Silver Nanoparticles Enhance Antimicrobial Efficacy of Antibiotics and Restore That Efficacy against the Melioidosis Pathogen
Abstract
1. Introduction
2. Results
2.1. Characterization of Silver Nanoparticles
2.2. Antimicrobial Susceptibility
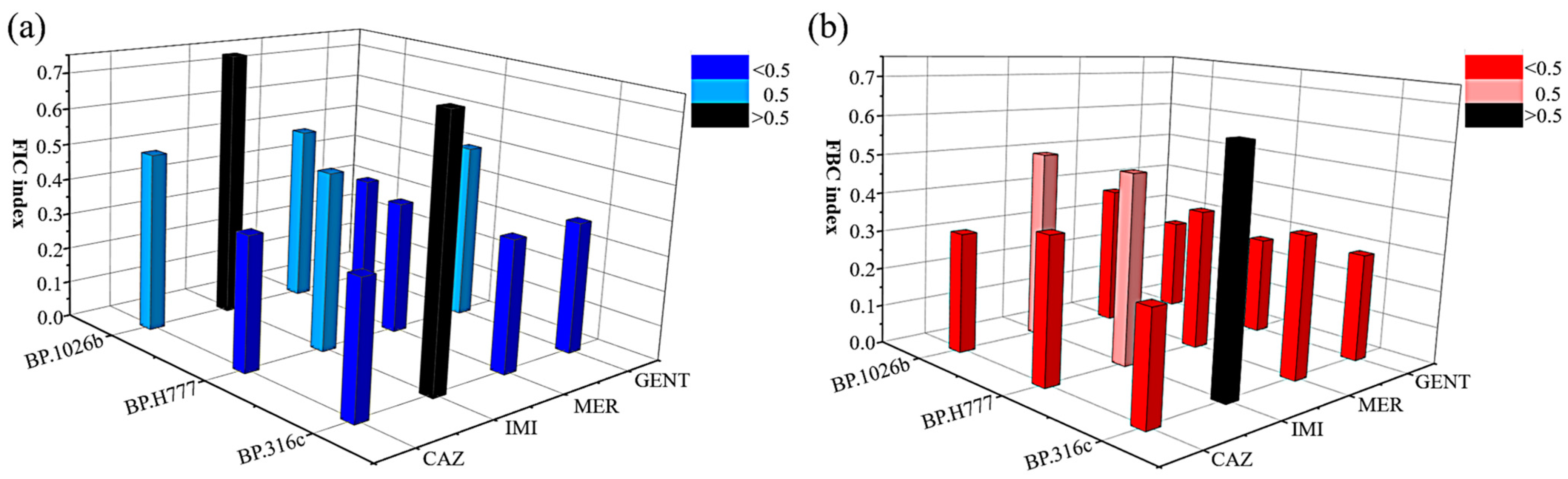
2.3. Synergistic Antibacterial Effects
2.4. Growth Curve of B. pseudomallei in the Presence of a Combination
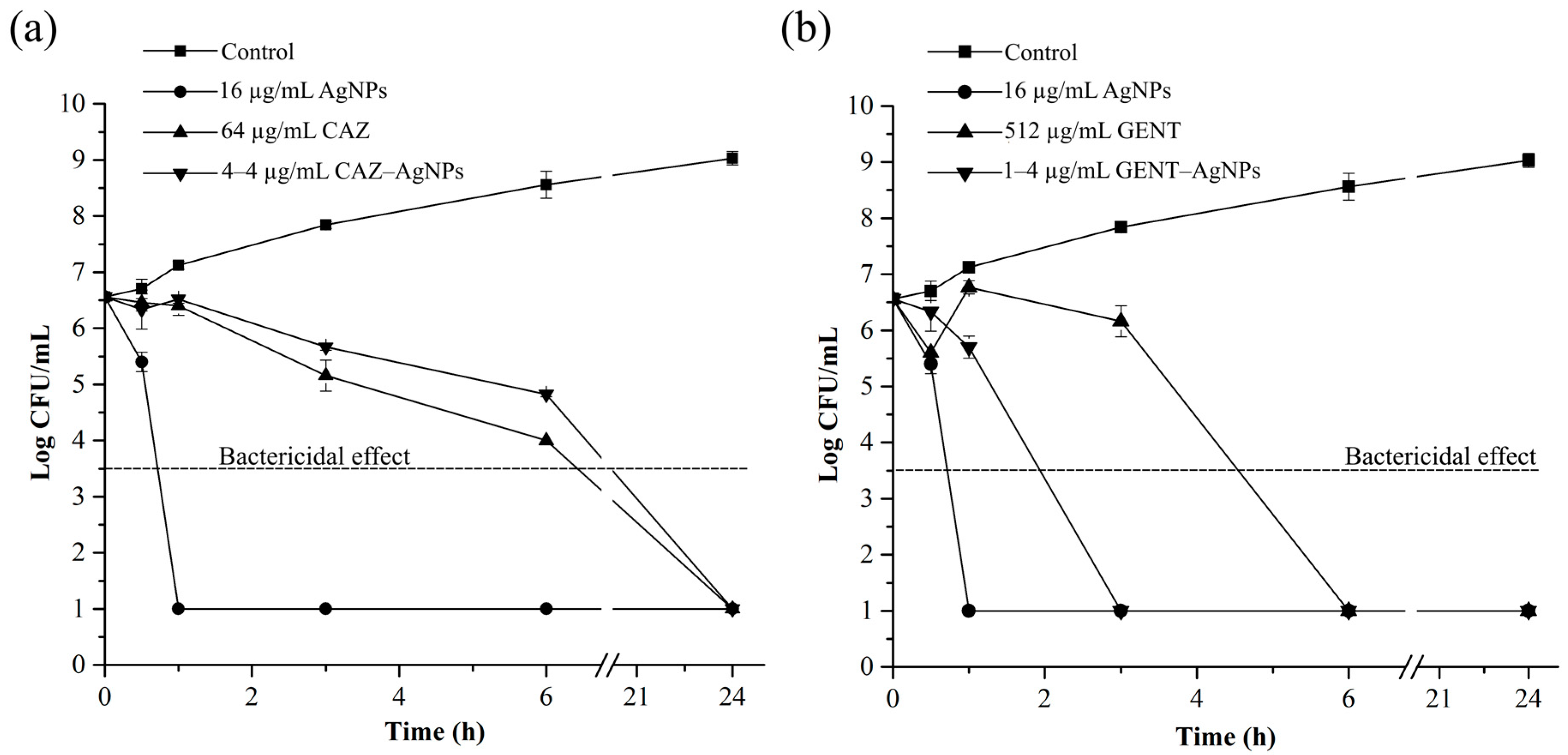
2.5. Time-Dependent Killing Efficiency of Combinations
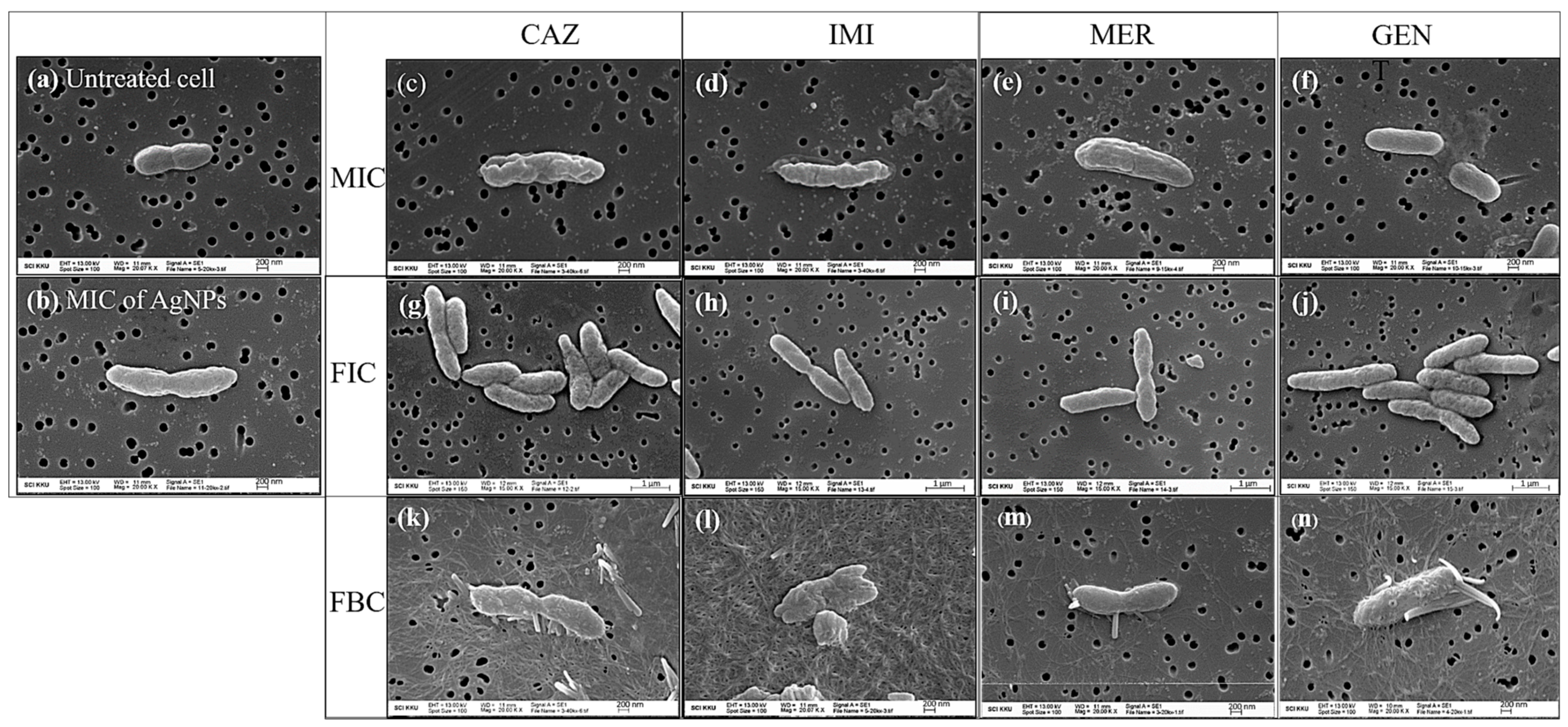
2.6. Cell Morphological Change
3. Discussion
4. Materials and Methods
4.1. Materials and Cell Culture
4.2. Preparation and Characterization of Silver Nanoparticles
4.3. Determination of the Minimum Inhibitory Concentration (MIC) and the Minimum Bactericidal Concentration (MBC)
4.4. Determination of Synergistic Antibacterial Effects
4.5. Bacterial Growth Curve of an Antibiotic with AgNPs Combination
4.6. Killing Kinetic Assay
4.7. Evaluating the Morphological Changes of the Bacterial Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Limmathurotsakul, D.; Golding, N.; Dance, D.A.; Messina, J.P.; Pigott, D.M.; Moyes, C.L.; Rolim, D.B.; Bertherat, E.; Day, N.P.; Peacock, S.J.; et al. Predicted Global Distribution of Burkholderia Pseudomallei and Burden of Melioidosis. Nat. Microbiol. 2016, 1, 120135. [Google Scholar] [CrossRef]
- White, N.J. Melioidosis. Lancet 2003, 361, 1715–1722. [Google Scholar] [CrossRef]
- Currie, B.J.; Fisher, D.A.; Howard, D.M.; Burrow, J.N.; Lo, D.; Selva-Nayagam, S.; Anstey, N.M.; Huffam, S.E.; Snelling, P.L.; Marks, P.J.; et al. Endemic Melioidosis in Tropical Northern Australia: A 10-Year Prospective Study and Review of the Literature. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2000, 31, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Hemarajata, P.; Baghdadi, J.D.; Hoffman, R.; Humphries, R.M. Burkholderia Pseudomallei: Challenges for the Clinical Microbiology Laboratory. J. Clin. Microbiol. 2016, 54, 2866–2873. [Google Scholar] [CrossRef]
- Viktorov, D.V.; Zakharova, I.B.; Podshivalova, M.V.; Kalinkina, E.V.; Merinova, O.A.; Ageeva, N.P.; Antonov, V.A.; Merinova, L.K.; Alekseev, V.V. High-Level Resistance to Fluoroquinolones and Cephalosporins in Burkholderia Pseudomallei and Closely Related Species. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, S103–S110. [Google Scholar] [CrossRef]
- Thibault, F.M.; Hernandez, E.; Vidal, D.R.; Girardet, M.; Cavallo, J.-D. Antibiotic Susceptibility of 65 Isolates of Burkholderia Pseudomallei and Burkholderia Mallei to 35 Antimicrobial Agents. J. Antimicrob. Chemother. 2004, 54, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Chantratita, N.; Rholl, D.A.; Sim, B.; Wuthiekanun, V.; Limmathurotsakul, D.; Amornchai, P.; Thanwisai, A.; Chua, H.H.; Ooi, W.F.; Holden, M.T.G.; et al. Antimicrobial Resistance to Ceftazidime Involving Loss of Penicillin-Binding Protein 3 in Burkholderia Pseudomallei. Proc. Natl. Acad. Sci. USA 2011, 108, 17165–17170. [Google Scholar] [CrossRef]
- Sarovich, D.S.; Price, E.P.; Von Schulze, A.T.; Cook, J.M.; Mayo, M.; Watson, L.M.; Richardson, L.; Seymour, M.L.; Tuanyok, A.; Engelthaler, D.M.; et al. Characterization of Ceftazidime Resistance Mechanisms in Clinical Isolates of Burkholderia Pseudomallei from Australia. PLoS ONE 2012, 7, e30789. [Google Scholar] [CrossRef]
- Sarovich, D.S.; Price, E.P.; Limmathurotsakul, D.; Cook, J.M.; Von Schulze, A.T.; Wolken, S.R.; Keim, P.; Peacock, S.J.; Pearson, T. Development of Ceftazidime Resistance in an Acute Burkholderia Pseudomallei Infection. Infect. Drug Resist. 2012, 5, 129–132. [Google Scholar] [CrossRef][Green Version]
- Simpson, A.J.; Suputtamongkol, Y.; Smith, M.D.; Angus, B.J.; Rajanuwong, A.; Wuthiekanun, V.; Howe, P.A.; Walsh, A.L.; Chaowagul, W.; White, N.J. Comparison of Imipenem and Ceftazidime as Therapy for Severe Melioidosis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1999, 29, 381–387. [Google Scholar] [CrossRef]
- Cheng, A.C.; Fisher, D.A.; Anstey, N.M.; Stephens, D.P.; Jacups, S.P.; Currie, B.J. Outcomes of Patients with Melioidosis Treated with Meropenem. Antimicrob. Agents Chemother. 2004, 48, 1763–1765. [Google Scholar] [CrossRef]
- Behera, B.; Prasad Babu, T.L.V.D.; Kamalesh, A.; Reddy, G. Ceftazidime Resistance in Burkholderia Pseudomallei: First Report from India. Asian Pac. J. Trop. Med. 2012, 5, 329–330. [Google Scholar] [CrossRef]
- Smith, M.D.; Wuthiekanun, V.; Walsh, A.L.; White, N.J. In-Vitro Activity of Carbapenem Antibiotics against Beta-Lactam Susceptible and Resistant Strains of Burkholderia Pseudomallei. J. Antimicrob. Chemother. 1996, 37, 611–615. [Google Scholar] [CrossRef][Green Version]
- Climo, M.W.; Patron, R.L.; Archer, G.L. Combinations of Vancomycin and Beta-Lactams Are Synergistic against Staphylococci with Reduced Susceptibilities to Vancomycin. Antimicrob. Agents Chemother. 1999, 43, 1747–1753. [Google Scholar] [CrossRef] [PubMed]
- Kanellakopoulou, K.; Sarafis, P.; Galani, I.; Giamarellou, H.; Giamarellos-Bourboulis, E.J. In Vitro Synergism of Beta-Lactams with Ciprofloxacin and Moxifloxacin against Genetically Distinct Multidrug-Resistant Isolates of Pseudomonas Aeruginosa. Int. J. Antimicrob. Agents 2008, 32, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Naghmouchi, K.; Le Lay, C.; Baah, J.; Drider, D. Antibiotic and Antimicrobial Peptide Combinations: Synergistic Inhibition of Pseudomonas Fluorescens and Antibiotic-Resistant Variants. Res. Microbiol. 2012, 163, 101–108. [Google Scholar] [CrossRef]
- Amani, J.; Barjini, K.A.; Moghaddam, M.M.; Asadi, A. In Vitro Synergistic Effect of the CM11 Antimicrobial Peptide in Combination with Common Antibiotics against Clinical Isolates of Six Species of Multidrug-Resistant Pathogenic Bacteria. Protein Pept. Lett. 2015, 22, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Almaaytah, A.; Alnaamneh, A.; Abualhaijaa, A.; Alshari’, N.; Al-Balas, Q. In Vitro Synergistic Activities of the Hybrid Antimicrobial Peptide MelitAP-27 in Combination with Conventional Antibiotics Against Planktonic and Biofilm Forming Bacteria. Int. J. Pept. Res. Ther. 2016, 22, 497–504. [Google Scholar] [CrossRef]
- Cho, Y.-S.; Oh, J.J.; Oh, K.-H. Synergistic Anti-Bacterial and Proteomic Effects of Epigallocatechin Gallate on Clinical Isolates of Imipenem-Resistant Klebsiella Pneumoniae. Phytomedicine 2011, 18, 941–946. [Google Scholar] [CrossRef]
- Haroun, M.F.; Al-Kayali, R.S. Synergistic Effect of Thymbra Spicata, L. Extracts with Antibiotics against Multidrug—Resistant Staphylococcus Aureus and Klebsiella Pneumoniae Strains. Iran. J. Basic Med. Sci. 2016, 19, 1193–1200. [Google Scholar] [PubMed]
- Ma, L.; Wu, J.; Wang, S.; Yang, H.; Liang, D.; Lu, Z. Synergistic Antibacterial Effect of Bi2S3 Nanospheres Combined with Ineffective Antibiotic Gentamicin against Methicillin-Resistant Staphylococcus Aureus. J. Inorg. Biochem. 2017, 168, 38–45. [Google Scholar] [CrossRef]
- Hemeg, H.A. Nanomaterials for Alternative Antibacterial Therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef]
- Jain, J.; Arora, S.; Rajwade, J.M.; Omray, P.; Khandelwal, S.; Paknikar, K.M. Silver Nanoparticles in Therapeutics: Development of an Antimicrobial Gel Formulation for Topical Use. Mol. Pharm. 2009, 6, 1388–1401. [Google Scholar] [CrossRef]
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Vija, H.; Käkinen, A.; Titma, T.; Heinlaan, M.; et al. Size-Dependent Toxicity of Silver Nanoparticles to Bacteria, Yeast, Algae, Crustaceans and Mammalian Cells In Vitro. PLoS ONE 2014, 9, e102108. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Wang, Y.W.; Tang, H.; Wu, D.; Liu, D.; Liu, Y.; Cao, A.; Wang, H. Enhanced Bactericidal Toxicity of Silver Nanoparticles by the Antibiotic Gentamicin. Environ. Sci. Nano 2016, 3, 788–798. [Google Scholar] [CrossRef]
- Morones-Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver Enhances Antibiotic Activity against Gram-Negative Bacteria. Sci. Transl. Med. 2013, 5, 190. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Smékalová, M.; Večeřová, R.; Bogdanová, K.; Röderová, M.; Kolář, M.; Kilianová, M.; Hradilová, Š.; Froning, J.P.; Havrdová, M.; et al. Silver Nanoparticles Strongly Enhance and Restore Bactericidal Activity of Inactive Antibiotics against Multiresistant Enterobacteriaceae. Colloids Surf. B Biointerfaces 2016, 142, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; McShan, D.; Zhang, Y.; Sinha, S.S.; Arslan, Z.; Ray, P.C.; Yu, H. Mechanistic Study of the Synergistic Antibacterial Activity of Combined Silver Nanoparticles and Common Antibiotics. Environ. Sci. Technol. 2016, 50, 8840–8848. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.-S.; Hwang, J.H.; Choi, H.; Kim, K.-J.; Lee, D.G. Synergistic Effects between Silver Nanoparticles and Antibiotics and the Mechanisms Involved. J. Med. Microbiol. 2012, 61, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.T.G.; Titball, R.W.; Peacock, S.J.; Cerdeño-Tárraga, A.M.; Atkins, T.; Crossman, L.C.; Pitt, T.; Churcher, C.; Mungall, K.; Bentley, S.D.; et al. Genomic Plasticity of the Causative Agent of Melioidosis, Burkholderia pseudomallei. Proc. Natl. Acad. Sci. USA 2004, 101, 14240–14245. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-Controlled Silver Nanoparticles Synthesized over the Range 5–100 Nm Using the Same Protocol and Their Antibacterial Efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Ma, Y.-W.; Wu, Z.-W.; Zhang, L.-H.; Zhang, J.; Jian, G.-S.; Pan, S. Theoretical Study of the Local Surface Plasmon Resonance Properties of Silver Nanosphere Clusters. Plasmonics 2013, 8, 1351–1360. [Google Scholar] [CrossRef]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobley, J.; Fernig, D.G. A Rapid Method to Estimate the Concentration of Citrate Capped Silver Nanoparticles from UV-Visible Light Spectra. Analyst 2014, 139, 4855–4861. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Methods for the Determination of Susceptibility of Bacteria to Antimicrobial Agents. Terminology. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 1998, 4, 291. [Google Scholar]
- Siritongsuk, P.; Hongsing, N.; Thammawithan, S.; Daduang, S.; Klaynongsruang, S.; Tuanyok, A.; Patramanon, R. Two-Phase Bactericidal Mechanism of Silver Nanoparticles against Burkholderia pseudomallei. PLoS ONE 2016, 11, e0168098. [Google Scholar] [CrossRef]
- Trinh, T.T.; Hoang, T.S.; Tran, D.A.; Trinh, V.T.; Göhler, A.; Nguyen, T.T.; Hoang, S.N.; Krumkamp, R.; Nguyen, L.T.N.; May, J.; et al. A Simple Laboratory Algorithm for Diagnosis of Melioidosis in Resource-Constrained Areas: A Study from North-Central Vietnam. Clin. Microbiol. Infect. 2018, 24, 84. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, J.C.; Jeon, G.E.; Kim, C.S.; Seo, J.H. Effect of the Size and Shape of Silver Nanoparticles on Bacterial Growth and Metabolism by Monitoring Optical Density and Fluorescence Intensity. Biotechnol. Bioprocess Eng. 2017, 22, 210–217. [Google Scholar] [CrossRef]
- Kalyani, R.; Chandra, V.; Vijaykumar, P.; Pammi, S.V.N.; Rajkumar, M.; Swamy, P.; Murthy, K. Biosynthesis of Silver Nanoparticles Using Annona Squamosa Leaf Extract with Synergistic Antibacterial Activity. Indian J. Pharm. Sci. 2019, 81, 1036–1044. [Google Scholar] [CrossRef]
- Mazur, P.; Skiba-Kurek, I.; Mrowiec, P.; Karczewska, E.; Drożdż, R. Synergistic ROS-Associated Antimicrobial Activity of Silver Nanoparticles and Gentamicin Against Staphylococcus Epidermidis. Int. J. Nanomed. 2020, 15, 3551–3562. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, M.; Affifi, M.; Amin, B. Control of Imipenem Resistant—Klebsiella Pneumoniae Pulmonary Infection by Oral Treatment Using a Combination of Mycosynthesized Ag-Nanoparticles and Imipenem. J. Radiat. Res. Appl. Sci. 2017, 10, 353–360. [Google Scholar] [CrossRef][Green Version]
- Li, P.; Li, J.; Wu, C.; Wu, Q.; Li, J. Synergistic Antibacterial Effect of β-Lactum Antibiotic Combined with Silver Nanoparticle. Nanotechnology 2005, 16, 1912–1917. [Google Scholar] [CrossRef]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic Synthesis of Silver Nanoparticles and Their Synergistic Effect with Antibiotics: A Study against Gram-Positive and Gram-Negative Bacteria. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 103–109. [Google Scholar] [CrossRef]
- Duran, N.; Marcato, P.; Conti, R.; Alves, O.; Costa, F.; Brocchi, M. Potential Use of Silver Nanoparticles on Pathogenic Bacteria, Their Toxicity and Possible Mechanisms of Action. Rev. J. Braz. Chem. Soc. 2010, 21, 949–959. [Google Scholar] [CrossRef]
- Jamaran, S.; Zarif, B.R. Synergistic Effect of Silver Nanoparticles with Neomycin or Gentamicin Antibiotics on Mastitis-Causing Staphylococcus aureus. Open J. Ecol. 2016, 6, 452–459. [Google Scholar] [CrossRef]
- Burtnick, M.N.; Brett, P.J.; Woods, D.E. Molecular and Physical Characterization of Burkholderia Mallei O Antigens. J. Bacteriol. 2002, 184, 849–852. [Google Scholar] [CrossRef]
- Tuanyok, A.; Stone, J.K.; Mayo, M.; Kaestli, M.; Gruendike, J.; Georgia, S.; Warrington, S.; Mullins, T.; Allender, C.J.; Wagner, D.M.; et al. Genetic and Molecular Basis of O-Antigenic Diversity in Burkholderia pseudomallei lipopolysaccharide. PLoS Negl. Trop. Dis. 2012, 6, e1453. [Google Scholar] [CrossRef]
- Cloutier, M.; Muru, K.; Ravicoularamin, G.; Gauthier, C. Polysaccharides from Burkholderia Species as Targets for Vaccine Development, Immunomodulation and Chemical Synthesis. Nat. Prod. Rep. 2018, 35, 1251–1293. [Google Scholar] [CrossRef] [PubMed]
- Msarah, M.; Yusoff, M.; Nurema, S.; Prabhakaran, P.; Ibrahim, I.; Wan Mohd Noor, W.S.A. Extreme Environment: Biofilms and Microbial Diversity. Malays. J. Microbiol. 2018, 14, 435–443. [Google Scholar] [CrossRef]
- Aka, S.T.; Haji, S.H. Sub-MIC of Antibiotics Induced Biofilm Formation of Pseudomonas Aeruginosa in the Presence of Chlorhexidine. J. Microbiol. Publ. Braz. Soc. Microbiol. 2015, 46, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Limoli, D.; Jones, C.; Wozniak, D. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef]
- Nadell, C.; Xavier, J.; Levin, S.; Foster, K. The Evolution of Quorum Sensing in Bacterial Biofilms. PLoS Biol. 2008, 6, e14. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.J.; Belenky, P.A.; Yang, J.H.; MacDonald, I.C.; Martell, J.D.; Takahashi, N.; Chan, C.T.Y.; Lobritz, M.A.; Braff, D.; Schwarz, E.G.; et al. Antibiotics Induce Redox-Related Physiological Alterations as Part of Their Lethality. Proc. Natl. Acad. Sci. USA 2014, 111, E2100–E2109. [Google Scholar] [CrossRef]
- Foti, J.; Devadoss, B.; Winkler, J.; Collins, J.; Walker, G. Oxidation of the Guanine Nucleotide Pool Underlies Cell Death by Bactericidal Antibiotics. Science 2012, 336, 315–319. [Google Scholar] [CrossRef]
- Ranieri, M.R.; Whitchurch, C.B.; Burrows, L.L. Mechanisms of Biofilm Stimulation by Subinhibitory Concentrations of Antimicrobials. Curr. Opin. Microbiol. 2018, 45, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Braga, P.C.; Sasso, M.D.; Sala, M.T. Sub-MIC Concentrations of Cefodizime Interfere with Various Factors Affecting Bacterial Virulence. J. Antimicrob. Chemother. 2000, 45, 15–25. [Google Scholar] [CrossRef]
- Yu, W.; Hallinen, K.M.; Wood, K.B. Interplay between Antibiotic Efficacy and Drug-Induced Lysis Underlies Enhanced Biofilm Formation at Subinhibitory Drug Concentrations. Antimicrob. Agents Chemother. 2018, 62, e01603–e01617. [Google Scholar] [CrossRef] [PubMed]
- Marti, S.; Puig, C.; Merlos, A.; Viñas, M.; de Jonge, M.I.; Liñares, J.; Ardanuy, C.; Langereis, J.D. Bacterial Lysis through Interference with Peptidoglycan Synthesis Increases Biofilm Formation by Nontypeable Haemophilus Influenzae. mSphere 2017, 2, e00329. [Google Scholar] [CrossRef] [PubMed]
- Jaimee, G.; Halami, P.M. Subinhibitory Concentrations of Gentamicin Trigger Expression of Aac (6′)Ie-Aph (2″)Ia, Chaperones and Biofilm-Related Genes in Lactobacillus Plantarum MCC3011. Res. Microbiol. 2017, 168, 722–731. [Google Scholar] [CrossRef]
- Hoffman, L.R.; D’Argenio, D.A.; MacCoss, M.J.; Zhang, Z.; Jones, R.A.; Miller, S.I. Aminoglycoside Antibiotics Induce Bacterial Biofilm Formation. Nature 2005, 436, 1171–1175. [Google Scholar] [CrossRef]
- Yang, Y.; Alvarez, P.J.J. Sublethal Concentrations of Silver Nanoparticles Stimulate Biofilm Development. Environ. Sci. Technol. Lett. 2015, 2, 221–226. [Google Scholar] [CrossRef]
- Saeki, E.K.; Yamada, A.Y.; de Araujo, L.A.; Anversa, L.; de Garcia, D.O.; de Souza, R.L.B.; Martins, H.M.; Kobayashi, R.K.T.; Nakazato, G. Subinhibitory Concentrations of Biogenic Silver Nanoparticles Affect Motility and Biofilm Formation in Pseudomonas Aeruginosa. Front. Cell. Infect. Microbiol. 2021, 11, 656984. [Google Scholar] [CrossRef]
- Limmathurotsakul, D.; Paeyao, A.; Wongratanacheewin, S.; Saiprom, N.; Takpho, N.; Thaipadungpanit, J.; Chantratita, N.; Wuthiekanun, V.; Day, N.P.J.; Peacock, S.J. Role of Burkholderia Pseudomallei Biofilm Formation and Lipopolysaccharide in Relapse of Melioidosis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014, 20, O854–O856. [Google Scholar] [CrossRef]
- Taweechaisupapong, S.; Kaewpa, C.; Arunyanart, C.; Kanla, P.; Homchampa, P.; Sirisinha, S.; Proungvitaya, T.; Wongratanacheewin, S. Virulence of Burkholderia Pseudomallei Does Not Correlate with Biofilm Formation. Microb. Pathog. 2005, 39, 77–85. [Google Scholar] [CrossRef]
- Mongkolrob, R.; Taweechaisupapong, S.; Tungpradabkul, S. Correlation between Biofilm Production, Antibiotic Susceptibility and Exopolysaccharide Composition in Burkholderia Pseudomallei BpsI, Ppk, and RpoS Mutant Strains. Microbiol. Immunol. 2015, 59, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Kwon, D.-N.; Kim, J.-H. Enhanced Antibacterial and Anti-Biofilm Activities of Silver Nanoparticles against Gram-Negative and Gram-Positive Bacteria. Nanoscale Res. Lett. 2014, 9, 373. [Google Scholar] [CrossRef] [PubMed]
- DeShazer, D. Genomic Diversity of Burkholderia Pseudomallei Clinical Isolates: Subtractive Hybridization Reveals a Burkholderia Mallei-Specific Prophage in B. Pseudomallei 1026b. J. Bacteriol. 2004, 186, 3938–3950. [Google Scholar] [CrossRef] [PubMed]
- Madhongsa, K.; Pasan, S.; Phophetleb, O.; Nasompag, S.; Thammasirirak, S.; Daduang, S.; Taweechaisupapong, S.; Lomize, A.L.; Patramanon, R. Antimicrobial Action of the Cyclic Peptide Bactenecin on Burkholderia Pseudomallei Correlates with Efficient Membrane Permeabilization. PLoS Negl. Trop. Dis. 2013, 7, e2267. [Google Scholar] [CrossRef]
- Kanthawong, S.; Nazmi, K.; Wongratanacheewin, S.; Bolscher, J.G.M.; Wuthiekanun, V.; Taweechaisupapong, S. In Vitro Susceptibility of Burkholderia Pseudomallei to Antimicrobial Peptides. Int. J. Antimicrob. Agents 2009, 34, 309–314. [Google Scholar] [CrossRef]
- Randall, L.B.; Dobos, K.; Papp-Wallace, K.M.; Bonomo, R.A.; Schweizer, H.P. Membrane-Bound PenA β-Lactamase of Burkholderia Pseudomallei. Antimicrob. Agents Chemother. 2015, 60, 1509–1514. [Google Scholar] [CrossRef]
- Mackay, M.L.; Milne, K.; Gould, I.M. Comparison of Methods for Assessing Synergic Antibiotic Interactions. Int. J. Antimicrob. Agents 2000, 15, 125–129. [Google Scholar] [CrossRef]
- Hwan, S.; Lee, H.-S.; Ryu, D.-S.; Choi, S.-J.; Lee, D.-S. Antibacterial Activity of Silver-Nanoparticles Against Staphylococcus Aureus and Escherichia Coli. Korean J. Microbiol. Biotechnol. 2010, 39, 77–85. [Google Scholar]


| Bacteria (Isolates) | MIC (μg/mL) | MBC (μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CAZ | IMI | MER | GENT | AgNPs | CAZ | IMI | MER | GENT | AgNPs | |
| * 1026b | 4 | 1 | 2 | 32 | 8 | 64 | 2 | 4 | 512 | 16 |
| ** H777 | 8 | 1 | 2 | 16 | 16 | 32 | 2 | 4 | 256 | 32 |
| *** 316c | 128 | 0.5 | 2 | 64 | 16 | 512 | 1 | 4 | 512 | 32 |
| Antibiotics | B. pseudomallei Isolates | MIC (µg/mL) | Decrease in the Concentration (-Fold) | FICI | Type of Interaction | |
|---|---|---|---|---|---|---|
| Alone | Combination | |||||
| Ceftazidime | 1026b | 4 | 1 (2) | 4 | 0.5 | S |
| H777 | 8 | 2 (2) | 4 | 0.375 | S | |
| 316c | 128 | 16 (4) | 8 | 0.375 | S | |
| Imipenem | 1026b | 1 | 0.5 (2) | 2 | 0.75 | I |
| H777 | 1 | 0.25 (4) | 4 | 0.5 | S | |
| 316c | 0.5 | 0.25 (4) | 2 | 0.75 | I | |
| Meropenem | 1026b | 2 | 0.5 (2) | 4 | 0.5 | S |
| H777 | 2 | 0.5 (2) | 4 | 0.375 | S | |
| 316c | 2 | 0.5 (2) | 4 | 0.375 | S | |
| Gentamicin | 1026b | 32 | 2 (2) | 16 | 0.312 | S |
| H777 | 16 | 4 (4) | 4 | 0.5 | S | |
| 316c | 64 | 16 (2) | 4 | 0.375 | S | |
| Antibiotic | B. pseudomallei Isolates | MBC (µg/mL) | Decrease in the Concentration (-Fold) | FBCI | Type of Interaction | |
|---|---|---|---|---|---|---|
| Alone | Combination | |||||
| Ceftazidime | 1026b | 64 | 4 (4) | 16 | 0.313 | S |
| H777 | 32 | 8 (4) | 4 | 0.375 | S | |
| 316c | 512 | 16 (8) | 32 | 0.281 | S | |
| Imipenem | 1026b | 2 | 0.5 (4) | 4 | 0.5 | S |
| H777 | 2 | 0.5 (8) | 4 | 0.5 | S | |
| 316c | 1 | 0.25 (4) | 2 | 0.625 | I | |
| Meropenem | 1026b | 4 | 1 (2) | 4 | 0.375 | S |
| H777 | 4 | 1 (4) | 4 | 0.375 | S | |
| 316c | 4 | 1 (4) | 4 | 0.375 | S | |
| Gentamicin | 1026b | 512 | 1 (4) | 512 | 0.252 | S |
| H777 | 256 | 4 (8) | 64 | 0.265 | S | |
| 316c | 512 | 16 (8) | 32 | 0.289 | S | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malawong, S.; Thammawithan, S.; Sirithongsuk, P.; Daduang, S.; Klaynongsruang, S.; Wong, P.T.; Patramanon, R. Silver Nanoparticles Enhance Antimicrobial Efficacy of Antibiotics and Restore That Efficacy against the Melioidosis Pathogen. Antibiotics 2021, 10, 839. https://doi.org/10.3390/antibiotics10070839
Malawong S, Thammawithan S, Sirithongsuk P, Daduang S, Klaynongsruang S, Wong PT, Patramanon R. Silver Nanoparticles Enhance Antimicrobial Efficacy of Antibiotics and Restore That Efficacy against the Melioidosis Pathogen. Antibiotics. 2021; 10(7):839. https://doi.org/10.3390/antibiotics10070839
Chicago/Turabian StyleMalawong, Sathit, Saengrawee Thammawithan, Pawinee Sirithongsuk, Sakda Daduang, Sompong Klaynongsruang, Pamela T. Wong, and Rina Patramanon. 2021. "Silver Nanoparticles Enhance Antimicrobial Efficacy of Antibiotics and Restore That Efficacy against the Melioidosis Pathogen" Antibiotics 10, no. 7: 839. https://doi.org/10.3390/antibiotics10070839
APA StyleMalawong, S., Thammawithan, S., Sirithongsuk, P., Daduang, S., Klaynongsruang, S., Wong, P. T., & Patramanon, R. (2021). Silver Nanoparticles Enhance Antimicrobial Efficacy of Antibiotics and Restore That Efficacy against the Melioidosis Pathogen. Antibiotics, 10(7), 839. https://doi.org/10.3390/antibiotics10070839






