Clearance of Gram-Negative Bacterial Pathogens from the Ocular Surface by Predatory Bacteria
Abstract
:1. Introduction
2. Results
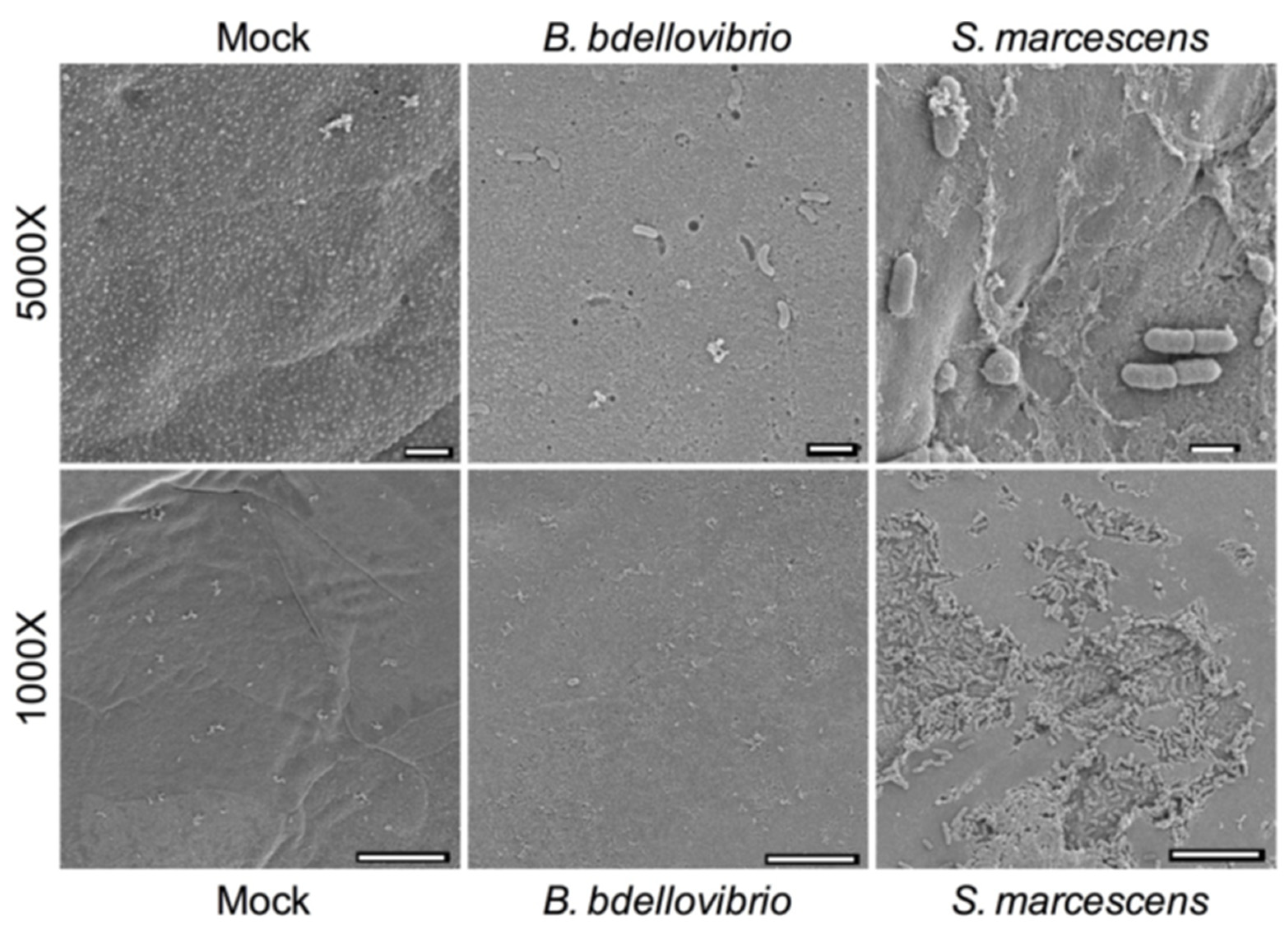
2.1. Scanning Electron Microscopy Visualization of B. bacteriovorus 109J with Porcine Corneas Ex Vivo
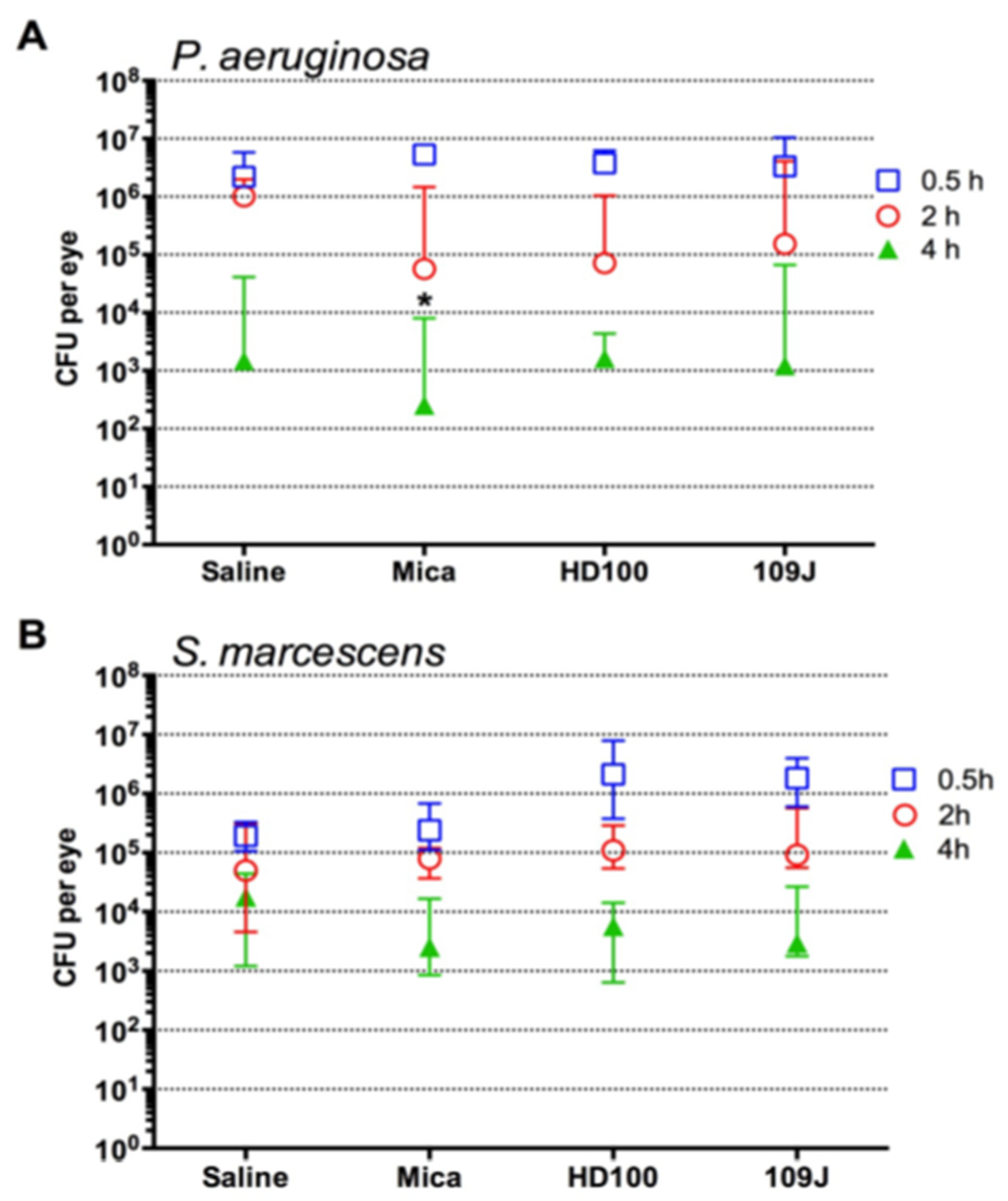
2.2. Clearance of Fluoroquinolone Resistant P. aeruginosa But Not S. marcescens from Rabbit Ocular Surfaces Was Facilitated by Instillation of Predatory Bacteria
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture
4.2. Scanning Electron Microscopy
4.3. In Vitro Predation Assay
4.4. Rabbit Ocular Surface Occupancy Model
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dwidar, M.; Monnappa, A.K.; Mitchell, R.J. The dual probiotic and antibiotic nature of Bdellovibrio bacteriovorus. BMB Rep. 2012, 45, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Sockett, R.E. Predatory lifestyle of Bdellovibrio bacteriovorus. Annu. Rev. Microbiol. 2009, 63, 523–539. [Google Scholar] [CrossRef]
- Kadouri, D.E.; To, K.; Shanks, R.M.; Doi, Y. Predatory bacteria: A potential ally against multidrug-resistant Gram-negative pathogens. PLoS ONE 2013, 8, e63397. [Google Scholar] [CrossRef] [Green Version]
- Shanks, R.M.; Davra, V.R.; Romanowski, E.G.; Brothers, K.M.; Stella, N.A.; Godboley, D.; Kadouri, D.E. An Eye to a Kill: Using Predatory Bacteria to Control Gram-Negative Pathogens Associated with Ocular Infections. PLoS ONE 2013, 8, e66723. [Google Scholar] [CrossRef] [Green Version]
- Pasternak, Z.; Njagi, M.; Shani, Y.; Chanyi, R.; Rotem, O.; Lurie-Weinberger, M.N.; Koval, S.; Pietrokovski, S.; Gophna, U.; Jurkevitch, E. In and out: An analysis of epibiotic vs periplasmic bacterial predators. ISME J. 2014, 8, 625–635. [Google Scholar] [CrossRef] [Green Version]
- Kadouri, D.; O’Toole, G.A. Susceptibility of biofilms to Bdellovibrio bacteriovorus attack. Appl. Environ. Microbiol. 2005, 71, 4044–4051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadouri, D.; Venzon, N.C.; O’Toole, G.A. Vulnerability of pathogenic biofilms to Micavibrio aeruginosavorus. Appl. Environ. Microbiol. 2007, 73, 605–614. [Google Scholar] [CrossRef] [Green Version]
- Dharani, S.; Kim, D.H.; Shanks, R.M.Q.; Doi, Y.; Kadouri, D.E. Susceptibility of colistin-resistant pathogens to predatory bacteria. Res. Microbiol. 2018, 169, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.C.; Gillette, R.K.; Maglasang, M.A.; Corn, E.A.; Tai, A.K.; Lazinski, D.W.; Shanks, R.M.Q.; Kadouri, D.E.; Camilli, A. High-throughput analysis of gene function in the bacterial predator Bdellovibrio bacteriovorus. mBio 2019, 10, e01040-19. [Google Scholar] [CrossRef] [Green Version]
- Romanowski, E.G.; Stella, N.A.; Brothers, K.M.; Yates, K.A.; Funderburgh, M.L.; Funderburgh, J.L.; Gupta, S.; Dharani, S.; Kadouri, D.E.; Shanks, R.M. Predatory bacteria are nontoxic to the rabbit ocular surface. Sci. Rep. 2016, 6, 30987. [Google Scholar] [CrossRef]
- Nakamura, M. Alteration of Shigella pathogenicity by other bacteria. Am. J. Clin. Nutr. 1972, 25, 1441–1451. [Google Scholar] [CrossRef]
- Boileau, M.J.; Mani, R.; Breshears, M.A.; Gilmour, M.; Taylor, J.D.; Clickenbeard, K.D. Efficacy of Bdellovibrio bacteriovorus 109J for the treatment of dairy calves with experimentally induced infectious bovine keratoconjunctivitis. Am. J. Vet. Res. 2016, 77, 1017–1028. [Google Scholar] [CrossRef]
- Shatzkes, K.; Chae, R.; Tang, C.; Ramirez, G.C.; Mukherjee, S.; Tsenova, L.; Connell, N.D.; Kadouri, D.E. Examining the safety of respiratory and intravenous inoculation of Bdellovibrio bacteriovorus and Micavibrio aeruginosavorus in a mouse model. Sci. Rep. 2015, 5, 12899. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Tang, C.; Tran, M.; Kadouri, D.E. Effect of predatory bacterio on human cell lines. PLoS ONE 2016, 11, e0161242. [Google Scholar] [CrossRef]
- Monnappa, A.K.; Bari, W.; Choi, S.Y.; Mitchell, R.J. Investigating the responses of human epithelial cells to predatory bacteria. Sci. Rep. 2016, 15, 33485. [Google Scholar] [CrossRef] [Green Version]
- Shatzkes, K.; Singleton, E.; Tang, C.; Zuena, M.; Shukla, S.; Gupta, S.; Dharani, S.; Onyile, O.; Rinaggio, J.; Connell, N.D.; et al. Predatory bacteria attenuate Klebsiella pneumoniae burden in rat lungs. mBio 2016, 7, e01847-16. [Google Scholar] [CrossRef] [Green Version]
- Findlay, J.S.; Flick-Smith, H.C.; Keyser, E.; Cooper, I.A.; Williamson, E.D.; Oyston, P.C.F. Predatory bacteria can protectx SKH-1 mice from a lethal plague challenge. Sci. Rep. 2019, 9, 7225. [Google Scholar] [CrossRef] [Green Version]
- Willis, R.A.; Moore, C.; Mazon-Moya, M.; Krokowski, S.; Lambert, C.; Till, R.; Mostowy, S.; Sockett, R.E. Injections of predatory bacteria work alongside host immune cells to treat Shigella infections in zebrafish larvae. Curr. Biol. 2016, 26, 3343–3351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Sheorey, H.; Taylor, H.R.; Vajpayee, R.B. Association between cultures of contact lens and corneal scraping in contact lens related microbial keratitis. Arch. Ophthalmol. 2007, 125, 1182–1185. [Google Scholar] [CrossRef] [Green Version]
- Green, M.; Sara, S.; Hughes, I.; Apel, A.; Stapleton, F. Trends in contact lens microbial keratitis 1999 to 2015: A retrospective clinical review. Clin. Exp. Ophthalmol. 2019, 47, 726–732. [Google Scholar] [CrossRef]
- Mah-Sadorra, J.H.; Najjar, D.M.; Rapuano, C.J.; Laibson, P.R.; Cohen, E.J. Serratia corneal ulcers: A retrospective clinical study. Cornea 2005, 24, 793–800. [Google Scholar] [CrossRef]
- Lakhundi, S.; Siddiqui, R.; Khan, N.A. Pathogenesis of microbial keratitis. Microb. Pathog. 2017, 104, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Hume, E.B.; Willcox, M.D. Emergence of Serratia marcescens as an ocular surface pathogen. Arch. Soc. Esp. Oftalmol. 2004, 79, 475–477. [Google Scholar]
- Hilliam, Y.; Kaye, S.; Winstanley, C. Pseudomonas aeruginosa and microbial keratitis. J. Med. Microbiol. 2020, 69, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, M.; Apel, A.; Stapleton, F. Risk factors and causative organisms in microbial keratitis. Cornea 2008, 27, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.D. Review of resistance of ocular isolates of Pseudomonas aeruginosa and staphylococci from keratitis to ciprofloxacin, gentamicin and cephalosporins. Clin. Exp. Optom. 2011, 94, 161–168. [Google Scholar] [CrossRef]
- Shen, E.P.; Hsieh, Y.T.; Chu, H.S.; Chang, S.C.; Hu, F.R. Correlation of Pseudomonas aeruginosa genotype with antibiotic susceptibility and clinical features of induced central keratitis. Investig. Ophthalmol. Vis. Sci. 2014, 56, 365–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazirani, J.; Wurity, S.; Ali, M.H. Multidrug-Resistant Pseudomonas aeruginosa Keratitis: Risk Factors, Clinical Characteristics, and Outcomes. Ophthalmology 2015, 122, 2110–2114. [Google Scholar] [CrossRef]
- Fernandes, M.; Vira, D.; Medikonda, R.; Kumar, N. Extensively and pan-drug resistant Pseudomonas aeruginosa keratitis: Clinical features, risk factors, and outcome. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 315–322. [Google Scholar] [CrossRef]
- Garcia, C.J.; Pericleous, A.; Elsayed, M.; Tran, M.; Gupta, S.; Callaghan, J.D.; Stella, N.A.; Franks, J.M.; Thibodeau, P.H.; Shanks, R.M.Q.; et al. Serralysin family metalloproteases protects Serratia marcescens from predation by the predatory bacteria Micavibrio aeruginosavorus. Sci. Rep. 2018, 8, 14025. [Google Scholar] [CrossRef]
- Shanks, R.M.; Stella, N.A.; Hunt, K.M.; Brothers, K.M.; Zhang, L.; Thibodeau, P.H. Identification of SlpB, a cytotoxic protease from Serratia marcescens. Infect. Immun. 2015, 83, 2907–2916. [Google Scholar] [CrossRef] [Green Version]
- Stella, N.A.; Callaghan, J.D.; Zhang, L.; Brothers, K.M.; Kowalski, R.P.; Huang, J.J.; Thibodeau, P.H.; Shanks, R.M.Q. SlpE is a calcium-dependent cytotoxic metalloprotease associated with clinical isolates of Serratia marcescens. Res. Microbiol. 2017, 168, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Stella, N.A.; Brothers, K.M.; Shanks, R.M.Q. Differential susceptibility of airway and ocular surface cell lines to FlhDC-mediated virulence factors PhlA and ShlA from Serratia marcescens. J. Med. Microbiol. 2021, 70, 001292. [Google Scholar] [CrossRef]
- Atterbury, R.J.; Hobley, L.; Till, R.; Lambert, C.; Capeness, M.J.; Lerner, T.R.; Fenton, A.K.; Barrow, P.; Sockett, R.E. Effects of orally administered Bdellovibrio bacteriovorus on the well-being and Salmonella colonization of young chicks. Appl. Environ. Microbiol. 2011, 77, 5794–5803. [Google Scholar] [CrossRef] [Green Version]
- Doan, T.; Akileswaran, L.; Andersen, D.; Johnson, B.; Ko, N.; Shrestha, A.; Shestopalov, V.; Lee, C.S.; Lee, A.Y.; Van Gelder, R.D. Paucibacterial microbiome and resident DNA virome of the healthy conjunctiva. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5116–5126. [Google Scholar] [CrossRef] [Green Version]
- Ozkan, J.; Willcox, M.D. The ocular microbiome: Molecular characterization of a unique and low microbial environment. Curr. Eye Res. 2019, 44, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, F.; Pignataro, D.; Lavano, M.A.; Santella, B.; Folliero, V.; Zannella, C.; Astarita, C.; Gagliano, C.; Franci, G.; Avitabile, T.; et al. Current evidence on the ocular surface microbiota and related diseases. Microorganisms 2020, 8, 1033. [Google Scholar] [CrossRef]
- Shatzkes, K.; Tang, C.; Singleton, E.; Shukla, S.; Zuena, M.; Gupta, S.; Dharani, S.; Rinaggio, J.; Connell, N.D.; Kadouri, D.E. Effect of predatory bacteria on the gut bacterial microbiota in rats. Sci. Rep. 2017, 7, 43483. [Google Scholar] [CrossRef]
- Lebba, V.; Santangelo, F.; Totino, V.; Nicoletti, M.; Gagliardi, A.; De Biase, R.V.; Cucchiara, S.; Nencioni, L.; Conte, M.P.; Schippa, S. Higher prevalence and abundance of Bdellovibrio bacteriovorus in the human gut of healthy subjects. PLoS ONE 2013, 8, e61608. [Google Scholar] [CrossRef] [Green Version]
- Dantas, P.E.; Uesugui, E.; Nishiwaki-Dantas, M.C.; Mimica, L.J. Antibacterial activity of anaesthetic solutions and preservatives: An in vitro comparative study. Cornea 2000, 19, 353–354. [Google Scholar] [CrossRef]
- Edwards, S.G.; Maggs, D.J.; Byrne, B.A.; Kass, P.H.; Lassaline, M.E. Effect of topical application of 0.5% proparacaine on corneal culture results from 33 dogs, 12 cats, and 19 horses with spontaneously arising ulcerative keratitis. Vet. Ophthalmol. 2019, 22, 415–422. [Google Scholar] [CrossRef]
- Mun, J.J.; Tam, C.; Kowbel, D.; Hawgood, S.; Barnett, M.J.; Evans, D.J.; Fleiszig, S.M. Clearance of Pseudomonas aeruginosa from a healthy ocular surface involves surfactant protein D and is compromised by bacterial elastase in a murine null-infection model. Infect. Immun. 2009, 77, 2392–2398. [Google Scholar] [CrossRef] [Green Version]
- St Leger, A.J.; Desai, J.V.; Drummond, R.A.; Kugadas, A.; Almaghrabi, F.; Silver, P.; Raychaudhuri, K.; Gadjeva, M.; Iwakura, Y.; Lionakis, M.S.; et al. An ocular commensal protects against corneal infection by driving an interleukin-17 response from mucosal γδ T cells. Immunity 2017, 47, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, R.P.; Pandya, A.N.; Karenchak, L.M.; Romanowski, E.G.; Husted, R.C.; Ritterband, D.C.; Shah, M.K.; Gordon, Y.J. An in vitro resistance study of levofloxacin, ciprofloxacin, and ofloxacin using keratitis isolates of Staphylococcus aureus and Pseudomonas aeruginosa. Ophthalmology 2001, 108, 1826–1829. [Google Scholar] [CrossRef]
- Kalivoda, E.J.; Stella, N.A.; Aston, M.A.; Fender, J.E.; Thompson, P.P.; Kowalski, R.P.; Shanks, R.M. Cyclic AMP negatively regulates prodigiosin production by Serratia marcescens. Res. Microbiol. 2010, 161, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Cockerill, F.R. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement; CLSI: Wayne, PA, USA, 2013. [Google Scholar]
- Kowalski, R.P.; Dhaliwal, D.K.; Karenchak, L.M.; Romanowski, E.G.; Mah, F.S.; Ritterband, D.C.; Gordon, Y.J. Gatifloxacin and moxifloxacin: An in vitro susceptibility comparison to levofloxacin, ciprofloxacin, and ofloxacin using bacterial keratitis isolates. Am. J. Ophthalmol. 2003, 136, 500–505. [Google Scholar] [CrossRef]
- Bertani, G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rittenberger, S.C. Nonidentity of Bdellovibrio bacteriovorus strains 109D and 109J. J. Bacteriol. 1972, 109, 432–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolp, H.; Starr, M.P. Bdellovibrio bacteriovorus Gen. Et Sp. N., A predatory, ectoparasitic, and bacteriolytic microorganism. Antonie Van Leeuwenhoek 1963, 29, 217–248. [Google Scholar] [CrossRef]
- Lambina, V.A.; Afinogenova, A.V.; Romay Penobad, Z.; Konovalova, S.M.; Andreev, L.V. New species of exoparasitic bacteria of the genus Micavibrio infecting gram-negative bacteria. Mikrobiologiia 1983, 52, 777–780. [Google Scholar]
- Brothers, K.M.; Stella, N.A.; Hunt, K.M.; Romanowski, E.G.; Liu, X.; Klarlund, J.K.; Shanks, R.M. Putting on the brakes: Bacterial impediment of wound healing. Sci. Rep. 2015, 5, 14003. [Google Scholar] [CrossRef]
- Xu, K.P.; Li, X.F.; Yu, F.S. Corneal organ culture model for assessing epithelial responses to surfactants. Toxicol. Sci. 2000, 58, 306–314. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanowski, E.G.; Gupta, S.; Pericleous, A.; Kadouri, D.E.; Shanks, R.M.Q. Clearance of Gram-Negative Bacterial Pathogens from the Ocular Surface by Predatory Bacteria. Antibiotics 2021, 10, 810. https://doi.org/10.3390/antibiotics10070810
Romanowski EG, Gupta S, Pericleous A, Kadouri DE, Shanks RMQ. Clearance of Gram-Negative Bacterial Pathogens from the Ocular Surface by Predatory Bacteria. Antibiotics. 2021; 10(7):810. https://doi.org/10.3390/antibiotics10070810
Chicago/Turabian StyleRomanowski, Eric G., Shilpi Gupta, Androulla Pericleous, Daniel E. Kadouri, and Robert M. Q. Shanks. 2021. "Clearance of Gram-Negative Bacterial Pathogens from the Ocular Surface by Predatory Bacteria" Antibiotics 10, no. 7: 810. https://doi.org/10.3390/antibiotics10070810






