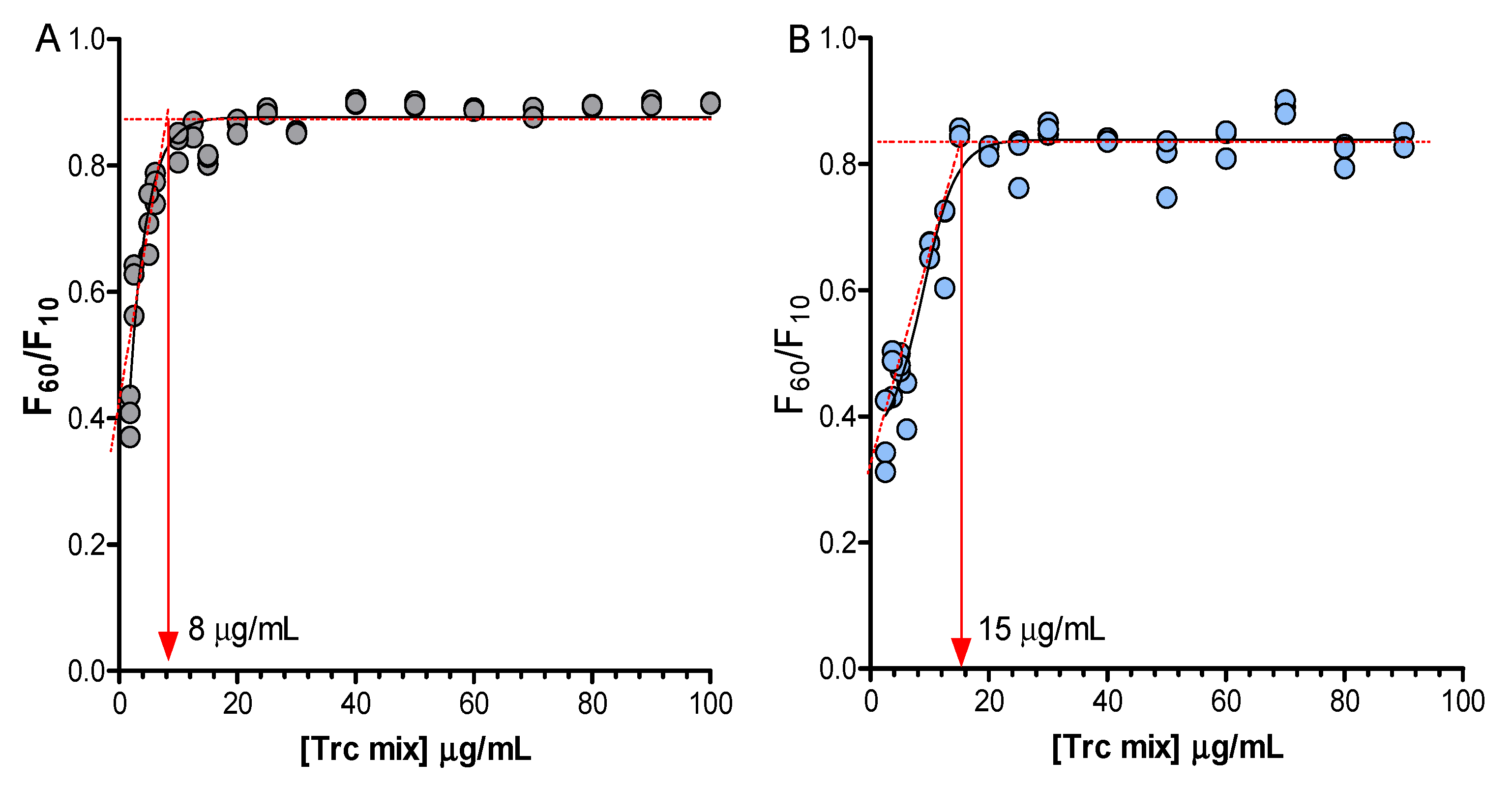
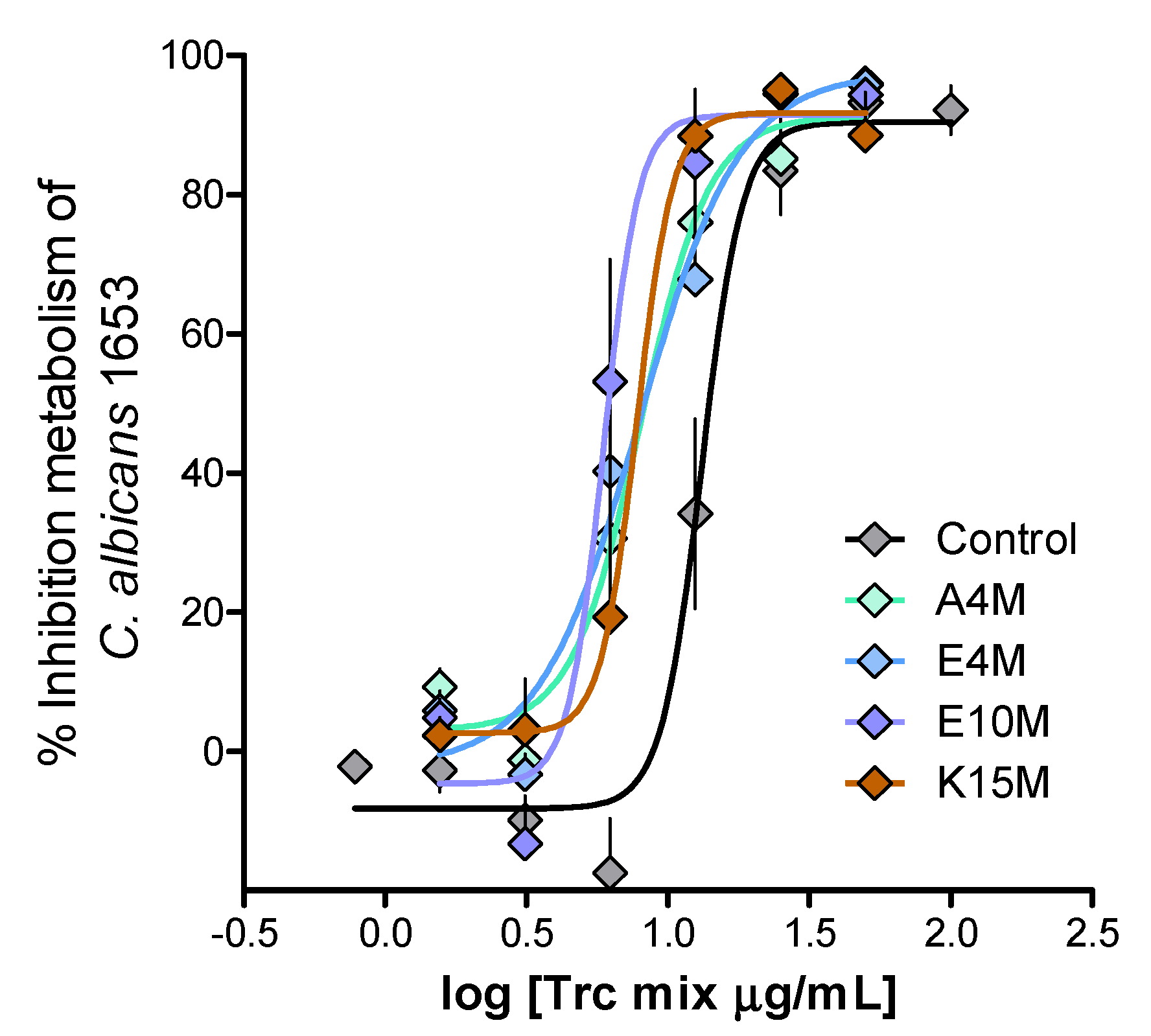
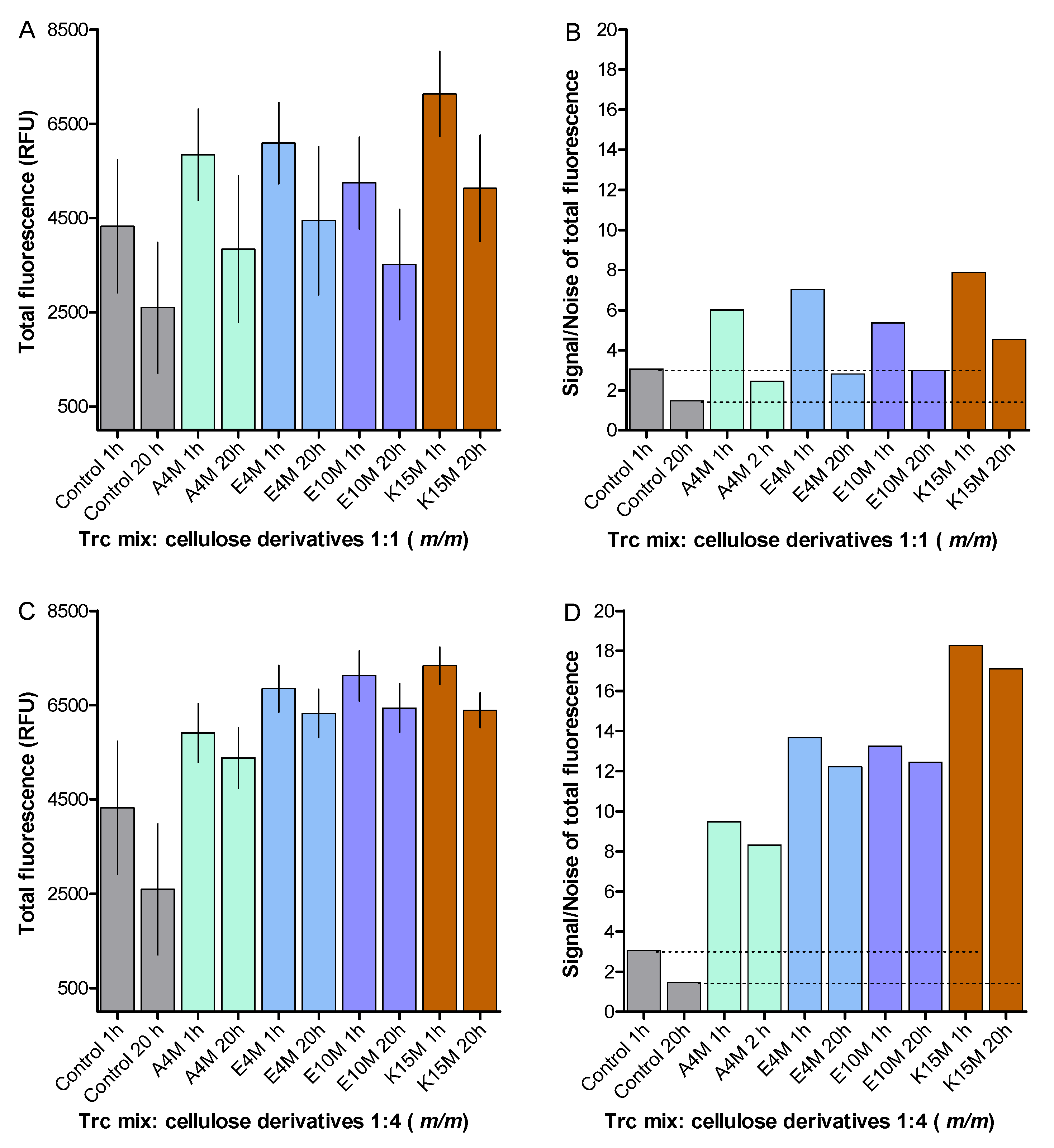
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Culture Media
3.3. Culturing and Growth Conditions of C. albicans and C. glabrata
3.4. Preparation of Formulations of Trc Mix
3.5. Fluorescence Spectroscopy
3.6. Anti-Candida Assays
3.7. Haemolytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Achkar, J.M.; Fries, B.C. Candida infections of the genitourinary tract. Clin. Microbiol. Rev. 2010, 23, 253–273. [Google Scholar] [CrossRef]
- Ruhnke, M. Epidemiology of Candida albicans infections and role of non-Candida albicans yeasts. Curr. Drug Targets 2006, 7, 495–504. [Google Scholar] [CrossRef]
- Brown, A.J.; Brown, G.D.; Netea, M.G.; Gow, N.A. Metabolism impacts upon Candida immunogenicity and pathogenicity at multiple levels. Trends Microbiol. 2014, 22, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Noble, S.M.; Gianetti, B.A.; Witchley, J.N. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat. Rev. Microbiol. 2017, 15, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Perfect, J.R. The antifungal pipeline: A reality check. Nat. Rev. Drug Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Taff, H.T.; Mitchell, K.F.; Edward, J.A.; Andes, D.R. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013, 8, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal biofilm resistance. Int. J. Microbiol. 2012, 2012, 528521. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, D.; Odds, F.C. Resistance of Candida species to antifungal agents: Molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2002, 2, 73–85. [Google Scholar] [CrossRef]
- Thevissen, K.; Kristensen, H.H.; Thomma, B.P.; Cammue, B.P.; François, I.E. Therapeutic potential of antifungal plant and insect defensins. Drug Discov. Today 2007, 12, 966–971. [Google Scholar] [CrossRef]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef]
- Troskie, A.M.; Rautenbach, M.; Delattin, N.; Vosloo, J.A.; Dathe, M.; Cammue, B.P.A.; Thevissen, K. Synergistic activity of the tyrocidines, antimicrobial cyclodecapeptides from Bacillus aneurinolyticus, with amphotericin B and caspofungin against Candida albicans biofilms. Antimicrob. Agents Chemother. 2014, 58, 3697–3707. [Google Scholar] [CrossRef] [PubMed]
- Troskie, A.M.; de Beer, A.; Vosloo, J.A.; Jacobs, K.; Rautenbach, M. Inhibition of agronomically relevant fungal phytopathogens by tyrocidines, cyclic antimicrobial peptides isolated from Bacillus aneurinolyticus. Microbiology 2014, 160, 2089–2101. [Google Scholar] [CrossRef]
- Rautenbach, M.; Troskie, A.M.; Vosloo, J.A. Antifungal peptides: To be or not to be membrane active. Biochimie 2016, 130, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Vriens, K.; Cammue, B.P.A.; Thevissen, K. Antifungal plant defensins: Mechanisms of action and production. Molecules 2014, 19, 12280–12303. [Google Scholar] [CrossRef]
- Vosloo, J.A.; Rautenbach, M. Following tyrothricin peptide production by Brevibacillus parabrevis with electrospray mass spectrometry. Biochimie 2020, 179, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.D.; Dubos, R.J. Bactericidal fractions from an aerobic sporulation bacillus. J. Biol. Chem. 1941, 136, 803–804. [Google Scholar] [CrossRef]
- Dubos, R.J. Studies on a bactericidal agent extracted from a soil bacillus. I. Preparation of the agent. Its activity in vitro. J. Exp. Med. 1939, 70, 1–10. [Google Scholar] [CrossRef]
- Dubos, R.J.; Hotchkiss, R.D.; Coburn, A.F. The effect of gramicidin and tyrocidine on bacterial metabolism. J. Biol. Chem. 1942, 146, 421–426. [Google Scholar] [CrossRef]
- Spathelf, B.M.; Rautenbach, M. Anti-listerial activity and structure-activity relationships of the six major tyrocidines, cyclic decapeptides from Bacillus aneurinolyticus. Bioorg. Med. Chem. 2009, 17, 5541–5548. [Google Scholar] [CrossRef]
- Mach, B.; Slayman, C.W. Mode of action of tyrocidine on Neupospora. Biochim. Biophys. Acta 1966, 124, 351–361. [Google Scholar] [CrossRef]
- Rautenbach, M.; Troskie, A.M.; Vosloo, J.A.; Dathe, M.E. Antifungal membranolytic activity of the tyrocidines against filamentous plant fungi. Biochimie 2016, 130, 122–131. [Google Scholar] [CrossRef]
- Rautenbach, M.; Vlok, N.M.; Stander, M.; Hoppe, H.C. Inhibition of malaria parasite blood stages by tyrocidines, membrane-active cyclic peptide antibiotics from Bacillus brevis. Biochim. Biophys. Acta Biomembr. 2007, 1768, 1488–1497. [Google Scholar] [CrossRef]
- Jokonya, S.; Langlais, M.; Leshabane, M.; Reader, P.W.; Vosloo, J.A.; Pfukwa, R.; Coertzen, D.; Birkholtz, L.M.; Rautenbach, M.; Klumperman, B. Poly (N-vinylpyrrolidone) antimalaria conjugates of membrane-disruptive peptides. Biomacromolecules 2020, 21, 5053–5066. [Google Scholar] [CrossRef]
- Rammelkamp, C.H.; Weinstein, L. Toxic effects of tyrothricin, gramicidin and tyrocidine. J. Infect. Dis. 1942, 71, 166–173. [Google Scholar] [CrossRef]
- Van Epps, H.L. René Dubos: Unearthing antibiotics. J. Exp. Med. 2006, 203, 259. [Google Scholar] [CrossRef] [PubMed]
- Paladini, A.; Craig, L.C. The chemistry of tyrocidine. III. The structure of tyrocidine A. J. Am. Chem. Soc. 1954, 76, 688–692. [Google Scholar] [CrossRef]
- Tang, X.J.; Thibault, P.; Boyd, R.K. Characterisation of the tyrocidine and gramicidin fractions of the tyrothricin complex from Bacillus brevis using liquid chromatography and mass spectrometry. Int. J. Mass Spectrom. Ion Process. 1992, 122, 153–179. [Google Scholar] [CrossRef]
- Beyer, C.F.; Gibbons, W.A.; Craig, L.C.; Longworth, J.W. Heterogeneous tryptophan environments in the cyclic peptides tyrocidines B and C. Phosphorescence studies. J. Biol. Chem. 1974, 249, 3204–3211. [Google Scholar] [CrossRef]
- Gibbons, W.A.; Beyer, C.F.; Dadok, J.; Sprecher, R.F.; Wyssbrod, H.R. Studies of individual amino acid residues of the decapeptide tyrocidine A by proton double-resonance difference spectroscopy in the correlation mode. Biochemistry 1975, 14, 420–429. [Google Scholar] [CrossRef]
- Kuo, M.-C.; Gibbons, W.A. Nuclear Overhauser effect and cross-relaxation rate determinations of dihedral and transannular interproton distances in the decapeptide tyrocidine A. Biophys. J. 1980, 32, 807–836. [Google Scholar] [CrossRef]
- Kuo, M.-C.; Gibbons, W.A. Determination of individual side-chain conformations, tertiary conformations, and molecular topography of tyrocidine A from scalar coupling constants and chemical shifts. Biochemistry 1979, 18, 5855–5867. [Google Scholar] [CrossRef]
- Loll, P.J.; Upton, E.C.; Nahoum, V.; Economou, N.J.; Cocklin, S. The high resolution structure of tyrocidine A reveals an amphipathic dimer. Biochim. Biophys. Acta Biomembr. 2014, 1838, 1199–1207. [Google Scholar] [CrossRef]
- Munyuki, G.; Jackson, G.E.; Venter, G.A.; Kövér, K.E.; Szilágyi, L.; Rautenbach, M.; Spathelf, B.M.; Bhattacharya, B.; Van Der Spoel, D. β-sheet structures and dimer models of the two major tyrocidines, antimicrobial peptides from Bacillus aneurinolyticus. Biochemistry 2013, 52, 7798–7806. [Google Scholar] [CrossRef]
- Appleby, J.C.; Knowles, E.; Pearson, J.; White, T. A preliminary study of the formation, assay and stability of tyrothricin. J. Gen. Microbiol. 1947, 1, 137–144. [Google Scholar] [CrossRef][Green Version]
- Helle, S.S.; Zandstra, P.W.; Cooper, D.G. Unusual surface tension behavior of an aqueous solution of gramicidin S. J. Colloid Interface Sci. 1992, 151, 130–135. [Google Scholar] [CrossRef]
- Ruttenberg, M.A.; King, T.; Craig, L.C. The use of the tyrocidines for the study of conformation and aggregation behavior. J. Am. Chem. Soc. 1965, 87, 4196–4198. [Google Scholar] [CrossRef] [PubMed]
- Ruttenberg, M.A.; King, T.P.; Craig, L.C. The chemistry of tyrocidine. VII. Studies on association behavior and implications regarding conformation. Biochemistry 1966, 5, 2857–2864. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.P.S.; Hancock, R.E.W. The relationship between peptide structure and antibacterial activity. Peptides 2003, 24, 1681–1691. [Google Scholar] [CrossRef]
- Williams, R.C.; Yphantis, D.A.; Craig, L.C. Noncovalent association of tyrocidine B. Biochemistry 1972, 11, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Rautenbach, M.; Kumar, V.; Vosloo, J.A.; Masoudi, Y.; van Wyk, R.J.; Stander, M.A. Oligomerisation of tryptocidine C, a Trp-rich cyclodecapeptide from the antimicrobial tyrothricin complex. Biochimie 2020, 181, 123–133. [Google Scholar] [CrossRef]
- Prenner, E.J.; Kay, C.M.; Mcelhaney, R.N.; Hodges, R.S. Conformation and interaction of the cyclic cationic antimicrobial peptides in lipid bilayers. J. Pept. Res. 2002, 60, 23–36. [Google Scholar]
- Marques, M.A.; Citron, D.M.; Wang, C.C. Development of Tyrocidine A analogues with improved antibacterial activity. Bioorg. Med. Chem. 2007, 15, 6667–6677. [Google Scholar] [CrossRef]
- van Rensburg, W. Characterization of Natural Antimicrobial Peptides Adsorbed to Different Matrices. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2015. Available online: http://scholar.sun.ac.za/handle/10019.1/97929 (accessed on 17 November 2020).
- Rautenbach, M.; van Rensburg, W. Method for Preventing or Treating Microbial Growth on a Manufactured Product. U.S. Patent 10,512,271, 24 December 2019. [Google Scholar]
- van Rensburg, W. The Tyrocidines in the Creation of Antimicrobial Cellulose and Sterilising Materials. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2020. [Google Scholar]
- Juhl, D.W.; Rensburg, W.; Bossis, X.; Vosloo, J.A.; Rautenbach, M.; Bechinger, B. Tyrocidine A interactions with saccharides investigated by CD and NMR spectroscopies. J. Pept. Sci. 2019, 25, e3163. [Google Scholar] [CrossRef] [PubMed]
- Paradies, H.H. Aggregation of tyrocidine in aqueous solutions. Biochem. Biophys. Res. Commun. 1979, 88, 810–817. [Google Scholar] [CrossRef]
- Vosloo, J.A. Optimised Bacterial Production and Characterisation of Natural Antimicrobial Peptides with Potential Application in Agriculture. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2016. Available online: http://scholar.sun.ac.za/handle/10019.1/98411 (accessed on 22 October 2020).
- Stefanini, I. Yeast-insect associations: It takes guts. Yeast 2018, 35, 315–330. [Google Scholar] [CrossRef]
- Li, P.; Seneviratne, C.J.; Alpi, E.; Vizcaino, J.A.; Jin, L. Delicate metabolic control and coordinated stress response critically determine antifungal tolerance of Candida albicans biofilm persisters. Antimicrob. Agents Chemother. 2015, 59, 6101–6112. [Google Scholar] [CrossRef]
- Lafleur, M.D.; Kumamoto, C.A.; Lewis, K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 2006, 50, 3839–3846. [Google Scholar] [CrossRef]
- Wuyts, J.; Van Dijck, P.; Holtappels, M. Fungal persister cells: The basis for recalcitrant infections? PLoS Pathog. 2018, 14, e1007301. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. (Ed.) Principles of Fluorescence Spectroscopy; Springer: Berlin, Germany, 2013; pp. 529–567. ISBN 9780387312781. [Google Scholar]
- Chattopadhyay, A.; Raghuraman, H. Application of fluorescence spectroscopy to membrane protein structure and dynamics. Curr. Sci. 2004, 87, 175–180. [Google Scholar]
- Chen, Y.; Barkley, M.D. Toward understanding tryptophan fluorescence in proteins. Biochemistry 1998, 37, 9976–9982. [Google Scholar] [CrossRef]
- Thies, M.; Paradies, H.H. Self-Assembly of tyrocidines in nanotubular structures. MRS Online Proc. Libr. Arch. 1997, 489. [Google Scholar] [CrossRef]
- Paradies, H.H.; Reichelt, H. Formation and structures of tyrocidine B oligomers in solution in the presence of water. AIP Adv. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Vosloo, J.A.; Stander, M.A.; Leussa, A.N.N.; Spathelf, B.M.; Rautenbach, M. Manipulation of the tyrothricin production profile of Bacillus aneurinolyticus. Microbiology 2013, 159, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Rautenbach, M.; Gerstner, G.D.; Vlok, N.M.; Kulenkampff, J.; Westerhoff, H.V. Analyses of dose—Response curves to compare the antimicrobial activity of model cationic α-helical peptides highlights the necessity for a minimum of two activity parameters. Anal. Biochem. 2006, 350, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Lask, S. Tyrothricin as an antibiotic. Arch. Surg. 1948, 56, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Wigger-Alberti, W.; Stauss-Grabo, M.; Grigo, K.; Atiye, S.; Williams, R.; Korting, H.C. Efficacy of a tyrothricin-containing wound gel in an abrasive wound model for superficial wounds. Skin Pharmacol. Physiol. 2013, 26, 52–56. [Google Scholar] [CrossRef]
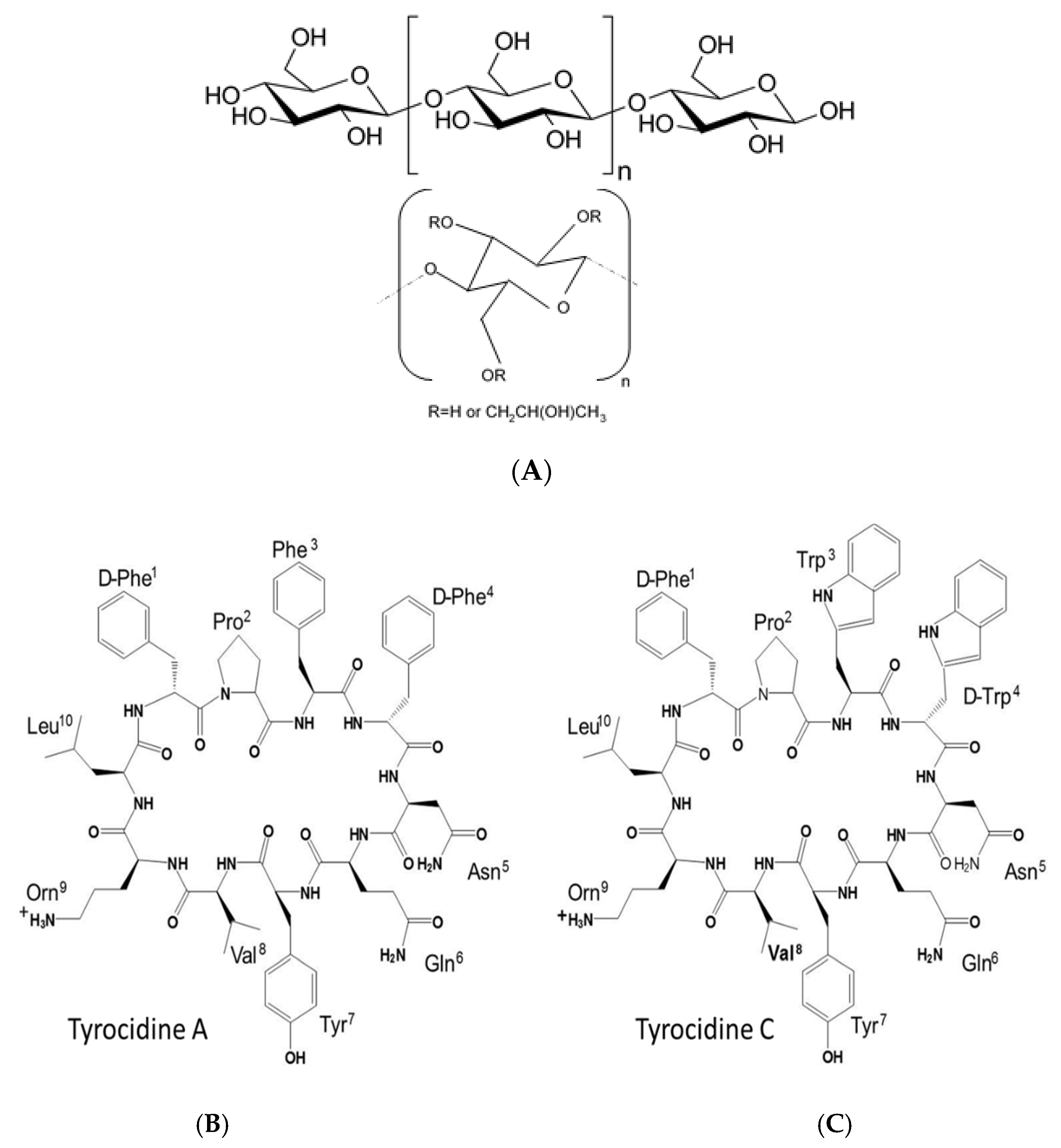



| Saccharide Derivative | Commercial ID Abbreviation | Modification | Viscosity Cps | Colour Code * |
|---|---|---|---|---|
| Methyl-cellulose | A4M | -CH3 | 3500–5600 | |
| Hydroxy-propyl-methyl-cellulose | E4M | -CH2OH/ -CH2CH(OH)CH3 | 2700–5040 | |
| Hydroxy-propyl-methyl- cellulose | E10M | -CH2OH/ -CH2CH(OH) CH3 | 7500–14,000 | |
| Hydroxy-propyl-cellulose | KLUE | -CH2OH | 200–600 | |
| Hydroxy-propyl- cellulose | KLUL | -CH2OH | 75–150 | |
| Hydroxy-propyl-methyl-cellulose | K15M | -CH2OH/ -CH2CH(OH)CH3 | 10,000–18,000 |
| Trc Mix Concentration in Formulation | |||
|---|---|---|---|
| Peptide Formulant | 50 µg/mL | 25 µg/mL | 12.5 µg/mL |
| Control | 4 * | 16 * | 52 * |
| A4M | 0 | 0 | 29 |
| E4M | 0 | 0 | 37 |
| E10M | 0 | 4 | 42 |
| KLUE | 0 | 0 | 58 |
| KLUL | 0 | 8 | 54 |
| K15M | 0 | 4 | 37 |
| IC50 ± SEM (µg/mL) | HC50 ± SEM (µg/mL) | Selectivity HC50/IC50 | |||||
|---|---|---|---|---|---|---|---|
| Formulant or Drug | Trc Mix: Formulant (m/m) | Fresh 1 h, n = 16 | Matured 20 h, n = 11–16 | Fresh 1 h, n = 3 | Matured 20 h, n = 3 | Fresh, 1 h | Matured 20 h |
| Control | 1:0 | 11.4 ± 1.3 * | 5.3 ± 0.4 | 14 ± 0.7 # | 12 ± 0.4 # | 1.2 | 2.2 |
| A4M | 1:1 | 8.6 ± 1.7 | 4.6 ± 0.0 | 13 ± 0.2 | 10 ± 0.2 | 1.5 | 2.2 |
| E4M | 1:1 | 6.6 ± 1.2 | 5.4 ± 0.5 | 13 ± 0.8 | 10 ± 0.2 | 2.0 | 1.9 |
| E10M | 1:1 | 8.1 ± 1.3 | 5.7 ± 0.6 | 12 ± 0.3 | 10 ± 0.2 | 1.4 | 1.7 |
| K15M | 1:1 | 8.9 ± 1.1 | 7.5 ± 1.3 | 13 ± 0.4 | 12 ± 0.2 | 1.5 | 1.6 |
| A4M | 1:2 | 8.9 ± 0.6 | 4.9 ± 0.2 | 14 ± 0.5 | 11 ± 0.7 | 1.6 | 1.7 |
| E4M | 1:2 | 10 ± 0.8 | 6.8 ± 1.2 | 16 ± 1.5 | 12 ± 0.5 | 1.5 | 2.2 |
| E10M | 1:2 | 9.2 ± 0.3 | 6.2 ± 0.5 | 15 ± 0.5 | 14 ± 0.6 | 1.7 | 2.2 |
| K15M | 1:2 | 8.9 ± 0.9 | 5.1 ± 0.2 | 13 ± 1.1 | 11 ± 0.3 | 1.4 | 2.2 |
| A4M | 1:4 | 9.6 ± 1.3 | 5.6 ± 0.5 | 17 ± 2.0 | 13 ± 0.5 | 1.7 | 2.4 |
| E4M | 1:4 | 8.8 ± 1.4 | 6.5 ± 0.9 | 16 ± 0.6 | 14 ± 0.5 | 1.8 | 2.1 |
| E10M | 1:4 | 7.0 ± 0.8 | 2.3 ± 0.7 | 14 ± 0.1 | 12 ± 0.3 | 1.9 | 5.3 |
| K15M | 1:4 | 8.2 ± 1.3 | 6.5 ± 1.2 | 14 ± 0.9 | 13 ± 0.3 | 1.7 | 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masoudi, Y.; van Rensburg, W.; Barnard-Jenkins, B.; Rautenbach, M. The Influence of Cellulose-Type Formulants on Anti-Candida Activity of the Tyrocidines. Antibiotics 2021, 10, 597. https://doi.org/10.3390/antibiotics10050597
Masoudi Y, van Rensburg W, Barnard-Jenkins B, Rautenbach M. The Influence of Cellulose-Type Formulants on Anti-Candida Activity of the Tyrocidines. Antibiotics. 2021; 10(5):597. https://doi.org/10.3390/antibiotics10050597
Chicago/Turabian StyleMasoudi, Yasamin, Wilma van Rensburg, Bernice Barnard-Jenkins, and Marina Rautenbach. 2021. "The Influence of Cellulose-Type Formulants on Anti-Candida Activity of the Tyrocidines" Antibiotics 10, no. 5: 597. https://doi.org/10.3390/antibiotics10050597
APA StyleMasoudi, Y., van Rensburg, W., Barnard-Jenkins, B., & Rautenbach, M. (2021). The Influence of Cellulose-Type Formulants on Anti-Candida Activity of the Tyrocidines. Antibiotics, 10(5), 597. https://doi.org/10.3390/antibiotics10050597







