A Review of Systemic Minocycline Side Effects and Topical Minocycline as a Safer Alternative for Treating Acne and Rosacea
Abstract
1. Introduction
2. Systemic Minocycline for the Treatment of Dermatological Diseases
2.1. Rosacea and Acne Vulgaris
2.2. Antibiotic Resistance to Topical Macrolide Antibiotics Historically Used to Treat Acne
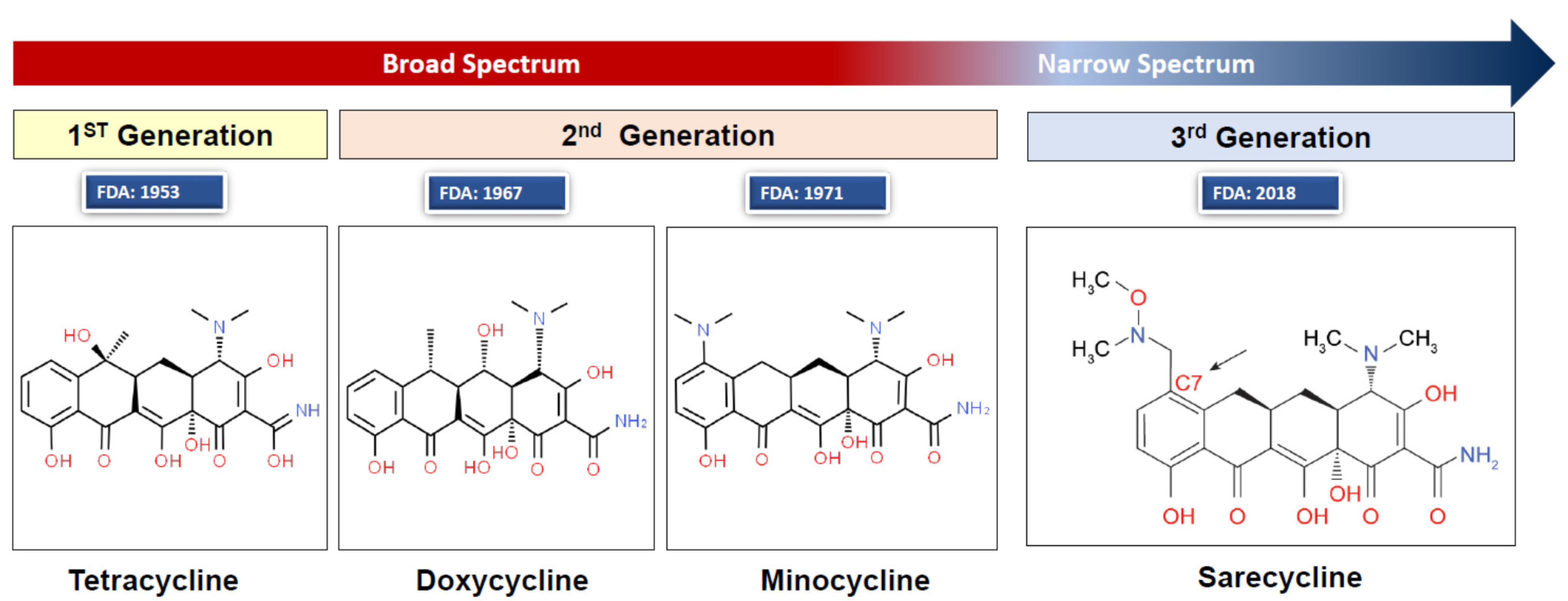
2.3. Treatment of Acne and Rosacea with the Tetracycline Class Antibiotics
2.4. Minocycline Dosage Forms Available in the United States
2.5. Pharmacokinetics of Systemic Minocycline
2.6. Efficacy of Systemic Minocycline for Treatment of Acne and Rosacea
3. Adverse Effects Caused by Systemic Minocycline and Contraindications
3.1. Adverse Effects of Oral Minocycline
3.1.1. Nervous System-Related
3.1.2. Skin-Related
3.1.3. Dentition-Related
3.1.4. Autoimmune-Related
3.1.5. Anaphylaxis
3.1.6. Respiratory System-Related
3.2. Contraindications
4. Interaction of Minocycline with Other Drugs
5. Topical Minocycline Development, Safety and Efficacy
5.1. Development of Topical Minocycline
5.2. Efficacy of Topical Minocycline
5.3. Pharmacokinetics of Topical Minocycline
5.4. Safety of Locally Administered Minocycline Using Topical Formulations
5.5. Bacterial Resistance to Topical Minocycline
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jukes, T.H. Some historical notes on chlortetracycline. Rev. Infect. Dis. 1985, 7, 702–707. [Google Scholar] [CrossRef]
- Jonas, M.; Cunha, B.A. Minocycline. Ther. Drug Monit. 1982, 4, 137–145. [Google Scholar] [CrossRef]
- Graber, E.M. Treating acne with the tetracycline class of antibiotics: A review. Dermatol. Rev. 2021. [Google Scholar] [CrossRef]
- Zhanel, G.; Critchley, I.; Lin, L.Y.; Alvandi, N. Microbiological Profile of Sarecycline, a Novel Targeted Spectrum Tetracycline for the Treatment of Acne Vulgaris. Antimicrob. Agents Chemother. 2018, 63, e01297–e01318. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef]
- Garrido-Mesa, N.; Zarzuelo, A.; Galvez, J. Minocycline: Far beyond an antibiotic. Br. J. Pharmacol. 2013, 169, 337–352. [Google Scholar] [CrossRef]
- Redin, G.S. Antibacterial activity in mice of minocycline, a new tetracycline. Antimicrob. Agents Chemother. 1966, 6, 371–376. [Google Scholar]
- Solodyn (Minocycline Hydrochloride) Extended Release Tablets. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/050808s000TOC.cfm (accessed on 13 June 2021).
- Minocycline (Topical). Available online: https://www.drugs.com/monograph/minocycline-topical.html (accessed on 18 February 2021).
- Sapadin, A.N.; Fleischmajer, R. Tetracyclines: Nonantibiotic properties and their clinical implications. J. Am. Acad. Dermatol. 2006, 54, 258–265. [Google Scholar] [CrossRef]
- Bikowski, J.B. Subantimicrobial dose doxycycline for acne and rosacea. Skinmed 2003, 2, 234–245. [Google Scholar] [CrossRef]
- Gether, L.; Overgaard, L.K.; Egeberg, A.; Thyssen, J.P. Incidence and prevalence of rosacea: A systematic review and meta-analysis. Br. J. Dermatol. 2018, 179, 282–289. [Google Scholar] [CrossRef]
- Buddenkotte, J.; Steinhoff, M. Recent advances in understanding and managing rosacea. F1000Research 2018, 7. [Google Scholar] [CrossRef]
- Farshchian, M.; Daveluy, S. Rosacea; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Huynh, T.T. Burden of Disease: The Psychosocial Impact of Rosacea on a Patient’s Quality of Life. Am. Health Drug Benefits 2013, 6, 348–354. [Google Scholar]
- Medgyesi, B.; Dajnoki, Z.; Beke, G.; Gaspar, K.; Szabo, I.L.; Janka, E.A.; Poliska, S.; Hendrik, Z.; Mehes, G.; Torocsik, D.; et al. Rosacea is Characterized by a Profoundly Diminished Skin Barrier. J. Investig. Dermatol. 2020, 140, 1938–1950. [Google Scholar] [CrossRef]
- Darlenski, R.; Kazandjieva, J.; Tsankov, N.; Fluhr, J.W. Acute irritant threshold correlates with barrier function, skin hydration and contact hypersensitivity in atopic dermatitis and rosacea. Exp. Dermatol. 2013, 22, 752–753. [Google Scholar] [CrossRef]
- Powell, F.C.; Ni Raghallaigh, S. Interventions for ‘rosacea’. Br. J. Dermatol. 2011, 165, 707–708. [Google Scholar] [CrossRef]
- Bhate, K.; Williams, H.C. Epidemiology of acne vulgaris. Br. J. Dermatol. 2013, 168, 474–485. [Google Scholar] [CrossRef]
- Tan, A.U.; Schlosser, B.J.; Paller, A.S. A review of diagnosis and treatment of acne in adult female patients. Int. J. Womens Dermatol. 2018, 4, 56–71. [Google Scholar] [CrossRef]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973. [Google Scholar] [CrossRef]
- Goodman, G. Acne and acne scarring - the case for active and early intervention. Aust. Fam. Physician 2006, 35, 503–504. [Google Scholar]
- Karadag, A.S.; Aslan Kayiran, M.; Wu, C.Y.; Chen, W.; Parish, L.C. Antibiotic resistance in acne: Changes, consequences and concerns. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 73–78. [Google Scholar] [CrossRef]
- Zeichner, J. An Update on the Use of Minocycline for the Treatment of Acne. Pract. Dermatol. 2020, 2020, 60–61. [Google Scholar]
- Alikhan, A.; Kurek, L.; Feldman, S.R. The role of tetracyclines in rosacea. Am. J. Clin. Dermatol. 2010, 11, 79–87. [Google Scholar] [CrossRef]
- Barbieri, J.S.; Bhate, K.; Hartnett, K.P.; Fleming-Dutra, K.E.; Margolis, D.J. Trends in Oral Antibiotic Prescription in Dermatology, 2008 to 2016. JAMA Dermatol. 2019, 155, 290–297. [Google Scholar] [CrossRef]
- Del Rosso, J.Q. Clinical Implications of Minocycline Use in Acne Vulgaris: Focus on Antimicrobial and Anti-inflammatory Properties. Cosmet. Dermatol. 2008, 21, 437–440. [Google Scholar]
- Del Rosso, J.Q. A status report on the use of subantimicrobial-dose doxycycline: A review of the biologic and antimicrobial effects of the tetracyclines. Cutis 2004, 74, 118–122. [Google Scholar] [PubMed]
- Leite, L.M.; Carvalho, A.G.; Ferreira, P.L.; Pessoa, I.X.; Goncalves, D.O.; Lopes Ade, A.; Goes, J.G.; Alves, V.C.; Leal, L.K.; Brito, G.A.; et al. Anti-inflammatory properties of doxycycline and minocycline in experimental models: An in vivo and in vitro comparative study. Inflammopharmacology 2011, 19, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Chosidow, O.; Poli, F.; Naline, E.; Advenier, C.; Revuz, J. Comedonal diffusion of minocycline in acne. Dermatology 1998, 196, 162. [Google Scholar] [CrossRef]
- Minocycline. Available online: https://go.drugbank.com/drugs/DB01017 (accessed on 26 February 2021).
- Minocycline Hydrochloride. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=050315 (accessed on 18 February 2021).
- Dinnendahl, V.; Minocyclin, F.U. Basisinformation über arzneiliche Wirkstoffe. In Arzneistoff-Profile; Dinnendahl, V., Minocyclin, F.U., Eds.; Govi Pharmazeutischer Verlag: Eschborn, Germany, 2010. (In German) [Google Scholar]
- Zhanel, G.G.; Adam, H.J.; Baxter, M.R.; Fuller, J.; Nichol, K.A.; Denisuik, A.J.; Lagace-Wiens, P.R.; Walkty, A.; Karlowsky, J.A.; Schweizer, F.; et al. Antimicrobial susceptibility of 22746 pathogens from Canadian hospitals: Results of the CANWARD 2007-11 study. J. Antimicrob. Chemother. 2013, 68, i7–i22. [Google Scholar] [CrossRef]
- Elewa, H.F.; Hilali, H.; Hess, D.C.; Machado, L.S.; Fagan, S.C. Minocycline for short-term neuroprotection. Pharmacotherapy 2006, 26, 515–521. [Google Scholar] [CrossRef]
- Brogden, R.N.; Speight, T.M.; Avery, G.S. Minocycline: A review of its antibacterial and pharmacokinetic properties and therapeutic use. Drugs 1975, 9, 251–291. [Google Scholar] [CrossRef]
- Fleischer, A.B., Jr.; Dinehart, S.; Stough, D.; Plott, R.T.; Solodyn Phase 2 Study Group; Solodyn Phase 3 Study Group. Safety and efficacy of a new extended-release formulation of minocycline. Cutis 2006, 78, 21–31. [Google Scholar]
- Dreno, B.; Moyse, D.; Alirezai, M.; Amblard, P.; Auffret, N.; Beylot, C.; Bodokh, I.; Chivot, M.; Daniel, F.; Humbert, P.; et al. Multicenter randomized comparative double-blind controlled clinical trial of the safety and efficacy of zinc gluconate versus minocycline hydrochloride in the treatment of inflammatory acne vulgaris. Dermatology 2001, 203, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Jernberg, C.; Lofmark, S.; Edlund, C.; Jansson, J.K. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 2010, 156, 3216–3223. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.S.H.; Akash, M.S.H. Toxicity of Antibiotics. In Antibiotics and Antimicrobial Resistance Genes in the Environment; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 234–252. [Google Scholar]
- Francino, M.P. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 2015, 6, 1543. [Google Scholar] [CrossRef]
- Oriel, J.D.; Waterworth, P.M. Effects of minocycline and tetracycline on the vaginal yeast flora. J. Clin. Pathol. 1975, 28, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Garner, S.E.; Eady, A.; Bennett, C.; Newton, J.N.; Thomas, K.; Popescu, C.M. Minocycline for acne vulgaris: Efficacy and safety. Cochrane Database Syst. Rev. 2012, CD002086. [Google Scholar] [CrossRef] [PubMed]
- Goulden, V.; Glass, D.; Cunliffe, W.J. Safety of long-term high-dose minocycline in the treatment of acne. Br. J. Dermatol. 1996, 134, 693–695. [Google Scholar] [CrossRef]
- Lebrun-Vignes, B.; Kreft-Jais, C.; Castot, A.; Chosidow, O.; French Network of Regional Centers of Pharmacovigilance. Comparative analysis of adverse drug reactions to tetracyclines: Results of a French national survey and review of the literature. Br. J. Dermatol. 2012, 166, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.E.; Knowles, S.R.; Shear, N.H. Comparative safety of tetracycline, minocycline, and doxycycline. Arch. Dermatol. 1997, 133, 1224–1230. [Google Scholar] [CrossRef]
- Hamilton, L.A.; Guarascio, A.J. Tetracycline Allergy. Pharmacy 2019, 7, 104. [Google Scholar] [CrossRef]
- MacNeil, M.; Haase, D.A.; Tremaine, R.; Marrie, T.J. Fever, lymphadenopathy, eosinophilia, lymphocytosis, hepatitis, and dermatitis: A severe adverse reaction to minocycline. J. Am. Acad. Dermatol. 1997, 36, 347–350. [Google Scholar] [CrossRef]
- Shankar, P.R. VigiAccess: Promoting public access to VigiBase. Indian J. Pharmacol. 2016, 48, 606–607. [Google Scholar] [CrossRef]
- Gump, D.W.; Ashikaga, T.; Fink, T.J.; Radin, A.M. Side effects of minocycline: Different dosage regimens. Antimicrob. Agents Chemother. 1977, 12, 642–646. [Google Scholar] [CrossRef][Green Version]
- Maubec, E.; Wolkenstein, P.; Loriot, M.A.; Wechsler, J.; Mulot, C.; Beaune, P.; Revuz, J.; Roujeau, J.C. Minocycline-induced DRESS: Evidence for accumulation of the culprit drug. Dermatology 2008, 216, 200–204. [Google Scholar] [CrossRef]
- Del Rosso, J.Q. Oral Doxycycline in the Management of Acne Vulgaris: Current Perspectives on Clinical Use and Recent Findings with a New Double-scored Small Tablet Formulation. J. Clin. Aesthetic Dermatol. 2015, 8, 19–26. [Google Scholar]
- Lindquist, M. VigiBase, the WHO Global ICSR Database System: Basic Facts. Ther. Innov. Regul. Sci. 2008, 42, 409–419. [Google Scholar] [CrossRef]
- Kircik, L.H. Doxycycline and minocycline for the management of acne: A review of efficacy and safety with emphasis on clinical implications. J. Drugs Dermatol. 2010, 9, 1407–1411. [Google Scholar]
- Pseudotumor Cerebri Information Page: What Research is Being Done? Available online: https://www.ninds.nih.gov/Disorders/All-Disorders/Pseudotumor-Cerebri-Information-Page (accessed on 18 February 2021).
- Donnet, A.; Dufour, H.; Graziani, N.; Grisoli, F. Minocycline and benign intracranial hypertension. Biomed. Pharmacother. 1992, 46, 171–172. [Google Scholar] [CrossRef]
- Thon, O.R.; Gittinger, J.W., Jr. Medication-Related Pseudotumor Cerebri Syndrome. Semin. Ophthalmol. 2017, 32, 134–143. [Google Scholar] [CrossRef]
- Kaabour, M.; Guerisse, F.; Mols, P.; Levy, S. Pseudotumor cerebri due to taking minocycline. Rev. Med. Brux. 2017, 38, 169–172. [Google Scholar]
- Fraser, C.L.; Biousse, V.; Newman, N.J. Minocycline-induced fulminant intracranial hypertension. Arch. Neurol. 2012, 69, 1067–1070. [Google Scholar] [CrossRef]
- Lee, A.G. Pseudotumor cerebri after treatment with tetracycline and isotretinoin for acne. Cutis 1995, 55, 165–168. [Google Scholar]
- Sierra, M.; David, A.S. Depersonalization: A selective impairment of self-awareness. Conscious. Cogn. 2011, 20, 99–108. [Google Scholar] [CrossRef]
- Cohen, P.R. Medication-associated depersonalization symptoms: Report of transient depersonalization symptoms induced by minocycline. South Med. J. 2004, 97, 70–73. [Google Scholar] [CrossRef]
- Shamout, Y.; Sigal, A.; Litvinov, I.V. Minocycline-induced transient depersonalization: A case report. SAGE Open Med. Case Rep. 2019, 7, 2050313X18823827. [Google Scholar] [CrossRef]
- Hasan, T.; Khan, A.U. Phototoxicity of the tetracyclines: Photosensitized emission of singlet delta dioxygen. Proc. Natl. Acad. Sci. USA 1986, 83, 4604–4606. [Google Scholar] [CrossRef]
- Monteiro, A.F.; Rato, M.; Martins, C. Drug-induced photosensitivity: Photoallergic and phototoxic reactions. Clin. Dermatol. 2016, 34, 571–581. [Google Scholar] [CrossRef]
- Moore, A.; Green, L.J.; Bruce, S.; Sadick, N.; Tschen, E.; Werschler, P.; Cook-Bolden, F.E.; Dhawan, S.S.; Forsha, D.; Gold, M.H.; et al. Once-Daily Oral Sarecycline 1.5 mg/kg/day Is Effective for Moderate to Severe Acne Vulgaris: Results from Two Identically Designed, Phase 3, Randomized, Double-Blind Clinical Trials. J. Drugs Dermatol. 2018, 17, 987–996. [Google Scholar]
- Filitis, D.C.; Graber, E.M. Minocycline-induced hyperpigmentation involving the oral mucosa after short-term minocycline use. Cutis 2013, 92, 46–48. [Google Scholar]
- Fiscus, V.; Hankinson, A.; Alweis, R. Minocycline-induced hyperpigmentation. J. Community Hosp. Intern. Med. Perspect. 2014, 4, 1. [Google Scholar] [CrossRef]
- Haskes, C.; Shea, M.; Imondi, D. Minocycline-Induced Scleral and Dermal Hyperpigmentation. Optom. Vis. Sci. 2017, 94, 436–442. [Google Scholar] [CrossRef]
- Geria, A.N.; Tajirian, A.L.; Kihiczak, G.; Schwartz, R.A. Minocycline-induced skin pigmentation: An update. Acta Dermatovenerol. Croat. 2009, 17, 123–126. [Google Scholar]
- Krause, W. Drug-induced hperpigemntation: A systematic review. J. Dtsch. Dermatol. Ges. 2013, 11, 644–651. [Google Scholar] [CrossRef]
- Kudrna, J.J.; Eisenbeisz, H.C.; Huot, C. Bilateral Tarsal Conjunctival Pigmentation After Eight Months of Minocycline Therapy. S. D. Med. 2020, 73, 360–365. [Google Scholar] [PubMed]
- Maloney, S.M.; Williams, B.K., Jr.; Shields, C.L. Long-term Minocycline Therapy With Scleral Pigmentation Simulating Melanocytosis. JAMA Ophthalmol. 2018, 136, e183088. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, K.; Tanemura, A.; Takafuji, M.; Hanaoka, Y.; Kiyohara, E.; Fujimoto, M. Case of minocycline-induced hyperpigmentation mimicking angiosarcoma. J. Dermatol. 2019, 46, e414–e416. [Google Scholar] [CrossRef]
- Katz, J.; Barak, S.; Shemer, J.; Langevitz, P.; Livneh, A. Black tongue associated with minocycline therapy. Arch. Dermatol. 1995, 131, 620. [Google Scholar]
- Tanzi, E.L.; Hecker, M.S. Minocycline-induced hyperpigmentation of the tongue. Arch. Dermatol. 2000, 136, 427–428. [Google Scholar] [CrossRef]
- Sanchez, A.R.; Rogers, R.S., III; Sheridan, P.J. Tetracycline and other tetracycline-derivative staining of the teeth and oral cavity. Int. J. Dermatol. 2004, 43, 709–715. [Google Scholar] [CrossRef]
- Good, M.L.; Hussey, D.L. Minocycline: Stain devil? Br. J. Dermatol. 2003, 149, 237–239. [Google Scholar] [CrossRef]
- Gait, R.C.; Affleck, A.G.; Leach, I.H.; Varma, S. Perinuclear antineutrophilic cytoplasmic antibody-positive polyarteritis nodosa secondary to minocycline treatment for acne vulgaris. J. Am. Acad. Dermatol. 2008, 58, S123–S124. [Google Scholar] [CrossRef]
- Elkayam, O.; Yaron, M.; Caspi, D. Minocycline-induced autoimmune syndromes: An overview. Semin. Arthritis Rheum. 1999, 28, 392–397. [Google Scholar] [CrossRef]
- Marzo-Ortega, H.; Baxter, K.; Strauss, R.M.; Drysdale, S.; Griffiths, B.; Misbah, S.A.; Gough, A.; Cunliffe, W.J.; Emery, P. Is minocycline therapy in acne associated with antineutrophil cytoplasmic antibody positivity? A cross-sectional study. Br. J. Dermatol. 2007, 156, 1005–1009. [Google Scholar] [CrossRef]
- Bonati, L.M.; Dover, J.S. Treating Acne With Topical Antibiotics: Current Obstacles and the Introduction of Topical Minocycline as a New Treatment Option. J. Drugs Dermatol. 2019, 18, 240–244. [Google Scholar]
- Brown, R.J.; Rother, K.I.; Artman, H.; Mercurio, M.G.; Wang, R.; Looney, R.J.; Cowen, E.W. Minocycline-induced drug hypersensitivity syndrome followed by multiple autoimmune sequelae. Arch. Dermatol. 2009, 145, 63–66. [Google Scholar] [CrossRef]
- Corneli, H.M. DRESS Syndrome: Drug Reaction With Eosinophilia and Systemic Symptoms. Pediatric Emerg. Care 2017, 33, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, K.K.; Bouchard, S.M.; Mohr, M.R.; Herre, J.M.; Salkey, K.S. Minocycline-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome with persistent myocarditis. J. Am. Acad. Dermatol. 2010, 62, 315–318. [Google Scholar] [CrossRef]
- Walsh, S.A.; Creamer, D. Drug reaction with eosinophilia and systemic symptoms (DRESS): A clinical update and review of current thinking. Clin. Exp. Dermatol. 2011, 36, 6–11. [Google Scholar] [CrossRef]
- Howell, E.; Paivanas, N.; Stern, J.; Vidula, H. Treatment of Acute Necrotizing Eosinophilic Myocarditis With Immunosuppression and Mechanical Circulatory Support. Circ. Heart Fail 2016, 9, e003665. [Google Scholar] [CrossRef]
- Kanno, K.; Sakai, H.; Yamada, Y.; Iizuka, H. Drug-induced hypersensitivity syndrome due to minocycline complicated by severe myocarditis. J. Dermatol. 2014, 41, 160–162. [Google Scholar] [CrossRef]
- Loner, C.A.; Crane, P.W. Use of Emergency Department Extracorporeal Membrane Oxygenation for Treatment of Acute Necrotizing Myocarditis. Clin. Pract. Cases Emerg. Med. 2019, 3, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.A.; Anadkat, M.J. Fever, eosinophilia, and death: A case of minocycline hypersensitivity. Cutis 2014, 93, 107–110. [Google Scholar]
- Diny, N.L.; Rose, N.R.; Cihakova, D. Eosinophils in Autoimmune Diseases. Front. Immunol. 2017, 8, 484. [Google Scholar] [CrossRef]
- Morikawa, D.; Hiraoka, E.; Obunai, K.; Norisue, Y. Myocarditis Associated with Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome: A Case Report and Review of the Literature. Am. J. Case Rep. 2018, 19, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Parneix-Spake, A.; Bastuji-Garin, S.; Lobut, J.B.; Erner, J.; Guyet-Rousset, P.; Revuz, J.; Roujeau, J.C. Minocycline as possible cause of severe and protracted hypersensitivity drug reaction. Arch. Dermatol. 1995, 131, 490–491. [Google Scholar] [CrossRef]
- Lan, J.; Lahoti, A.; Lew, D.B. A severe case of minocycline-induced DRESS resulting in liver transplantation and autoimmune sequelae. Ann. Allergy Asthma Immunol. 2016, 116, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Descamps, V.; Valance, A.; Edlinger, C.; Fillet, A.M.; Grossin, M.; Lebrun-Vignes, B.; Belaich, S.; Crickx, B. Association of human herpesvirus 6 infection with drug reaction with eosinophilia and systemic symptoms. Arch. Dermatol. 2001, 137, 301–304. [Google Scholar] [PubMed]
- Solhjoo, M.; Bansal, P.; Goyal, A.; Chauhan, K. Drug-Induced Lupus Erythematosus; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Knowles, S.R.; Shapiro, L.; Shear, N.H. Serious adverse reactions induced by minocycline. Report of 13 patients and review of the literature. Arch. Dermatol. 1996, 132, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J. Severe complication of a commonly prescribed drug: Minocycline-induced lupus. J. Am. Board Fam. Pract. 2002, 15, 239–241. [Google Scholar]
- Dunphy, J.; Oliver, M.; Rands, A.L.; Lovell, C.R.; McHugh, N.J. Antineutrophil cytoplasmic antibodies and HLA class II alleles in minocycline-induced lupus-like syndrome. Br. J. Dermatol. 2000, 142, 461–467. [Google Scholar] [CrossRef]
- Schlienger, R.G.; Bircher, A.J.; Meier, C.R. Minocycline-induced lupus. Dermatology 2000, 200, 223–231. [Google Scholar] [CrossRef]
- Clark, A.K.; Shi, V.Y.; Sivamani, R.K. Unique urticarial presentation of minocycline-induced lupus erythematosus. Dermatol. Online J. 2017, 23. [Google Scholar] [CrossRef]
- Bettge, A.M.; Gross, G.N. A serum sickness-like reaction to a commonly used acne drug. JAAPA 2008, 21, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Levenson, T.; Masood, D.; Patterson, R. Minocycline-induced serum sickness. Allergy Asthma Proc. 1996, 17, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Puyana, J.; Urena, V.; Quirce, S.; Fernandez-Rivas, M.; Cuevas, M.; Fraj, J. Serum sickness-like syndrome associated with minocycline therapy. Allergy 1990, 45, 313–315. [Google Scholar] [CrossRef]
- Malakar, S.; Dhar, S.; Shah Malakar, R. Is serum sickness an uncommon adverse effect of minocycline treatment? Arch. Dermatol. 2001, 137, 100–101. [Google Scholar] [PubMed]
- Lenert, P.; Icardi, M.; Dahmoush, L. ANA (+) ANCA (+) systemic vasculitis associated with the use of minocycline: Case-based review. Clin. Rheumatol. 2013, 32, 1099–1106. [Google Scholar] [CrossRef]
- Tehrani, R.; Nash-Goelitz, A.; Adams, E.; Dahiya, M.; Eilers, D. Minocycline-induced cutaneous polyarteritis nodosa. J. Clin. Rheumatol. 2007, 13, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Munoz-Furlong, A.; Campbell, R.L.; Adkinson, N.F., Jr.; Bock, S.A.; Branum, A.; Brown, S.G.; Camargo, C.A., Jr.; Cydulka, R.; Galli, S.J.; et al. Second symposium on the definition and management of anaphylaxis: Summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J. Allergy Clin. Immunol. 2006, 117, 391–397. [Google Scholar] [CrossRef]
- Jang, J.W.; Bae, Y.J.; Kim, Y.G.; Jin, Y.J.; Park, K.S.; Cho, Y.S.; Moon, H.B.; Kim, T.B. A case of anaphylaxis to oral minocycline. J Korean Med. Sci. 2010, 25, 1231–1233. [Google Scholar] [CrossRef]
- Okano, M.; Imai, S. Anaphylactoid symptoms due to oral minocycline. Acta Derm. Venereol. 1996, 76, 164. [Google Scholar] [CrossRef]
- Bartal, C.; Sagy, I.; Barski, L. Drug-induced eosinophilic pneumonia: A review of 196 case reports. Medicine 2018, 97, e9688. [Google Scholar] [CrossRef]
- Hung, S.W. Minocycline-induced acute eosinophilic pneumonia: A case report and review of the literature. Respir. Med. Case Rep. 2015, 15, 110–114. [Google Scholar] [CrossRef][Green Version]
- Philit, F.; Etienne-Mastroianni, B.; Parrot, A.; Guerin, C.; Robert, D.; Cordier, J.F. Idiopathic acute eosinophilic pneumonia: A study of 22 patients. Am. J. Respir. Crit. Care Med. 2002, 166, 1235–1239. [Google Scholar] [CrossRef]
- Buendia-Roldan, I.; Santiago-Ruiz, L.; Perez-Rubio, G.; Mejia, M.; Rojas-Serrano, J.; Ambrocio-Ortiz, E.; Benitez-Valdez, G.; Selman, M.; Falfan-Valencia, R. A major genetic determinant of autoimmune diseases is associated with the presence of autoantibodies in hypersensitivity pneumonitis. Eur. Respir. J. 2020, 56, 1901380. [Google Scholar] [CrossRef]
- Greenberger, P.A. Hypersensitivity pneumonitis: A fibrosing alveolitis produced by inhalation of diverse antigens. J. Allergy Clin. Immunol. 2019, 143, 1295–1301. [Google Scholar] [CrossRef]
- Watts, M.M.; Grammer, L.C. Hypersensitivity pneumonitis. Allergy Asthma Proc. 2019, 40, 425–428. [Google Scholar] [CrossRef]
- Solodyn. Available online: https://www.rxlist.com/solodyn-drug.htm#description (accessed on 13 June 2021).
- Drugs and Lactation Database (LactMed). Minocycline. Available online: https://www.ncbi.nlm.nih.gov/books/NBK501031/ (accessed on 24 July 2020).
- Pai, M.P.; Momary, K.M.; Rodvold, K.A. Antibiotic drug interactions. Med. Clin. N. Am. 2006, 90, 1223–1255. [Google Scholar] [CrossRef]
- Minocycline Drug Interactions. Available online: https://www.drugs.com/drug-interactions/minocycline.html (accessed on 27 February 2021).
- Questions and Answers for Consumers on Doxycycline. Available online: https://www.fda.gov/drugs/bioterrorism-and-drug-preparedness/questions-and-answers-consumers-doxycycline (accessed on 30 March 2021).
- Dickinson, B.D.; Altman, R.D.; Nielsen, N.H.; Sterling, M.L.; American Medical Association Council on Scientific Affairs. Drug interactions between oral contraceptives and antibiotics. Obstet. Gynecol. 2001, 98, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Neely, J.L.; Abate, M.; Swinker, M.; D’Angio, R. The effect of doxycycline on serum levels of ethinyl estradiol, norethindrone, and endogenous progesterone. Obstet. Gynecol. 1991, 77, 416–420. [Google Scholar]
- Foamix Pharmaceuticals Ltd. Foamix Receives FDA Approval of AMZEEQ™ Topical Minocycline Treatment for Millions of Moderate to Severe Acne Sufferers; Foamix Pharmaceuticals Ltd.: New York, NY, USA, 2019. [Google Scholar]
- Minocycline foam (Amzeeq) for acne. Med. Lett. Drugs Ther. 2020, 62, 68–70.
- Onge, E.S.; Mobley, W.C. Minocycline Topical Foam: A New Drug for the Treatment of Acne. Ann. Pharmacother. 2021, 55, 105–110. [Google Scholar] [CrossRef]
- Minocycline foam (Zilxi) for rosacea. Med. Lett. Drugs Ther. 2020, 62, 179–180.
- Draelos, Z.D. Vehicle Effects on the Rosacea Skin Barrier. J. Drugs Dermatol. 2021, 20, 630–632. [Google Scholar] [CrossRef]
- Hovione. Hovione Announces Successful End-of-phase 2 Meeting With the FDA and Outlines Phase 3 Program for Minocycline Topical Gel; Hovione: New York, NY, USA, 2019. [Google Scholar]
- Webster, G.; Draelos, Z.D.; Graber, E.; Lee, M.S.; Dhawan, S.; Salman, M.; Magrath, G.N. A multicentre, randomized, double-masked, parallel group, vehicle-controlled phase IIb study to evaluate the safety and efficacy of 1% and 3% topical minocycline gel in patients with papulopustular rosacea. Br. J. Dermatol. 2020, 183, 471–479. [Google Scholar] [CrossRef]
- BioPharmX Corporation. BioPharmX Announces Positive Topline Results from Phase 2b Trial of BPX-04 for Papulopustular Rosacea; BioPharmX Corporation: New York, NY, USA, 2019. [Google Scholar]
- Ma, Y.; Song, J.; Almassri, H.N.S.; Zhang, D.; Zhang, T.; Cheng, Y.; Wu, X. Minocycline-loaded PLGA electrospun membrane prevents alveolar bone loss in experimental peridontitis. Drug Deliv. 2020, 27, 151–160. [Google Scholar] [CrossRef]
- Schmid, J.L.; Kirchberg, M.; Sarembe, S.; Kiesow, A.; Sculean, A.; Mader, K.; Buchholz, M.; Eick, S. In Vitro Evaluation of Antimicrobial Activity of Minocycline Formulations for Topical Application in Periodontal Therapy. Pharmaceutics 2020, 12, 352. [Google Scholar] [CrossRef]
- Yang, Z.; Liang, X.; Jiang, X.; Guo, J.; Tao, Y.; Wang, S.; Cao, Y.; Gui, S. Development and Evaluation of Minocycline Hydrochloride-Loaded In Situ Cubic Liquid Crystal for Intra-Periodontal Pocket Administration. Molecules 2018, 23, 2275. [Google Scholar] [CrossRef]
- Marto, J.M.; Gouveia, L.F.; Goncalves, L.M.D.; Ribeiro, H.M.; Almeida, A.J. Design of minocycline-containing starch nanocapsules for topical delivery. J. Microencapsul. 2018, 35, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Marto, J.; Duarte, A.; Simoes, S.; Goncalves, L.M.; Gouveia, L.F.; Almeida, A.J.; Ribeiro, H.M. Starch-Based Pickering Emulsions as Platforms for Topical Antibiotic Delivery: In Vitro and In Vivo Studies. Polymers 2019, 11, 108. [Google Scholar] [CrossRef]
- Gold, L.S.; Dhawan, S.; Weiss, J.; Draelos, Z.D.; Ellman, H.; Stuart, I.A. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: Results of 2 randomized, double-blind, phase 3 studies. J. Am. Acad. Dermatol. 2019, 80, 168–177. [Google Scholar] [CrossRef]
- Gold, L.S.; Del Rosso, J.Q.; Kircik, L.; Bhatia, N.D.; Hooper, D.; Nahm, W.K.; Stuart, I. Minocycline 1.5% foam for the topical treatment of moderate to severe papulopustular rosacea: Results of 2 phase 3, randomized, clinical trials. J. Am. Acad. Dermatol. 2020, 82, 1166–1173. [Google Scholar] [CrossRef]
- Alexis, A.; Del Rosso, J.Q.; Desai, S.R.; Downie, J.B.; Draelos, Z.D.; Feser, C.; Forconi, R.; Fowler, J.F., Jr.; Gold, M.; Kaufman-Janette, J.; et al. BPX-01 Minocycline Topical Gel Shows Promise for the Treatment of Moderate-to-severe Inflammatory Acne Vulgaris. J. Clin. Aesthet. Dermatol. 2018, 11, 25–35. [Google Scholar]
- BPX-04. Available online: https://www.biopharmx.com/pipeline/bpx-04-rosacea/ (accessed on 19 February 2021).
- BioPharmX Corporation. BioPharmX Receives Concurrence from FDA on Phase 3 Acne Study Plans; BioPharmX Corporation: New York, NY, USA, 2019. [Google Scholar]
- Del Rosso, J.Q.; Webster, G.F.; Jackson, M.; Rendon, M.; Rich, P.; Torok, H.; Bradshaw, M. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J. Am. Acad. Dermatol. 2007, 56, 791–802. [Google Scholar] [CrossRef]
- Oge, L.K.; Muncie, H.L.; Phillips-Savoy, A.R. Rosacea: Diagnosis and Treatment. Am. Fam. Physician 2015, 92, 187–196. [Google Scholar]
- Cherian, P.; Gunson, T.; Borchard, K.; Tai, Y.; Smith, H.; Vinciullo, C. Oral antibiotics versus topical decolonization to prevent surgical site infection after Mohs micrographic surgery—A randomized, controlled trial. Dermatol. Surg. 2013, 39, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Hwang, M.R.; Kim, J.O.; Lee, J.H.; Kim, Y.I.; Kim, J.H.; Chang, S.W.; Jin, S.G.; Kim, J.A.; Lyoo, W.S.; et al. Gel characterisation and in vivo evaluation of minocycline-loaded wound dressing with enhanced wound healing using polyvinyl alcohol and chitosan. Int. J. Pharm. 2010, 392, 232–240. [Google Scholar] [CrossRef]
- Kassem, A.A.; Ismail, F.A.; Naggar, V.F.; Aboulmagd, E. Comparative study to investigate the effect of meloxicam or minocycline HCl in situ gel system on local treatment of periodontal pockets. AAPS PharmSciTech 2014, 15, 1021–1028. [Google Scholar] [CrossRef]
- Schwartz, B.S.; Graber, C.J.; Diep, B.A.; Basuino, L.; Perdreau-Remington, F.; Chambers, H.F. Doxycycline, not minocycline, induces its own resistance in multidrug-resistant, community-associated methicillin-resistant Staphylococcus aureus clone USA300. Clin. Infect. Dis. 2009, 48, 1483–1484. [Google Scholar] [CrossRef]
- Jones, T.M.; Ellman, H.; deVries, T. Pharmacokinetic Comparison of Once-Daily Topical Minocycline Foam 4% vs Oral Minocycline for Moderate-to-Severe Acne. J. Drugs Dermatol. 2017, 16, 1022–1028. [Google Scholar]
- Raoof, T.J.; Hooper, D.; Moore, A.; Zaiac, M.; Sullivan, T.; Kircik, L.; Lain, E.; Jankicevic, J.; Stuart, I. Efficacy and safety of a novel topical minocycline foam for the treatment of moderate to severe acne vulgaris: A phase 3 study. J. Am. Acad. Dermatol. 2020, 82, 832–837. [Google Scholar] [CrossRef]
- Dias, O.M.; Nascimento, E.; Chate, R.C.; Kairalla, R.A.; Baldi, B.G. Eosinophilic pneumonia: Remember topical drugs as a potential etiology. J. Bras. Pneumol. 2018, 44, 522–524. [Google Scholar] [CrossRef]
- Austin, B.A.; Fleischer, A.B., Jr. The extinction of topical erythromycin therapy for acne vulgaris and concern for the future of topical clindamycin. J. Dermatolog. Treat. 2017, 28, 145–148. [Google Scholar] [CrossRef]
- Paik, J. Topical Minocycline Foam 4%: A Review in Acne Vulgaris. Am. J. Clin. Dermatol. 2020, 21, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, J.; McLaughlin, R.; Del Rosso, J.; Weiss, J.; Baldwin, H.; Webster, G.; Leyden, J.; Zhao, X.; Read, A.; Drlica, K.; et al. Assessing Bacterial Susceptibility to FMX101 4% Topical Minocycline Foam. In Proceedings of the Society for Investigative Dermatology, Chicago, IL, USA, 8–11 May 2019; 2019; pp. 8–11. [Google Scholar]
- Amzeeq. Available online: https://www.drugs.com/pro/amzeeq.html (accessed on 4 April 2021).
- Time to say goodbye to minocycline? Drug Ther. Bull. 2013, 51, 49. [CrossRef]
- Minocycline. Available online: https://www.nice.org.uk/advice/ktt11/resources/minocycline-pdf-58757947696069 (accessed on 19 February 2021).
- Lac, D.; Hermsmeier, M.; Chen, X.; Yam, N.; Yamamoto, A.; Huang, S.; Sawant, T.; Chan, K.F.; Nagavarapu, U. Topical minocycline formulations: Evaluation and comparison of dermal uptake efficacy. Int. J. Pharm. X 2019, 1, 100009. [Google Scholar] [CrossRef]
| Brand | Dosage Forms | Manufacturer | Indications |
|---|---|---|---|
| Cleeravue-M | Oral tablets, extended release | StoneBridge Pharma | Moderate-to-severe acne vulgaris. |
| Dynacin | Oral capsule | Medicis, The Dermatology Co.; Par Pharmaceutical, Inc. | Acne, Rocky Mountain spotted fever, typhus fever, Q fever, tick fevers, respiratory tract infections, lymphogranuloma venereum psittacosis, trachoma, inclusion conjunctivitis, nongonococcal urethritis, endocervical, or rectal infections, relapsing fever, chancroid, plague, tularemia, cholera, brucellosis, bartonellosis, granuloma inguinale, etc. |
| Minocin | Oral capsule (pellet-filled) IV solution (reconstituted) | Bausch Rempex Pharms | Same as Dynacin. |
| Minolira | Oral tablet, extended release (24 h) | EPI Health | Inflammatory lesions of non-nodular moderate-to-severe acne vulgaris in patients 12 years of age and older. |
| Solodyn | Oral tablet, extended release (24 h) | Medicis | Inflammatory lesions of non-nodular moderate-to-severe acne vulgaris in patients 12 years of age and older. |
| Ximino | Oral capsule, extended release (24 h) | Journey Medical Corporation | Inflammatory lesions of non-nodular moderate-to-severe acne vulgaris in patients 12 years of age and older. |
| Arestin | Oral Powder (microspheres) | OraPharma | Periodontitis |
| Generic | Oral tablet Oral capsule Oral tablet, extended release (24 h) | Several labs | Same as the brand minocycline formulations. |
| Target | Very Common (>10%) | Common (1–10%) | Rare (0.01–1%) | Very Rare (<0.01%) | Others and/or Frequency Not Reported |
|---|---|---|---|---|---|
| Nervous system | Headache (up to 23%) | Dizziness, somnolence, tinnitus, vertigo, mood alteration | Hypoesthesia, IIH, paresthesia, intracranial hypertension, impaired/decreased hearing, sedation, ataxia, vestibular reactions | Bulging fontanels (in infants) | Convulsions |
| Skin | Urticaria, rash, pruritus, erythematous rash | Angioedema, alopecia, erythema, fixed drug eruptions, hyperpigmentation, photosensitivity, cutaneous vasculitis, maculopapular rash, DRESS | Exfoliative dermatitis, hyperpigmentation of nails/nail beds, Stevens–Johnson syndrome, toxic epidermal necrolysis | Sweet’s syndrome (acute febrile neutrophilic dermatosis), anaphylactoid purpura, angioneurotic edema | |
| Gastrointestinal system | Nausea, vomits, teeth discoloration, diarrhea, abdominal pain | Dry mouth, dysphagia, dyspepsia, colitis, | Candidiasis, enamel hypoplasia, enterocolitis, esophagitis, esophageal ulcerations, glossitis, pancreatitis, oral mucosa discoloration | Inflammatory lesions in the oral and anogenital regions | |
| Musculo- skeletal system | Arthralgia, myalgia, MIL, arthritis | Joint stiffness, joint swelling, myopathy, hypersensitivity-associated rhabdomyolysis | Joint discoloration | Severe acute myopathy | |
| Hepatobiliary system | Abnormal hepatic function, hepatitis | Increased liver enzymes, autoimmune hepatitis, hepatic cholestasis, hepatic failure, hyperbilirubinemia, jaundice, liver injury | Fulminant hepatitis | Autoimmune hepatitis with lupus-like symptoms, acute hypersensitivity hepatitis associated with eosinophilia and dermatitis | |
| Respiratory system | Dyspnea | Cough, interstitial lung disease, pulmonary infiltration, eosinophilic pneumonia, bronchospasm, HP, pneumonitis, pleural effusions | Exacerbation of asthma | Pulmonary lupus, relapsing acute respiratory failure | |
| Immune system | Hypersensitivity | SSLR, ANCA-positive vasculitis | Immunosuppression | Positive ANCA titers, polyarteritis nodosa, ANCA-positive crescentic glomerulonephritis, autoimmune hepatitis, necrotizing vasculitis and systemic reactions | |
| Blood and lymphatic systems | Eosinophilia, leukopenia, neutropenia, thrombocytopenia, hemolytic anemia, pancytopenia, agranulocytosis | Thrombocytosis | ANCA-positive vasculitis, | ||
| Cardiovascular system | Palpitations, tachycardia, myocarditis, pericarditis, cardiac arrest, polyarteritis nodosa, vasculitis, hypotension, hypertension | Acute cardiac failure | |||
| Metabolic system | Anorexia | Dehydration | Hyperphosphatemia | Acidosis in patients with renal dysfunction | |
| Endocrine system | Hyperthyroidism, thyroiditis, hypothyroidism, autoimmune thyroiditis | Brown-black microscopic thyroid discoloration | Discolored breast secretions | ||
| Renal and genitourinary systems | Acute kidney injury, azotemia, increased blood and serum urea, interstitial nephritis, acute renal failure | Balanitis, vulvovaginitis | Deleterious effects on spermatogenesis | ||
| Others (hypersensitivity reactions) | Anaphylaxis/anaphylactoid reaction (including shock, death) | Pulmonary infiltrates, night sweats, fever, eosinophilia, severe CNS -pulmonary HSR, EP with relapsing acute respiratory failure, late-onset drug fever |
| Drug | Drug Type | Effect |
|---|---|---|
| Major drug interactions—highly clinically significant, avoid combinations | ||
| Acitretin, etretinate, isotretinoin, tretinoin, vitamin A | Retinoids, vitamin A derivatives | IIH caused by increased pressure in the brain, may lead to permanent vision loss |
| Aminolevulinic acid | Endogenous non-proteinogenic amino acid | Both drugs increase photosensitivity and may lead to severe sunburns |
| BCG | Bacillus Calmette–Guérin (BCG vaccine) | Minocycline reduces the antitumor activity of BCG in the bladder |
| Cholera vaccine (live) Typhoid vaccine (live) | Vaccines | Minocycline may reduce the effect of the vaccine |
| Leflunomide | Immunosuppressive disease-modifying antirheumatic drug | Liver injury |
| Lomitapide | Enzyme inhibitor | |
| Mipomersen | Antisense oligonucleotide inhibitor of apo B (cholesterol-lowering) | |
| Pexidartinib | Tyrosine kinase inhibitor | |
| Teriflunomide | Active metabolite of leflunomide | |
| Methoxyflurane | Ether, anesthetic | Kidney problems |
| Moderate drug interactions—avoid combinations except in special circumstances | ||
| Aluminum, calcium, magnesium salts | Interfere with minocycline absorption | |
| Aminophylline | Compound of the bronchodilator theophylline with ethylenediamine (ratio 2:1) | Minocycline increases the effect of aminophylline |
| Penicillin and derivatives | Penicillins | Minocycline may decrease the effect of penicillins |
| Anisindione | Synthetic anticoagulant | Minocycline may increase the hypoprothrombinemic effects of warfarin and similar anticoagulants. |
| Dicoumarol | Natural anticoagulant | |
| Warfarin | Derivative from dicoumarol | |
| Asparaginase | Enzyme (treatment of leukemia) | Liver injury |
| Bedaquiline | Enzyme inhibitor (treatment of multidrug-resistant TB) | |
| Brentuximab vedotin | Antibody-drug conjugate (treatment of some lymphomas) | |
| Clofarabine | Purine nucleoside antimetabolite (cancer treatment) | |
| Daclizumab | Humanized monoclonal antibody (treatment of relapsing forms of MS) | |
| Efavirenz | Antiretroviral (AIDS treatment) | |
| Epirubicin | Anthracycline (cancer treatment) | |
| Idelalisib | Enzyme inhibitor (blood cancer treatment) | |
| Interferon-β | Cytokine (MS treatment) | |
| Naltrexone | Opiate antagonist (treatment of addictions) | |
| Remdesivir | Nucleotide analogue prodrug, antiviral | |
| Thioguanine | Anticancer chemotherapy drug | |
| Trabectedin | Chemotherapy drug | |
| Atracurium, cisatracurium, mivacurium, pancuronium, rocuronium, succinylcholine, vecuronium | Neuromuscular blocking agents | Minocycline may increase the effect of these drugs, leading to respiratory depression and muscle weakness |
| Balsalazide | Anti-inflammatory | Minocycline may reduce the effect of balsalazide |
| Bismuth-, iron- or zinc-containing preparations, lanthanum salts | Chelation of minocycline, which may reduce its effective concentration | |
| Digitoxin | Natural cardiac glucoside (cancer therapy) | Minocycline may increase the serum levels of digitoxin and digoxin |
| Digoxin | Natural cardiac glucoside (treatment of heart conditions) | |
| Dihydroergotamine, ergonovine, ergotamine, methylergonovine, methysergide maleate | Ergot alkaloids (vasoconstrictors) | Tetracyclines may increase the plasma concentrations and toxicity of ergot alkaloids, leading to liver injury |
| Ethinyl estradiol | Estrogen (birth control) | Minocycline and other antibiotics may impair the contraceptive effect of estrogens in some rare individuals |
| Insulin and analogues | Hormone | Minocycline may enhance the hypoglycemic effect of insulin |
| Methotrexate | Chemotherapy agent, immunosuppressant | Tetracycline may elevate or reduce serum methotrexate concentrations |
| Methoxsalen | Psoralen (photosensitizing agent) | Increased photosensitivity |
| Methyl aminolevulinate (topical) | Prodrug (photosensitizing agent) | |
| Porfimer | Mixture of porphyrin oligomers (photosensitizing agent) | |
| Verteporfin | Benzoporphyrin derivative (photosensitizing agent) | |
| Mycophenolate mofetil | Immunosuppressant | Minocycline may reduce the immunosuppressive effects of mycophenolic acid |
| Oxtriphylline | Salt of choline and theophylline (bronchodilator) | Minocycline may decrease theophylline plasma clearance and increase theophylline levels |
| Theophylline | Bronchodilator | |
| Sodium acetate, bicarbonate, citrate, lactate Thrometamine | Organic amine proton acceptor (treatment of metabolic acidosis) | These compounds may decrease the effect of minocycline due to alkalinization of the urine |
| Minor drug interactions—minimize combined use, consider alternative drugs, if combined, institute monitoring plan | ||
| Acetazolamide, amiloride, bendroflumethiazide, benzthiazide, bumetanide, chlorothiazide, chlorthalidone, dichlorphenamide, ethacrynic acid, furosemide, glycerine, hydrochlorothiazide, indapamide, mannitol, methazolamide, methyclothiazide, metolazone, polythiazide, spironolactone, torsemide, triamterene, trichlormethiazide | Diuretics | Decreased renal function |
| Colestipol | Bile acid sequestrant (lowers blood cholesterol) | May reduce absorption of minocycline |
| Didanosine | Antiretroviral | |
| Lithium | Treatment of bipolar and depressive disorders | Minocycline may increase the plasma concentrations of lithium. Rarely, IIH has been reported when minocycline is co-administered with lithium |
| Brand/Phase | Manufacturer | Dosage forms | Minocycline Form | Indications |
|---|---|---|---|---|
| Amzeeq (FMX101)/market | Vyne Therapeutics | Topical foam (4%) | Minocycline HCl | Treatment of inflammatory lesions of non-nodular moderate-to-severe acne vulgaris in patients 9 years of age and older. |
| Zilxi/market | Vyne Therapeutics | Topical foam (1.5%) | Minocycline HCl | Treatment of inflammatory lesions of rosacea in adults. |
| BPX-01/IIb | BiopharmX | Hydrophilic gel (1% and 2%) | Minocycline HCl | Treatment of acne vulgaris |
| BPX-04/IIb | BiopharmX | Hydrophilic gel (1% and 2%) | Minocycline HCl | Treatment of papulopustular rosacea |
| HY01/IIb | Hovione | Anhydrous gel (1% and 3%) | Crystalline minocycline base | Treatment of papulopustular rosacea |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, A.M.; Marto, J.M.; Johnson, J.L.; Graber, E.M. A Review of Systemic Minocycline Side Effects and Topical Minocycline as a Safer Alternative for Treating Acne and Rosacea. Antibiotics 2021, 10, 757. https://doi.org/10.3390/antibiotics10070757
Martins AM, Marto JM, Johnson JL, Graber EM. A Review of Systemic Minocycline Side Effects and Topical Minocycline as a Safer Alternative for Treating Acne and Rosacea. Antibiotics. 2021; 10(7):757. https://doi.org/10.3390/antibiotics10070757
Chicago/Turabian StyleMartins, Ana M., Joana M. Marto, Jodi L. Johnson, and Emmy M. Graber. 2021. "A Review of Systemic Minocycline Side Effects and Topical Minocycline as a Safer Alternative for Treating Acne and Rosacea" Antibiotics 10, no. 7: 757. https://doi.org/10.3390/antibiotics10070757
APA StyleMartins, A. M., Marto, J. M., Johnson, J. L., & Graber, E. M. (2021). A Review of Systemic Minocycline Side Effects and Topical Minocycline as a Safer Alternative for Treating Acne and Rosacea. Antibiotics, 10(7), 757. https://doi.org/10.3390/antibiotics10070757








