Hepcidin Protects Yellow Catfish (Pelteobagrus fulvidraco) against Aeromonas veronii-Induced Ascites Disease by Regulating Iron Metabolism
Abstract
1. Introduction
2. Results
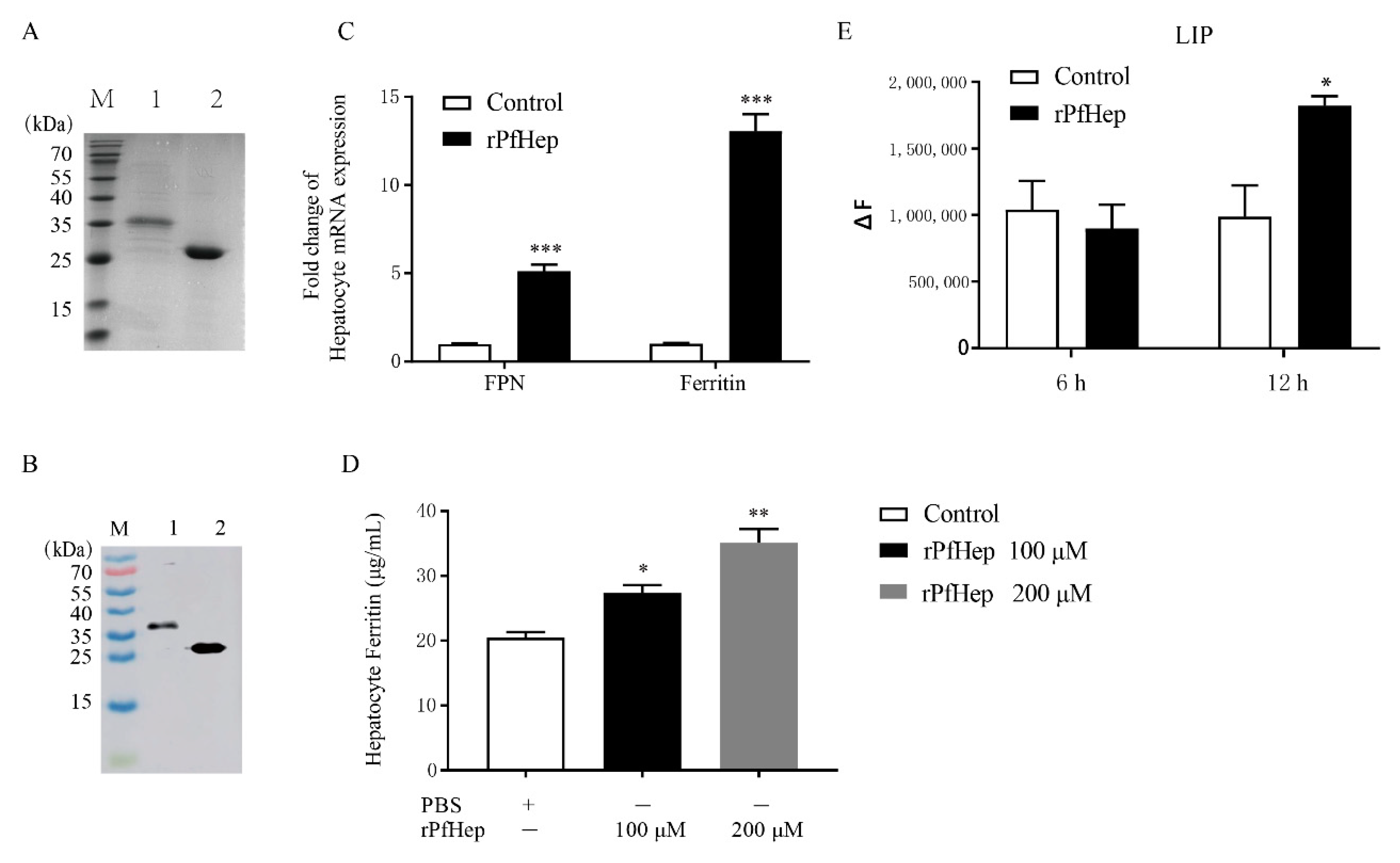
2.1. Activity of Purified rPfHep
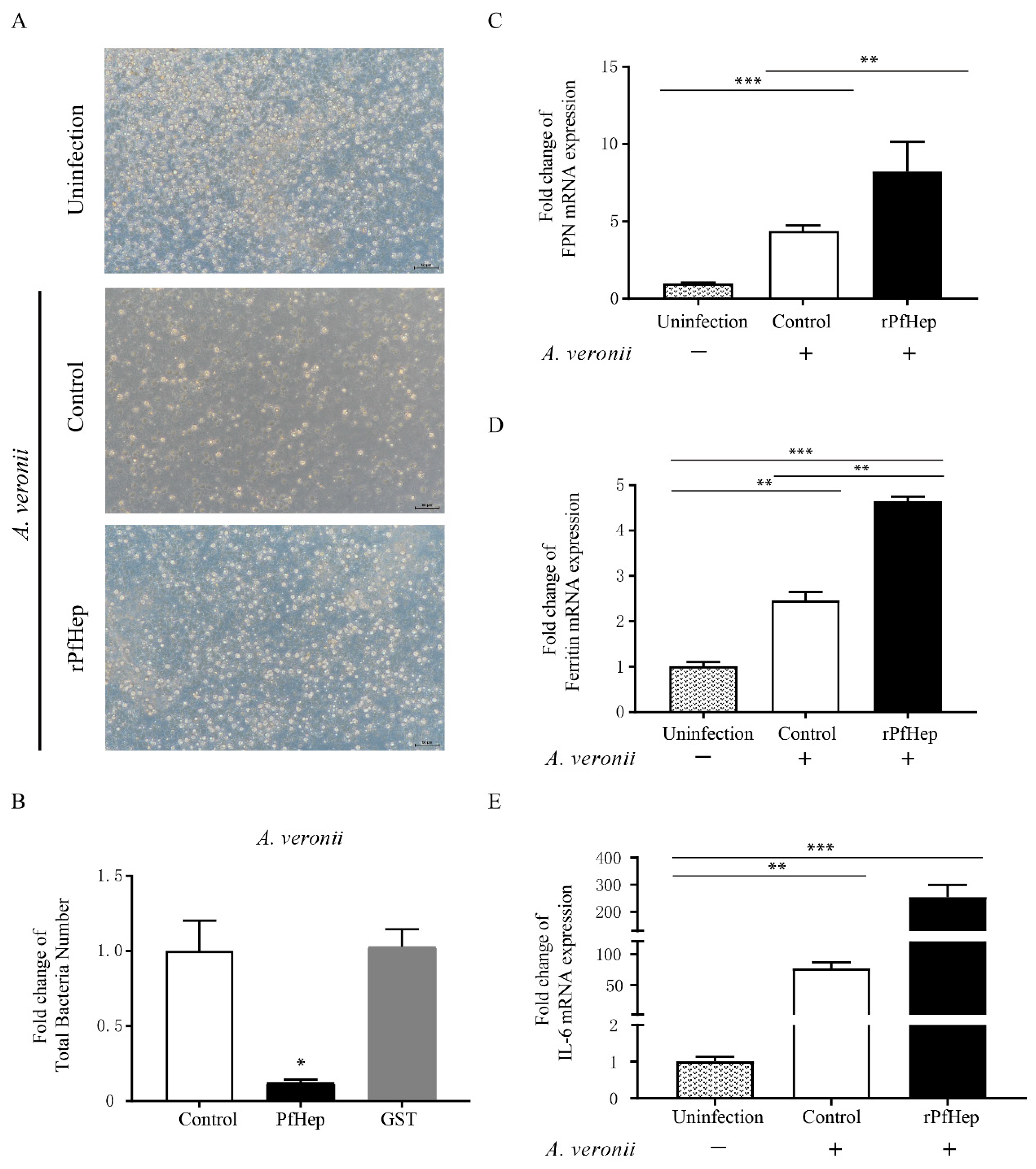
2.2. Promotion of Bacterial Defense by rPfHep in P. fulvidraco Primary Hepatocytes
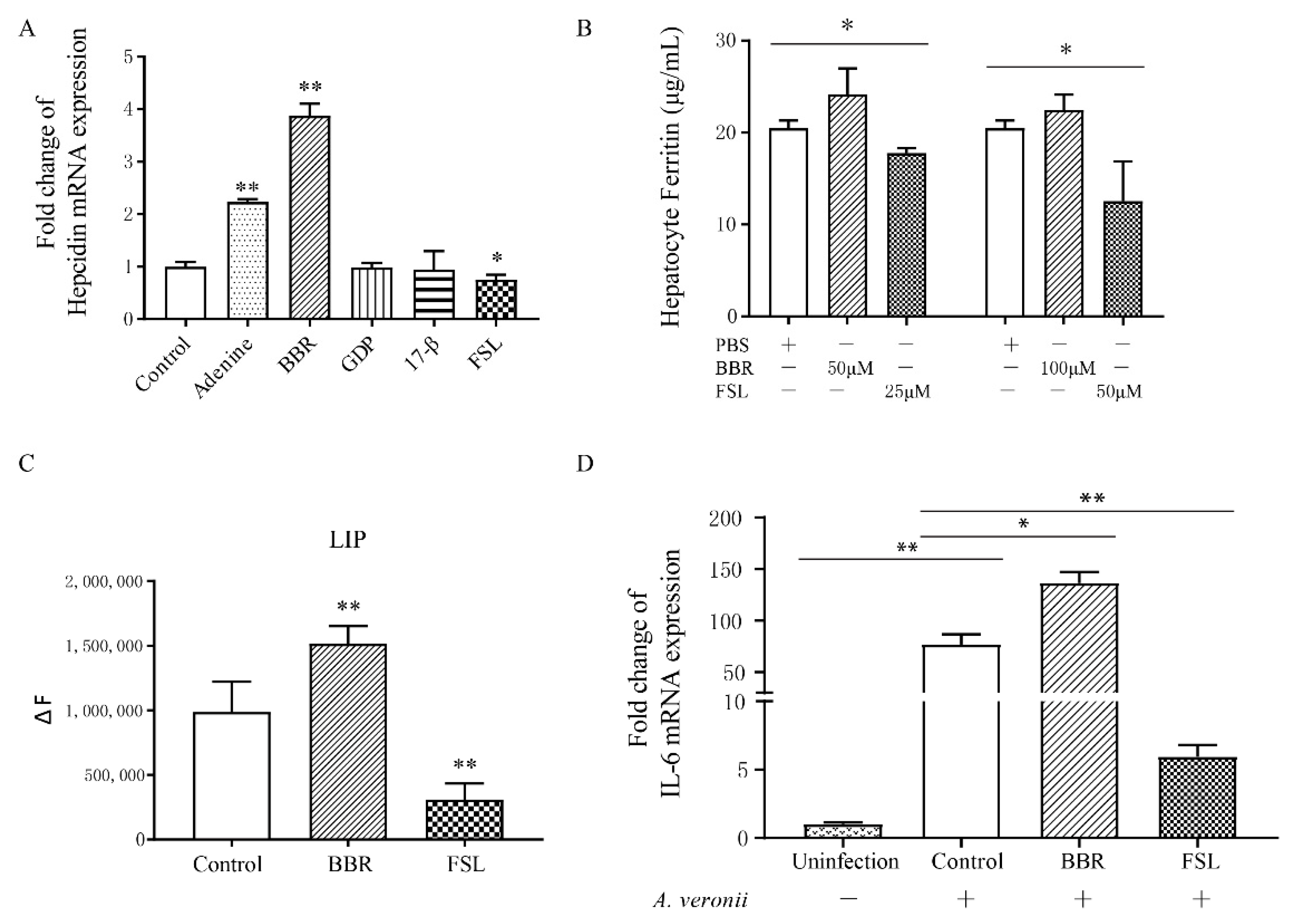
2.3. Identification of BBR and FSL as Hepcidin Agonists and Antagonists
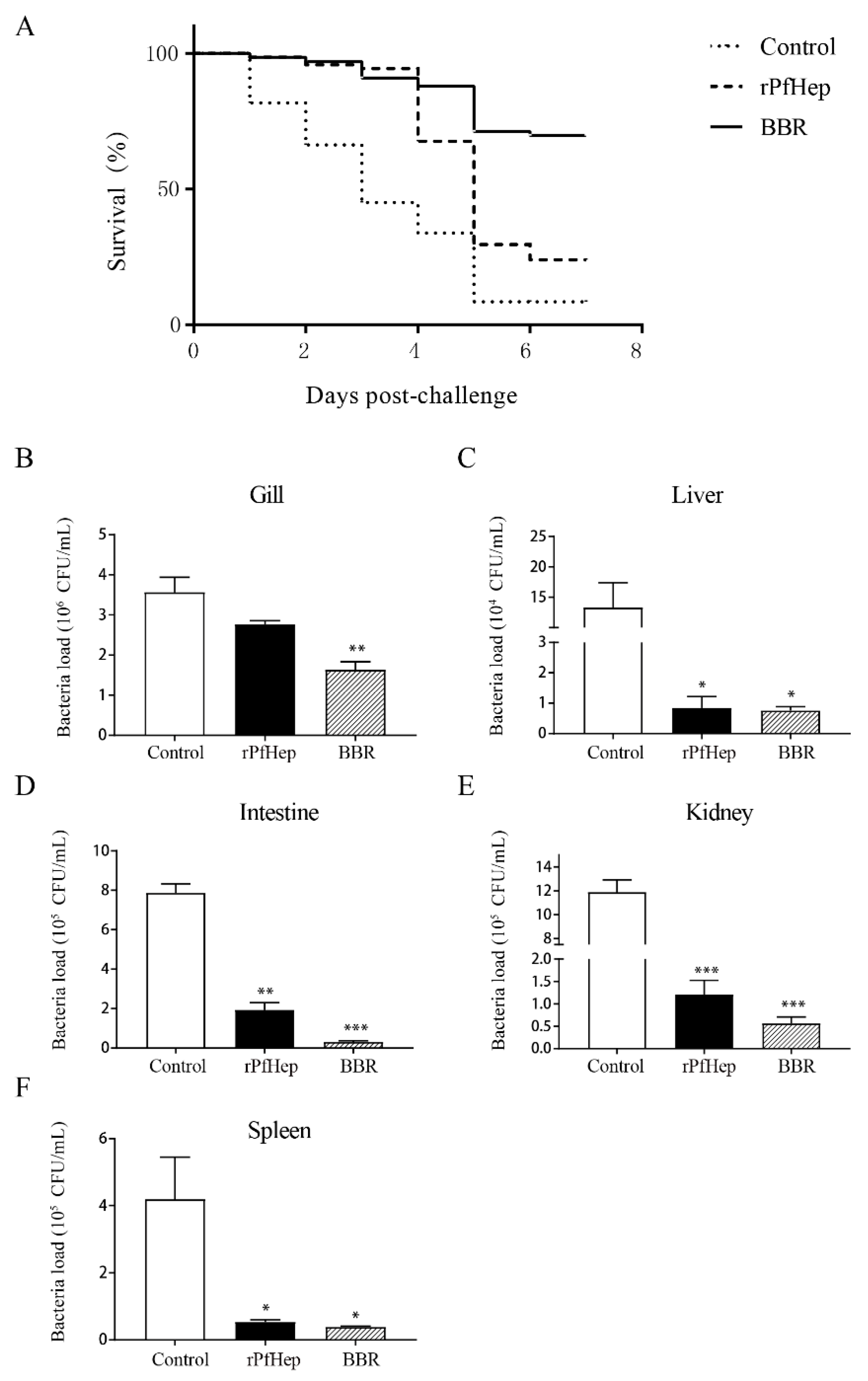
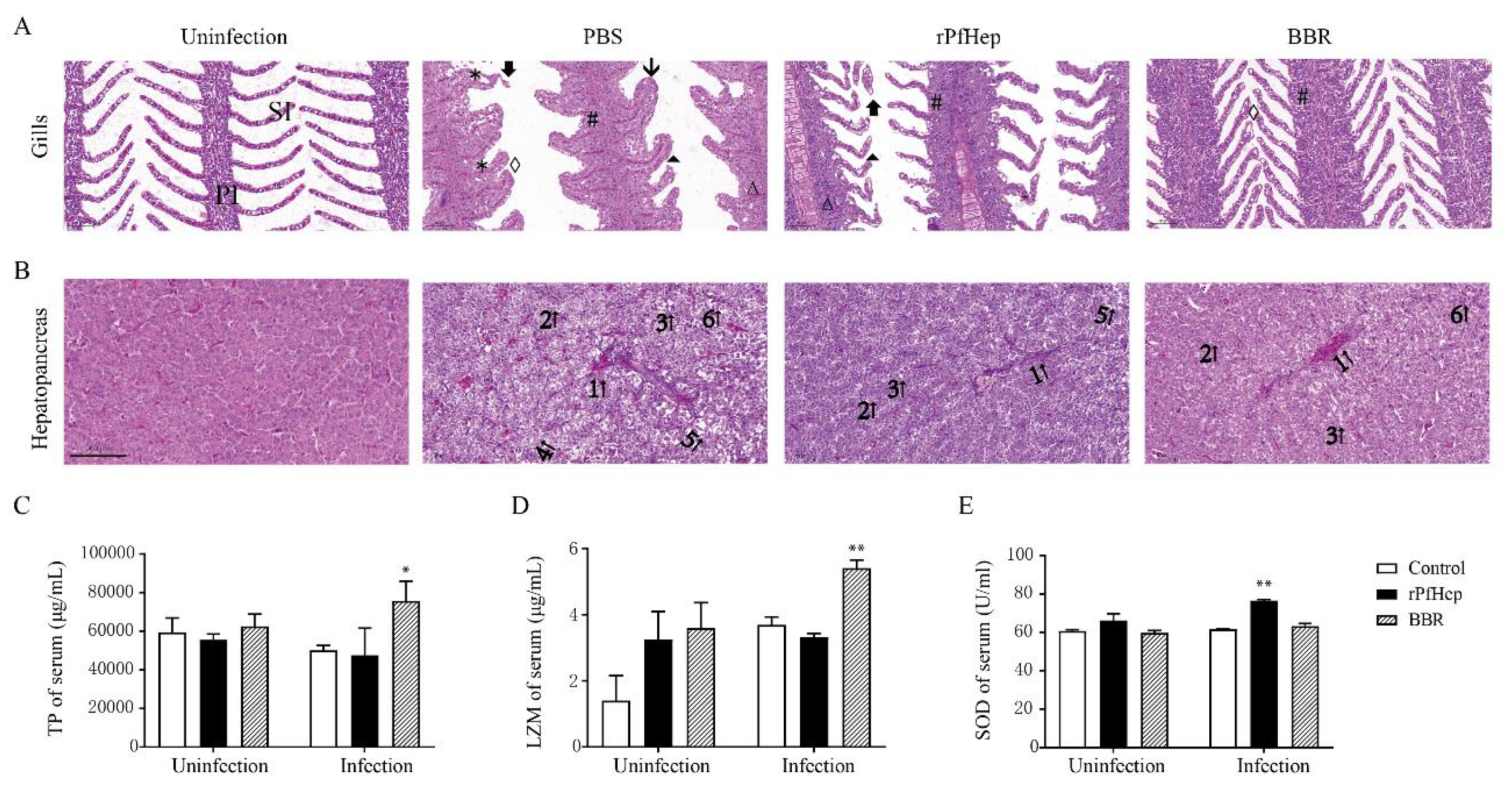
2.4. Reduction in Mortality and Tissue Bacterial Load by rPfHep and BBR in Yellow Catfish
2.5. Alleviation of A. veronii-Induced Inflammation by rPfHep and BBR
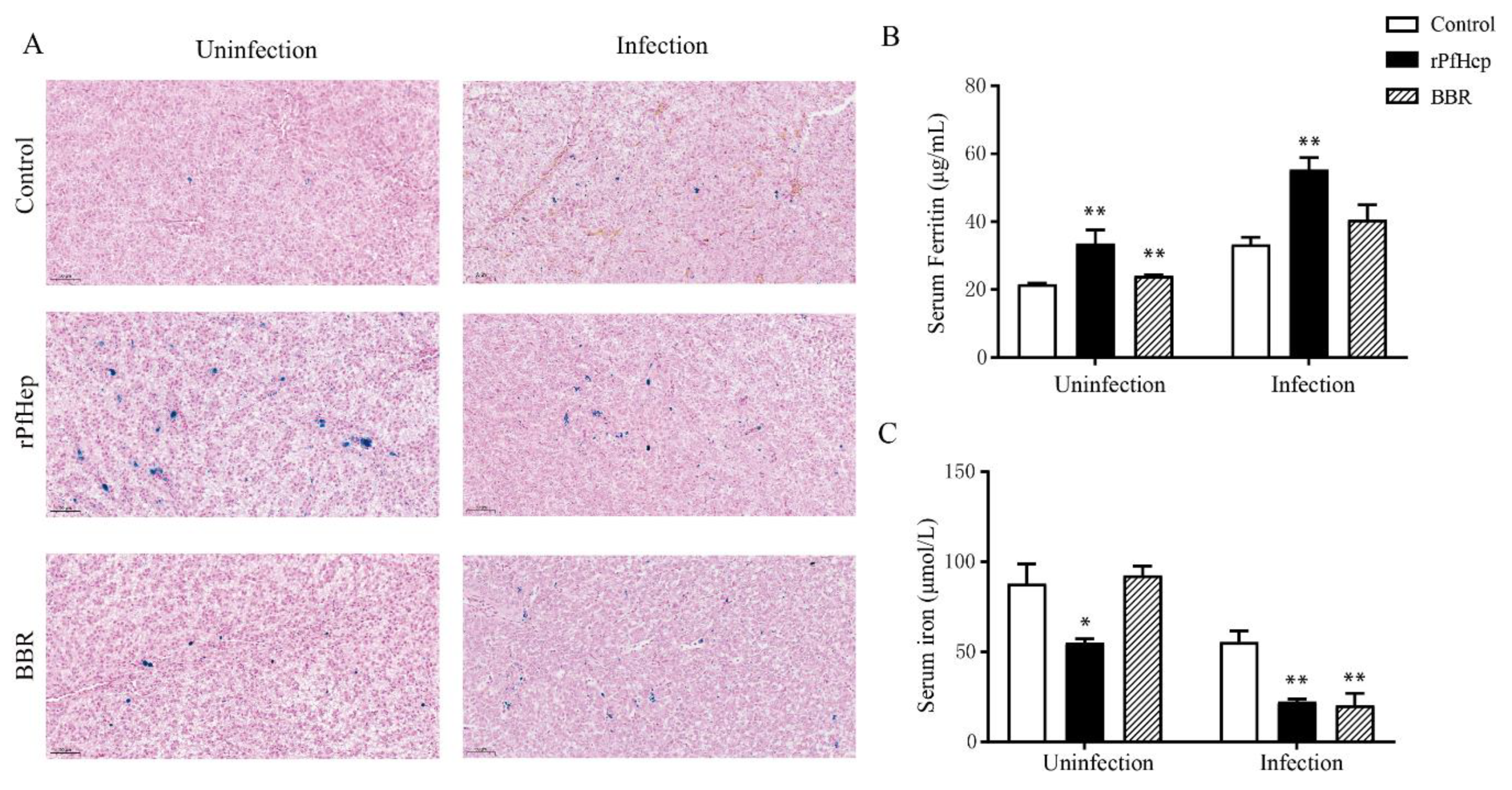
2.6. Regulation of Individual Iron Levels by rPfHep and BBR
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Hepatocyte Isolation
4.3. Bacterial Activity
4.4. Expression and Purification of Recombinant Hepcidin
4.5. Western Blot Analysis
4.6. Intracellular Iron Measurement
4.7. RNA Extraction and qPCR Analysis
4.8. Tissue Staining
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BBR | Berberine Hydrochloride |
| FSL | Fursultiamine |
| GDP | Guanosine 5′-diphosphate |
| RPS | Relative Percentage Survival |
| BSA | Bovine Serum Albumin |
| RBC | Red Blood Cells |
| ARG | Antibiotic Resistance Genes |
| LIP | Labile Iron Pool |
References
- Chen, H.J.; Yuan, G.L.; Su, J.G.; Liu, X.L. Hematological and immune genes responses in yellow catfish (Pelteobagrus fulvidraco) with septicemia induced by Edwardsiella ictaluri. Fish Shellfish Immunol. 2020, 97, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, Y.; Zhang, J.; Yuan, G.; Liu, X.; Ai, T.; Su, J. Astragalus polysaccharides, chitosan and poly(I:C) obviously enhance inactivated Edwardsiella ictaluri vaccine potency in yellow catfish Pelteobagrus fulvidraco. Fish Shellfish Immunol. 2019, 87, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Bravo, A.; Figueras, M.J. An Update on the Genus Aeromonas: Taxonomy, Epidemiology, and Pathogenicity. Microorganisms 2020, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- De Silva, L.A.D.S.; Wickramanayake, M.V.K.S.; Heo, G.J. Virulence and antimicrobial resistance potential of Aeromonas spp. associated with shellfish. Lett. Appl. Microbiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Geng, Y.; Wang, K.Y.; Chen, D.F.; Huang, X.L.; Ou, Y.P.; He, C.L.; Zhong, Z.J.; Lai, W.M. Aeromonas veronii Infection in Cultured Channel Catfish, Ictalurus punctatus, in Southwest China. Isr. J. Aquac. Bamidgeh 2016, 68, 20839. [Google Scholar]
- Yang, Q.; Zhao, M.; Wang, K.-Y.; Wang, J.; He, Y.; Wang, E.-L.; Liu, T.; Chen, D.-F.; Lai, W. Multidrug-Resistant Aeromonas veronii Recovered from Channel Catfish (Ictalurus punctatus) in China: Prevalence and Mechanisms of Fluoroquinolone Resistance. Microb. Drug Resist. 2017, 23, 473–479. [Google Scholar] [CrossRef]
- Long, B.; Wang, J.; He, Y.; Zhao, M.; Wang, E.; Cui, J.; Deng, L.; Liu, T.; Zeng, Y.; Wang, K.; et al. Isolation, identification and pathogenicity of Aeromonas veronii isolated from Micropterus salmoides. Chin. J. Vet. Sci. 2016, 36, 48–55. [Google Scholar]
- Zhao, B.-R.; Zheng, Y.; Gao, J.; Wang, X.-W. Maturation of an Antimicrobial Peptide Inhibits Aeromonas hydrophila Infection in Crayfish. J. Immunol. 2020, 204, 487–497. [Google Scholar] [CrossRef]
- Gonzalez-Serrano, C.J.; Santos, J.A.; Garcia-Lopez, M.L.; Otero, A. Virulence markers in Aeromonas hydrophila and Aeromonas veronii biovar sobria isolates from freshwater fish and from a diarrhoea case. J. Appl. Microbiol. 2002, 93, 414–419. [Google Scholar] [CrossRef]
- Preena, P.G.; Swaminathan, T.R.; Kumar, V.J.R.; Singh, I.S.B. Antimicrobial resistance in aquaculture: A crisis for concern. Biologia 2020, 75, 1497–1517. [Google Scholar] [CrossRef]
- Piotrowska, M.; Popowska, M. The prevalence of antibiotic resistance genes among Aeromonas species in aquatic environments. Ann. Microbiol. 2014, 64, 921–934. [Google Scholar] [CrossRef]
- Guida, C.; Altamura, S.; Klein, F.A.; Galy, B.; Boutros, M.; Ulmer, A.J.; Hentze, M.W.; Muckenthaler, M.U. A novel inflammatory pathway mediating rapid hepcidin-independent hypoferremia. Blood 2015, 125, 2265–2275. [Google Scholar] [CrossRef]
- Skaar, E.P. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. 2010, 6, e1000949. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Valore, E.V.; Waring, A.J.; Ganz, T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J. Biol. Chem. 2001, 276, 7806–7810. [Google Scholar] [CrossRef] [PubMed]
- Pigeon, C.; Ilyin, G.; Courselaud, B.; Leroyer, P.; Turlin, B.; Brissot, P.; Loréal, O. A New Mouse Liver-specific Gene, Encoding a Protein Homologous to Human Antimicrobial Peptide Hepcidin, Is Overexpressed during Iron Overload. J. Biol. Chem. 2001, 276, 7811–7819. [Google Scholar] [CrossRef]
- Krause, A.; Neitz, S.; Magert, H.J.; Schulz, A.; Forssmann, W.G.; Schulz-Knappe, P.; Adermann, K. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000, 480, 147–150. [Google Scholar] [CrossRef]
- Shike, H.; Lauth, X.; Westerman, M.E.; Ostland, V.E.; Carlberg, J.M.; Van Olst, J.C.; Shimizu, C.; Bulet, P.; Burns, J.C. Bass hepcidin is a novel antimicrobial peptide induced by bacterial challenge. Eur. J. Biochem. 2002, 269, 2232–2237. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. The role of hepcidin in iron metabolism. Acta Haematol. 2009, 122, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef]
- Casu, C.; Nemeth, E.; Rivella, S. Hepcidin agonists as therapeutic tools. Blood 2018, 131, 1790–1794. [Google Scholar] [CrossRef]
- Poli, M.; Asperti, M.; Ruzzenenti, P.; Regoni, M.; Arosio, P. Hepcidin antagonists for potential treatments of disorders with hepcidin excess. Front. Pharmacol. 2014, 5, 86. [Google Scholar] [CrossRef]
- Bayne, C.J.; Gerwick, L. The acute phase response and innate immunity of fish. Dev. Comp. Immunol. 2001, 25, 725–743. [Google Scholar] [CrossRef]
- Neves, J.V.; Wilson, J.M.; Rodrigues, P.N.S. Transferrin and ferritin response to bacterial infection: The role of the liver and brain in fish. Dev. Comp. Immunol. 2009, 33, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Brissot, P.; Ropert, M.; Le Lan, C.; Loreal, O. Non-transferrin bound iron: A key role in iron overload and iron toxicity. Biochim. Biophys. Acta 2012, 1820, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.-C.S.; Arsiwala, T.; Mohsen, M.; Vogel, M.; Manolova, V.; Bachmann, M.F. On Iron Metabolism and Its Regulation. Int. J. Mol. Sci. 2021, 22, 4591. [Google Scholar] [CrossRef]
- Lopez-Soto, F.; Leon-Sicairos, N.; Reyes-Lopez, M.; Serrano-Luna, J.; Ordaz-Pichardo, C.; Pina-Vazquez, C.; Ortiz-Estrada, G.; de la Garza, M. Use and endocytosis of iron-containing proteins by Entamoeba histolytica trophozoites. Infect. Genet. Evolut. 2009, 9, 1038–1050. [Google Scholar] [CrossRef]
- Phan-Aram, P.; Mahasri, G.; Kayansamruaj, P.; Amparyup, P.; Srisapoome, P. Immune Regulation, but Not Antibacterial Activity, Is a Crucial Function of Hepcidins in Resistance against Pathogenic Bacteria in Nile Tilapia (Oreochromis niloticus Linn.). Biomolecules 2020, 10, 1132. [Google Scholar] [CrossRef]
- Chen, J.; Nie, L.; Chen, J. Mudskipper (Boleophthalmus pectinirostris) Hepcidin-1 and Hepcidin-2 Present Different Gene Expression Profile and Antibacterial Activity and Possess Distinct Protective Effect against Edwardsiella tarda Infection. Probiotics Antimicrob. Proteins 2018, 10, 176–185. [Google Scholar] [CrossRef]
- Hu, Y.; Kurobe, T.; Liu, X.; Zhang, Y.A.; Su, J.; Yuan, G. Hamp Type-1 Promotes Antimicrobial Defense via Direct Microbial Killing and Regulating Iron Metabolism in Grass Carp (Ctenopharyngodon idella). Biomolecules 2020, 10, 825. [Google Scholar] [CrossRef]
- Malerba, M.; Louis, S.; Cuvellier, S.; Shambat, S.M.; Hua, C.; Gomart, C.; Fouet, A.; Ortonne, N.; Decousser, J.-W.; Zinkernagel, A.S.; et al. Epidermal hepcidin is required for neutrophil response to bacterial infection. J. Clin. Investig. 2020, 130, 329–334. [Google Scholar] [CrossRef]
- Wei, X.; Sarath Babu, V.; Lin, L.; Hu, Y.; Zhang, Y.; Liu, X.; Su, J.; Li, J.; Zhao, L.; Yuan, G. Hepcidin protects grass carp (Ctenopharyngodon idellus) against Flavobacterium columnare infection via regulating iron distribution and immune gene expression. Fish Shellfish Immunol. 2018, 75, 274–283. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, J.; Qu, Z.; Zou, Q.; Liu, X.; Su, J.; Fu, X.; Yuan, G. Administration of dietary recombinant hepcidin on grass carp (Ctenopharyngodon idella) against Flavobacterium columnare infection under cage aquaculture conditions. Fish Shellfish Immunol. 2020, 99, 27–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Wu, Q.; Wang, H.; Zhao, L.; Wang, X.; Mu, M.; Xie, E.; He, X.; Shao, D.; et al. Adenine alleviates iron overload by cAMP/PKA mediated hepatic hepcidin in mice. J. Cell. Physiol. 2018, 233, 7268–7278. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Guo, W.; Liu, X.; Liu, S.; Yin, H. Icariin regulates systemic iron metabolism by increasing hepatic hepcidin expression through Stat3 and Smad1/5/8 signaling. Int. J. Mol. Med. 2016, 37, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jian, J.; Katz, S.; Abramson, S.B.; Huang, X. 17beta-Estradiol inhibits iron hormone hepcidin through an estrogen responsive element half-site. Endocrinology 2012, 153, 3170–3178. [Google Scholar] [CrossRef] [PubMed]
- Angmo, S.; Tripathi, N.; Abbat, S.; Sharma, S.; Singh, S.S.; Halder, A.; Yadav, K.; Shukla, G.; Sandhir, R.; Rishi, V.; et al. Identification of Guanosine 5′-diphosphate as Potential Iron Mobilizer: Preventing the Hepcidin-Ferroportin Interaction and Modulating the Interleukin-6/Stat-3 Pathway. Sci. Rep. 2017, 7, 40097. [Google Scholar] [CrossRef] [PubMed]
- Fung, E.; Sugianto, P.; Hsu, J.; Damoiseaux, R.; Ganz, T.; Nemeth, E. High-Throughput screening of small molecules identifies hepcidin antagonists. Mol. Pharmacol. 2013, 83, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Canali, S.; Zumbrennen-Bullough, K.B.; Core, A.B.; Wang, C.-Y.; Nairz, M.; Bouley, R.; Swirski, F.K.; Babitt, J.L. Endothelial cells produce bone morphogenetic protein 6 required for iron homeostasis in mice. Blood 2017, 129, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-N.; Xin, Z.-Z.; Zhang, D.-Z.; Jiang, S.-H.; Chai, X.-Y.; Wang, Z.-F.; Li, C.-F.; Zhou, C.-L.; Tang, B.-P. cDNA cloning and expression analysis of a hepcidin gene from yellow catfish Pelteobagrus fulvidraco (Siluriformes: Bagridae). Fish Shellfish Immunol. 2017, 60, 247–254. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Iron homeostasis in host defence and inflammation. Nat. Rev. Immunol. 2015, 15, 500–510. [Google Scholar] [CrossRef]
- Ganz, T.; Longo, D.L. Anemia of Inflammation. N. Engl. J. Med. 2019, 381, 1148–1157. [Google Scholar] [CrossRef]
- Mu, Y.; Huo, J.; Guan, Y.; Fan, D.; Xiao, X.; Li, Q.; Mu, P.; Ao, J.; Chen, X. An improved genome assembly for Larimichthys crocea reveals hepcidin gene expansion with diversified regulation and function. Fish Shellfish Immunol. 2019, 91, 459. [Google Scholar] [CrossRef]
- Neves, J.V.; Caldas, C.; Vieira, I.; Ramos, M.F.; Rodrigues, P.N.S. Multiple Hepcidins in a Teleost Fish, Dicentrarchus labrax: Different Hepcidins for Different Roles. J. Immunol. 2015, 195, 2696–2709. [Google Scholar] [CrossRef] [PubMed]
- Masso-Silva, J.A.; Diamond, G. Antimicrobial Peptides from Fish. Pharmaceuticals 2014, 7, 265–310. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, X.; Chen, X.; Yu, S.; Chai, Y.; Zhai, T.; Zhu, Q. Molecular characterization and functional analysis of the hepcidin gene from roughskin sculpin (Trachidermus fasciatus). Fish Shellfish Immunol. 2017, 68, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Zhang, P.; Zhang, Q.; Zhang, J. Two hepcidins from spotted scat (Scatophagus argus) possess antibacterial and antiviral functions in vitro. Fish Shellfish Immunol. 2016, 50, 191–199. [Google Scholar] [CrossRef]
- Krewulak, K.D.; Vogel, H.J. Structural biology of bacterial iron uptake. Biochim. Biophys. Acta Biomembr. 2008, 1778, 1781–1804. [Google Scholar] [CrossRef] [PubMed]
- da Costa, J.P.; Cova, M.; Ferreira, R.; Vitorino, R. Antimicrobial peptides: An alternative for innovative medicines? Appl. Microbiol. Biotechnol. 2015, 99, 2023–2040. [Google Scholar] [CrossRef]
- Wang, G. Human antimicrobial peptides and proteins. Pharmaceuticals 2014, 7, 545–594. [Google Scholar] [CrossRef]
- Li, W.; Pan, X.; Cheng, W.; Cheng, Y.; Yin, Y.; Chen, J.; Xu, G.; Xie, L. Serum biochemistry, histology and transcriptomic profile analysis reflect liver inflammation and damage following dietary histamine supplementation in yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2018, 77, 83–90. [Google Scholar] [CrossRef]
- Kanamori, Y.; Murakami, M.; Matsui, T.; Funaba, M. Hepcidin expression in liver cells: Evaluation of mRNA levels and transcriptional regulation. Gene 2014, 546, 50–55. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wooldridge, K.G.; Williams, P.H. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol. Rev. 1993, 12, 325–348. [Google Scholar] [CrossRef]
- Valenzuela-Munoz, V.; Boltana, S.; Gallardo-Escarate, C. Uncovering iron regulation with species-specific transcriptome patterns in Atlantic and coho salmon during a Caligus rogercresseyi infestation. J. Fish Dis. 2017, 40, 1169–1184. [Google Scholar] [CrossRef]
- Braden, L.M.; Koop, B.F.; Jones, S.R.M. Signatures of resistance to Lepeophtheirus salmonis include a T(H)2-type response at the louse-salmon interface. Dev. Comp. Immunol. 2015, 48, 178–191. [Google Scholar] [CrossRef]
- Ganz, T. Iron and infection. Int. J. Hematol. 2018, 107, 7–15. [Google Scholar] [CrossRef]
- Koorts, A.M.; Viljoen, M. Ferritin and ferritin isoforms II: Protection against uncontrolled cellular proliferation, oxidative damage and inflammatory processes. Arch. Physiol. Biochem. 2007, 113, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.M.; Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta 1996, 1275, 161–203. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Regulation of iron metabolism by hepcidin. Annu. Rev. Nutr. 2006, 26, 323–342. [Google Scholar] [CrossRef]
- Alolga, R.N.; Fan, Y.; Zhang, G.; Li, J.; Zhao, Y.-J.; Kakila, J.L.; Chen, Y.; Li, P.; Qi, L.-W. Pharmacokinetics of a multicomponent herbal preparation in healthy Chinese and African volunteers. Sci. Rep. 2015, 5, 12961. [Google Scholar] [CrossRef]
- Feng, R.; Shou, J.-W.; Zhao, Z.-X.; He, C.-Y.; Ma, C.; Huang, M.; Fu, J.; Tan, X.-S.; Li, X.-Y.; Wen, B.-Y.; et al. Transforming berberine into its intestine-absorbable form by the gut microbiota. Sci. Rep. 2015, 5, 12155. [Google Scholar] [CrossRef]
- Zhu, L.; Gu, P.; Shen, H. Protective effects of berberine hydrochloride on DSS-induced ulcerative colitis in rats. Int. Immunopharmacol. 2019, 68, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, A.; Xie, J.; Ji, C. In vitro antibacterial effect of berberine hydrochloride and enrofloxacin to fish pathogenic bacteria. Aquac. Res. 2010, 41, 1095–1100. [Google Scholar] [CrossRef]
- Su, M.; Tang, R.; Wang, H.; Lu, L. Suppression effect of plant-derived berberine on cyprinid herpesvirus 2 proliferation and its pharmacokinetics in Crucian carp (Carassius auratus gibelio). Antivir. Res. 2021, 186, 105000. [Google Scholar] [CrossRef]
- Hien Van, D.; Hoseinifar, S.H.; Jaturasitha, S.; Dawood, M.A.O.; Harikrishnan, R. The effects of berberine powder supplementation on growth performance, skin mucus immune response, serum immunity, and disease resistance of Nile tilapia (Oreochromis niloticus) fingerlings. Aquaculture 2020, 520. [Google Scholar] [CrossRef]
- Yong, Z.; Nan, J.; Jia, Z.; Yuding, F.; Wenzhi, L.; Kaige, S.; Lingbing, Z. Isolation and identification of pathogenic bacterium from ascites disease of yellow catfish, Pelteobagrus fulvidraco. Chin. Fish. Qua. Stand. 2019, 9, 18–26. [Google Scholar]
- Zhuo, M.-Q.; Luo, Z.; Wu, K.; Zhu, Q.-L.; Zheng, J.-L.; Zhang, L.-H.; Chen, Q.-L. Regulation of insulin on lipid metabolism in freshly isolated hepatocytes from yellow catfish (Pelteobagrus fulvidraco). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 177, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-J.; Lee, D.-Y.; Wang, H.-C.; Kang, S.-T.; Hwang, P.-P.; Kou, G.-H.; Huang, M.-F.; Chang, G.-D.; Lo, C.-F. White Spot Syndrome Virus Protein Kinase 1 Defeats the Host Cell’s Iron-Withholding Defense Mechanism by Interacting with Host Ferritin. J. Virol. 2015, 89, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, H.; Shi, W.; Shen, Z.; Shen, H.; Li, M. Hyperinsulinemia induces hepatic iron overload by increasing liver TFR1 via the PI3K/IRP2 pathway. J. Mol. Endocrinol. 2014, 53, 381–392. [Google Scholar] [CrossRef]
| Gene | Primer | Primer Sequence (5′-3′) |
|---|---|---|
| PfHep | Forward | CGCGGATCCGCAGTACCTTTCTCTCAGAATG |
| Reverse | CCCAAGCTTTTAGAACCTGCAGCAGAACC | |
| Ferroportin (FPN) | Forward | AAAACGCTCGGCTCAAAGTG |
| Reverse | GTAGCAAAACGTCAGCAGCC | |
| Hepcidin | Forward | GCAGTACCTTTCTCTCAGAATG |
| Reverse | TTAGAACCTGCAGCAGAACC | |
| Ferritin | Forward | TGTCAAACGGCACTTCCTCA |
| Reverse | TGTCATTGGTGCATCCCACTT | |
| IL-6 | Forward | CTCCAGACCAGAAGTGGGTTGA |
| Reverse | CCCTTATAGGCGTAAATAGTCGTGTT | |
| β-actin | Forward | TTCGCTTGGAGATGATGCT |
| Reverse | CGTGCTCAATGGGGTACT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, M.; Kuang, R.; Wang, W.; Yu, Y.; Ai, T.; Liu, X.; Su, J.; Yuan, G. Hepcidin Protects Yellow Catfish (Pelteobagrus fulvidraco) against Aeromonas veronii-Induced Ascites Disease by Regulating Iron Metabolism. Antibiotics 2021, 10, 848. https://doi.org/10.3390/antibiotics10070848
Fu M, Kuang R, Wang W, Yu Y, Ai T, Liu X, Su J, Yuan G. Hepcidin Protects Yellow Catfish (Pelteobagrus fulvidraco) against Aeromonas veronii-Induced Ascites Disease by Regulating Iron Metabolism. Antibiotics. 2021; 10(7):848. https://doi.org/10.3390/antibiotics10070848
Chicago/Turabian StyleFu, Manquan, Rui Kuang, Weicheng Wang, Yunzhen Yu, Taoshan Ai, Xiaoling Liu, Jianguo Su, and Gailing Yuan. 2021. "Hepcidin Protects Yellow Catfish (Pelteobagrus fulvidraco) against Aeromonas veronii-Induced Ascites Disease by Regulating Iron Metabolism" Antibiotics 10, no. 7: 848. https://doi.org/10.3390/antibiotics10070848
APA StyleFu, M., Kuang, R., Wang, W., Yu, Y., Ai, T., Liu, X., Su, J., & Yuan, G. (2021). Hepcidin Protects Yellow Catfish (Pelteobagrus fulvidraco) against Aeromonas veronii-Induced Ascites Disease by Regulating Iron Metabolism. Antibiotics, 10(7), 848. https://doi.org/10.3390/antibiotics10070848







