Impact of a Nosocomial COVID-19 Outbreak on a Non-COVID-19 Nephrology Ward during the First Wave of the Pandemic in Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Institutional Setting and Study Period
2.2. Study Objectives
2.3. Variables and Data Sources
2.4. Clinical Classification of COVID-19 and Definition of Case
2.5. Intervention Methods
- -
- Review of medical files, laboratory records, and radiographic findings of confirmed cases;
- -
- Review of inpatient electronic tracking system to determine the movement of cases within and between units and to identify the exact location of beds occupied;
- -
- Scrutiny of staff records to understand work areas and shift patterns of infected staff;
- -
- Personal and telephone interviews with HCWs involved in the care of affected patients and preparation of a contact list for PCR studies;
- -
- Closure of affected ward with no admission for the first 2 weeks of the outbreak;
- -
- Banning of transfers to other wards;
- -
- Weekly PCR testing of all patients and HCWs (including cleaning staff) in the affected ward;
- -
- Mandatory confinement for HCWs with a positive PCR or who were close contacts with positive cases as per the hospital protocol;
- -
- Transfer of patients with a positive PCR to COVID-19 isolation wards;
- -
- Daily safety meeting with all staff involved in the outbreak;
- -
- Detailed analyses of human behaviors and interactions, room sizes, and ventilation characteristics of the ward and common areas.
2.6. Statistical Analysis
3. Results
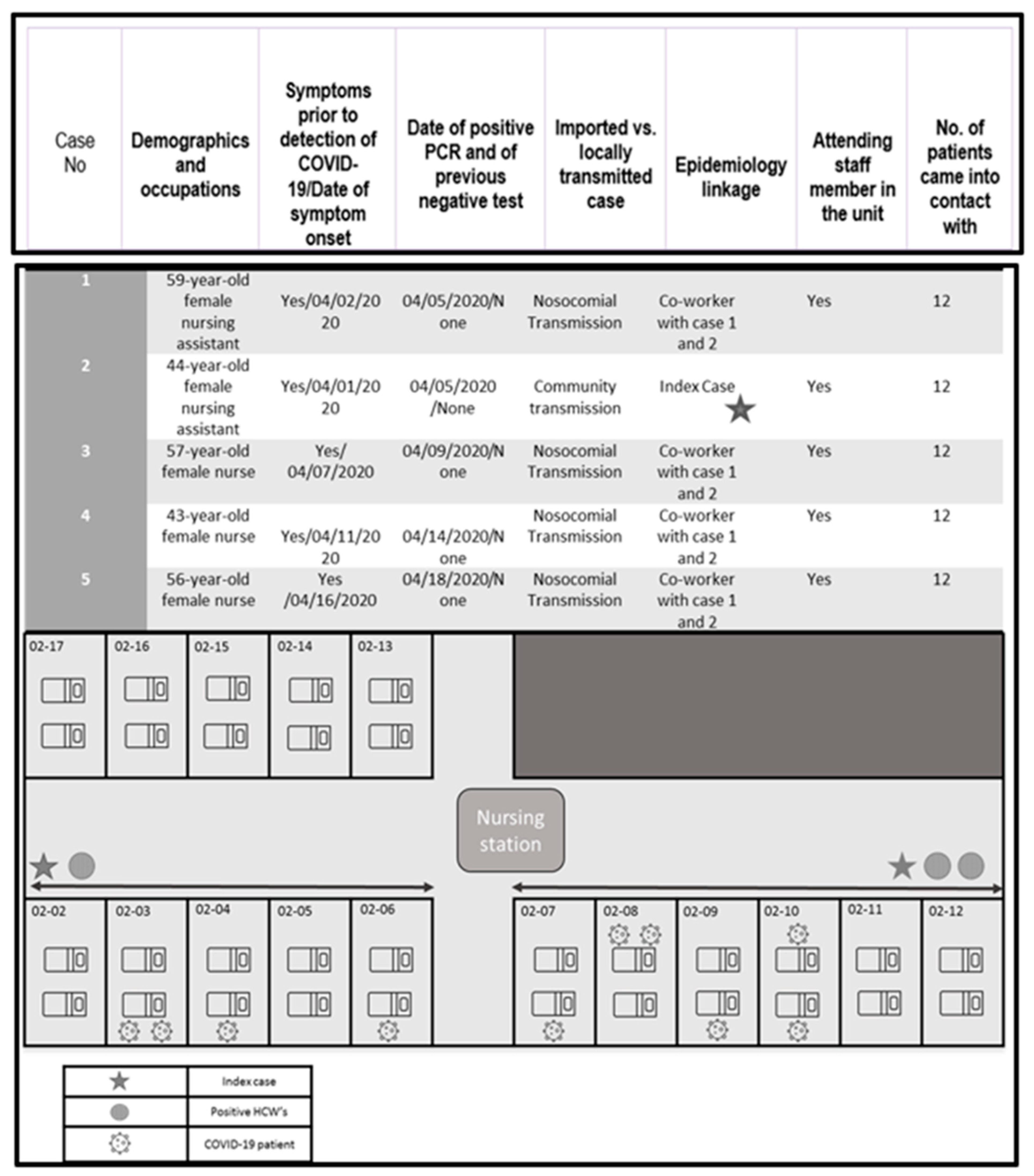
3.1. Epidemiological Curve
3.2. Case Detection and Epidemiological Investigation
3.3. Outbreak Management
- -
- Quarantine (14 days of preventive isolation for close-contact HCWs);
- -
- Transfer of positive patients to COVID isolation ward;
- -
- Implementation of preventive droplet and contact measures for all patients remaining in the ward, applicable to anyone entering the room: hand hygiene and use of surgical mask (or FFP2 mask for aerosol-generating procedures), eye protection (for aerosol-generating procedures or procedures with a risk of splash), gown (and apron for splash protection), and gloves;
- -
- Twice-weekly training sessions for all HCWs linked to the ward to emphasize the importance of preventing cross-infection and reinforce proper use of personal protection equipment (PPE) and hand hygiene. Before the COVID-19 pandemic, this ward had high compliance of infection control measures: great managing with isolation precautions, appropriate glove use, good hand hygiene compliance rate (2017: 87%; 2018: 87%; 2019: 69%), and when the pandemic started, universal masking was carried out for all HCWs; unfortunately, the infection control program was not available full time for conducting observations in non-COVID-19 units during the first part of the pandemic—in order to that, we have no data of compliance rates for this time;
- -
- Enhanced cleaning and disinfection: regular cleaning, followed by disinfection with 0.1% sodium hypochlorite (1000 ppm) of patient rooms (once a day), frequently touched surfaces, and all equipment in the affected ward (twice a day);
- -
- Weekly PCR tests for patients and HCWs on the ward;
- -
- Testing of proper operation of ventilation systems and adequate supplies of acceptable-quality indoor air adapted to occupancy at any given time;
- -
- These measures controlled the outbreak, and no new cases were detected up to the end of the study period (31 May).
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Chun-Quan China Medical Treatment Expert Group for Covid-19; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Situation Update Worldwide, as of 29 April 2021, (n.d.). Available online: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases (accessed on 29 April 2021).
- Coronavirus SARS-CoV-2. Canal Salut, (n.d.). Available online: https://canalsalut.gencat.cat/ca/salut-a-z/c/coronavirus-2019-ncov/ (accessed on 1 May 2021).
- Black, J.R.M.; Bailey, C.; Przewrocka, J.; Dijkstra, K.K.; Swanton, C. COVID-19: The case for health-care worker screening to prevent hospital transmission. Lancet 2020, 395, 1418–1420. [Google Scholar] [CrossRef]
- Islam, M.S.; Rahman, K.M.; Sun, Y.; Qureshi, M.O.; Abdi, I.; Chughtai, A.A.; Seale, H. Current knowledge of COVID-19 and infection prevention and control strategies in healthcare settings: A global analysis. Infect. Control. Hosp. Epidemiol. 2020, 41, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061. [Google Scholar] [CrossRef] [PubMed]
- Aghaizu, A.; Elam, G.; Ncube, F.; Thomson, G.; Szilágyi, E.; Eckmanns, T.; Poulakou, G.; Catchpole, M. Preventing the next ’SARS’—European healthcare workers’ attitudes towards monitoring their health for the surveillance of newly emerging infections: Qualitative study. BMC Public Health 2011, 11, 541. [Google Scholar] [CrossRef]
- Li, Y.-K.; Peng, S.; Li, L.-Q.; Wang, Q.; Ping, W.; Zhang, N.; Fu, X.-N. Clinical and Transmission Characteristics of Covid-19—A Retrospective Study of 25 Cases from a Single Thoracic Surgery Department. Curr. Med Sci. 2020, 40, 295–300. [Google Scholar] [CrossRef]
- Burrer, S.L.; De Perio, M.A.; Hughes, M.M.; Kuhar, D.T.; Luckhaupt, S.E.; McDaniel, C.J.; Porter, R.M.; Silk, B.; Stuckey, M.J.; Walters, M. Characteristics of Health Care Personnel with COVID-19 — United States, February 12–April 9, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 477–481. [Google Scholar] [CrossRef]
- Morawska, L.; Milton, D.K. It Is Time to Address Airborne Transmission of Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2020, 2311–2314. [Google Scholar] [CrossRef]
- Jayaweera, M.; Perera, H.; Gunawardana, B.; Manatunge, J. Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environ. Res. 2020, 188, 109819. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Jimenez, J.L.; A Prather, K.; Tufekci, Z.; Fisman, D.; Schooley, R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet 2021, 397, 1603–1605. [Google Scholar] [CrossRef]
- Ye, G.; Lin, H.; Chen, S.; Wang, S.; Zeng, Z.; Wang, W.; Zhang, S.; Rebmann, T.; Li, Y.; Pan, Z.; et al. Environmental contamination of SARS-CoV-2 in healthcare premises. J. Infect. 2020, 81, e1–e5. [Google Scholar] [CrossRef]
- Arons, M.M.; Hatfield, K.M.; Reddy, S.C.; Kimball, A.; James, A.; Jacobs, J.R.; Taylor, J.; Spicer, K.; Bardossy, A.C.; Oakley, L.P.; et al. Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility. N. Engl. J. Med. 2020, 382, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.W.X.; Tan, Y.K.; Chia, P.Y.; Lee, T.H.; Ng, O.T.; Wong, M.S.Y.; Marimuthu, K. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA 2020, 323, 1610. [Google Scholar] [CrossRef] [PubMed]
- Wee, L.E.; Sim, X.Y.J.; Conceicao, E.P.; Aung, M.K.; Goh, J.Q.; Yeo, D.W.T.; Gan, W.H.; Chua, Y.Y.; Wijaya, L.; Tan, T.T.; et al. Containment of COVID-19 cases among healthcare workers: The role of surveillance, early detection, and outbreak management. Infect. Control. Hosp. Epidemiol. 2020, 41, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Infectious Diseases Society of America Guidelines on Infection Prevention in Patients with Suspected or Known COVID-19, (n.d.). Available online: https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/ (accessed on 19 May 2020).
- Wang, Y.; Wang, Y.; Chen, Y.; Qin, Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J. Med. Virol. 2020, 92, 568–576. [Google Scholar] [CrossRef]
- Crespo, M.; Pérez-Sáez, M.J.; Redondo-Pachón, D.; Llinàs-Mallol, L.; Montero, M.M.; Villar-García, J.; Arias-Cabrales, C.; Buxeda, A.; Burballa, C.; Vázquez, S.; et al. COVID-19 in elderly kidney transplant recipients. Arab. Archaeol. Epigr. 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Arenas, M.D.; Crespo, M.; Pérez-Sáez, M.J.; Collado, S.; Redondo-Pachón, D.; Llinàs-Mallol, L.; Montero, M.M.; Villar-García, J.; Arias-Cabrales, C.; Barbosa, F.; et al. Clinical Profiles in Renal Patients with COVID-19. J. Clin. Med. 2020, 9, 2665. [Google Scholar] [CrossRef]
- Stone, S.P.; Cooper, B.S.; Kibbler, C.C.; Cookson, B.D.; A Roberts, J.; Medley, G.F.; Duckworth, G.; Lai, R.; Ebrahim, S.; Brown, E.M.; et al. The ORION statement: Guidelines for transparent reporting of outbreak reports and intervention studies of nosocomial infection. Lancet Infect. Dis. 2007, 7, 282–288. [Google Scholar] [CrossRef]
- Charlson, M.E.; Charlson, R.E.; Peterson, J.C.; Marinopoulos, S.S.; Briggs, W.M.; Hollenberg, J.P. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J. Clin. Epidemiol. 2008, 61, 1234–1240. [Google Scholar] [CrossRef]
- Kawana, A.; Teruya, K.; Kirikae, T.; Sekiguchi, J.-I.; Kato, Y.; Kuroda, E.; Horii, K.; Saito, S.-I.; Ohara, H.; Kuratsuji, T.; et al. “Syndromic surveillance within a hospital” for the early detection of a nosocomial outbreak of acute respiratory infection. Jpn. J. Infect. Dis. 2006, 59, 377. [Google Scholar]
- Infection Prevention and Control and Preparedness for COVID-19 in Healthcare Settings—Third Update, (n.d.). Available online: https://www.ecdc.europa.eu/en/publications-data/infection-prevention-and-control-and-preparedness-covid-19-healthcare-settings (accessed on 19 May 2020).
- A Treibel, T.; Manisty, C.; Burton, M.; McKnight, Á.; Lambourne, J.; Augusto, J.B.; Couto-Parada, X.; Cutino-Moguel, T.; Noursadeghi, M.; Moon, J.C. COVID-19: PCR screening of asymptomatic health-care workers at London hospital. Lancet 2020, 395, 1608–1610. [Google Scholar] [CrossRef]
- Rickman, H.M.; Rampling, T.; Shaw, K.; Martinez-Garcia, G.; Hail, L.; Coen, P.; Shahmanesh, M.; Shin, G.Y.; Nastouli, E.; Houlihan, C.F. Nosocomial Transmission of Coronavirus Disease 2019: A Retrospective Study of 66 Hospital-acquired Cases in a London Teaching Hospital. Clin. Infect. Dis. 2021, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.; Collins, J.; Barlow-Pay, F.; Rickard, F.; Bruce, E.; Verduri, A.; Quinn, T.; Mitchell, E.; Price, A.; Vilches-Moraga, A.; et al. Nosocomial COVID-19 infection: Examining the risk of mortality. The COPE-Nosocomial Study (COVID in Older PEople). J. Hosp. Infect. 2020, 106, 376–384. [Google Scholar] [CrossRef]
- Jewkes, S.V.; Zhang, Y.; Nicholl, D.J. Nosocomial spread of COVID-19: Lessons learned from an audit on a stroke/neurology ward in a UK district general hospital. Clin. Med. 2020, 20, e173–e177. [Google Scholar] [CrossRef] [PubMed]
- Van Praet, J.T.; Claeys, B.; Coene, A.-S.; Floré, K.; Reynders, M. Prevention of nosocomial COVID-19: Another challenge of the pandemic. Infect. Control. Hosp. Epidemiol. 2020, 41, 1355–1356. [Google Scholar] [CrossRef] [PubMed]

| SARS-CoV-2-Positive Hospitalized Patients (n = 10/31) | SARS-CoV-2-Positive Healthcare Workers (n = 5/43) | |
|---|---|---|
| Age * years, mean (SD) | 68.7 (15.5) | 52 (7.8) |
| Sex | ||
| Male | 5 (50%) | - |
| Female | 5 (50%) | 5 (100%) |
| Days from admission to positive PCR, median (IQR) | 10.5 (6–22) | |
| Underlying diseases | ||
| No comorbidities | 2 (20) | 5 (100%) |
| Charlson Comorbidity Index ≥ 2 | 8 (80) | 0 |
| Clinical presentation | ||
| Symptoms | ||
| Fever | 7 (70%) | 5 (100%) |
| Cough | 5 (50%) | 3 (60%) |
| Fatigue | 8 (80%) | 3 (60%) |
| Dyspnea | 7 (70%) | 0 |
| Diarrhea | 2 (20%) | 2 (40%) |
| Asymptomatic | 2 (20%) | 0 |
| Radiographic findings | 7 (70%) | 0 |
| All-cause mortality | ||
| Died | 5 (50%) | 0 |
| Alive | 5 (50%) | 5 (100%) |
| Mortality due to COVID-19 | ||
| No | 1 (10%) | - |
| Yes | 4 (40%) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montero, M.M.; Hidalgo López, C.; López Montesinos, I.; Sorli, L.; Barrufet Gonzalez, C.; Villar-García, J.; Güerri-Fernández, R.; Herranz, M.; Crespo, M.; Arenas Jiménez, M.D.; et al. Impact of a Nosocomial COVID-19 Outbreak on a Non-COVID-19 Nephrology Ward during the First Wave of the Pandemic in Spain. Antibiotics 2021, 10, 619. https://doi.org/10.3390/antibiotics10060619
Montero MM, Hidalgo López C, López Montesinos I, Sorli L, Barrufet Gonzalez C, Villar-García J, Güerri-Fernández R, Herranz M, Crespo M, Arenas Jiménez MD, et al. Impact of a Nosocomial COVID-19 Outbreak on a Non-COVID-19 Nephrology Ward during the First Wave of the Pandemic in Spain. Antibiotics. 2021; 10(6):619. https://doi.org/10.3390/antibiotics10060619
Chicago/Turabian StyleMontero, María Milagro, Carlota Hidalgo López, Inmaculada López Montesinos, Luisa Sorli, Cristina Barrufet Gonzalez, Judith Villar-García, Roberto Güerri-Fernández, Milagros Herranz, Marta Crespo, María Dolores Arenas Jiménez, and et al. 2021. "Impact of a Nosocomial COVID-19 Outbreak on a Non-COVID-19 Nephrology Ward during the First Wave of the Pandemic in Spain" Antibiotics 10, no. 6: 619. https://doi.org/10.3390/antibiotics10060619
APA StyleMontero, M. M., Hidalgo López, C., López Montesinos, I., Sorli, L., Barrufet Gonzalez, C., Villar-García, J., Güerri-Fernández, R., Herranz, M., Crespo, M., Arenas Jiménez, M. D., Pascual, J., González Juanes, C., & Horcajada, J. P. (2021). Impact of a Nosocomial COVID-19 Outbreak on a Non-COVID-19 Nephrology Ward during the First Wave of the Pandemic in Spain. Antibiotics, 10(6), 619. https://doi.org/10.3390/antibiotics10060619







