Antibacterial Activity of Boswellia sacra Flueck. Oleoresin Extract against Porphyromonas gingivalis Periodontal Pathogen
Abstract
:1. Introduction
2. Results
2.1. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.2. Antibacterial Activity of the Frankincense Oleoresin Extract
2.3. Bacterial Growth Curve
2.4. Measurement of Nucleic Acid Leakage
2.5. Bacterial Cell Surface Hydrophobicity
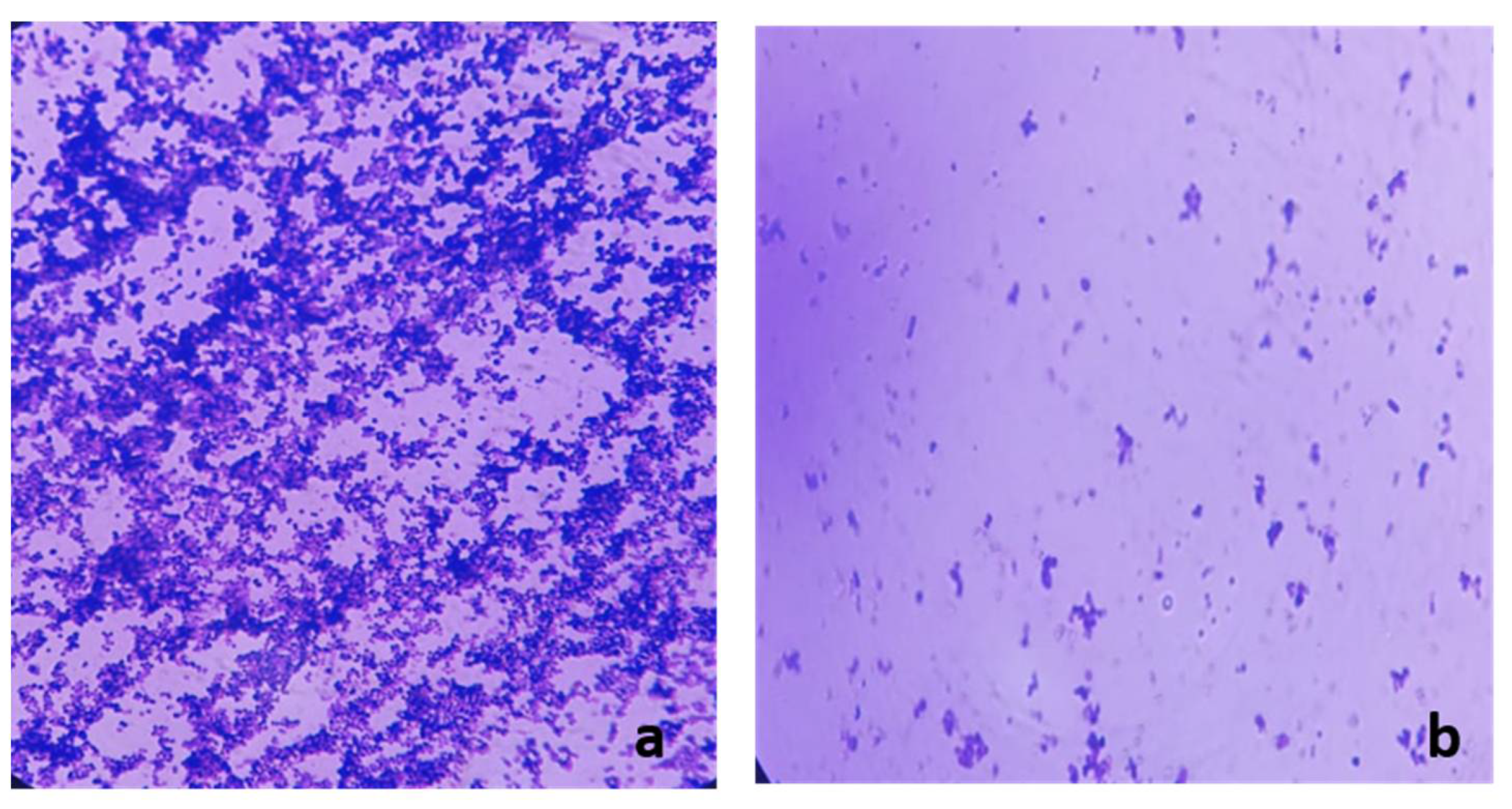
2.6. Antibiofilm Activity of Frankincense Extract
2.7. Extracellular Polysaccharide Measurement
2.8. Examination of the Biofilm Morphology by Light Microscope and Scanning Electron Microscope (SEM)
2.9. Quantitative RT-PCR
2.10. Cytotoxicity Assay
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals and Cell Lines
4.3. GC-MS Data Analysis
4.4. Identification of Compounds
4.5. Selection of Patients
4.6. Ethical Statement
4.7. Bacterial Isolation
4.8. Antibacterial Screening
4.9. Determination of MIC Values
4.10. Bacterial Growth Curve
4.11. Measurement of Nucleic Acid Leakage
4.12. Bacterial Cell Surface Hydrophobicity
4.13. Antibiofilm Activity of Frankincense Extract
4.14. Measurement of the Extracellular Polysaccharides
4.15. Examination of Biofilm Morphology by Light Microscope and SEM
4.16. qRT-PCR
4.17. Cytotoxicity Assay
4.18. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef]
- Dahlen, G.; Basic, A.; Bylund, J. Importance of virulence factors for the persistence of oral bacteria in the inflamed gingival crevice and in the pathogenesis of periodontal disease. J. Clin. Med. 2019, 8, 1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A multifaceted disease of tooth-supporting tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef] [Green Version]
- Parahitiyawa, N.; Scully, C.; Leung, W.; Yam, W.; Jin, L.; Samaranayake, L. Exploring the oral bacterial flora: Current status and future directions. Oral Dis. 2010, 16, 136–145. [Google Scholar] [CrossRef]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef]
- Bostanci, N.; Belibasakis, G.N. Porphyromonas gingivalis: An invasive and evasive opportunistic oral pathogen. FEMS Microbiol. Lett. 2012, 333, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Enersen, M.; Nakano, K.; Amano, A. Porphyromonas gingivalis fimbriae. J. Oral Microbiol. 2013, 5, 20265. [Google Scholar] [CrossRef]
- Fournier-Larente, J.; Morin, M.-P.; Grenier, D. Green tea catechins potentiate the effect of antibiotics and modulate adherence and gene expression in Porphyromonas gingivalis. Arch. Oral Biol. 2016, 65, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Sheets, S.M.; Robles-Price, A.G.; McKenzie, R.M.; Casiano, C.A.; Fletcher, H.M. Gingipain-dependent interactions with the host are important for survival of Porphyromonas gingivalis. Front. Biosci. 2008, 13, 3215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, Y.; Morozumi, T.; Saito, A.; Yoshimura, A.; Kakuta, E.; Suzuki, F.; Nishimura, F.; Takai, H.; Kobayashi, H.; Noguchi, K. Prospective Longitudinal Changes in the Periodontal Inflamed Surface Area Following Active Periodontal Treatment for Chronic Periodontitis. J. Clin. Med. 2021, 10, 1165. [Google Scholar] [CrossRef] [PubMed]
- Aljateeli, M.; Koticha, T.; Bashutski, J.; Sugai, J.V.; Braun, T.M.; Giannobile, W.V.; Wang, H.L. Surgical periodontal therapy with and without initial scaling and root planing in the management of chronic periodontitis: A randomized clinical trial. J. Clin. Periodontol. 2014, 41, 693–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamani, S.; Jansson, L. Oral hygiene behaviour change during the nonsurgical periodontal treatment phase. Open Dent. J. 2012, 6, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taba, M.; Kinney, J.; Kim, A.S.; Giannobile, W.V. Diagnostic biomarkers for oral and periodontal diseases. Dent. Clin. 2005, 49, 551–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, D.; Robinson, P. Correlation of histometric, microbial, and clinical indicators of periodontal disease status before and after root planing. J. Clin. Periodontol. 1987, 14, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Bedran, T.B.L.; de Oliveira, G.J.P.L.; Spolidorio, L.C.; Cirelli, J.A.; Spolidorio, D.P. Comparison of two different methods for detecting periodontal pathogenic bacteria. Braz. J. Oral Sci. 2016, 15, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Al-Hamdoni, S.A.; Al-Rawi, A.M. A Comparison Between Different Susceptibility Test Methods to Evaluate the Antibacterial Activity of Olibanum and Alum Against the “Red Complex” Periodontal Pathogens. Iraqi J. Sci. 2020, 61, 913–1925. [Google Scholar] [CrossRef]
- Abdelaziz, A.; Sonbol, F.; Elbanna, T.; El-Ekhnawy, E. Exposure to sublethal concentrations of benzalkonium chloride induces antimicrobial resistance and cellular changes in Klebsiellae pneumoniae clinical isolates. Microb. Drug Resist. 2019, 25, 631–638. [Google Scholar] [CrossRef]
- Mahavy, C.E.; Duez, P.; ElJaziri, M.; Rasamiravaka, T. African Plant-Based Natural Products with Antivirulence Activities to the Rescue of Antibiotics. Antibiotics 2020, 9, 830. [Google Scholar] [CrossRef]
- Beceiro, A.; Tomás, M.; Bou, G. Antimicrobial resistance and virulence: A successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 2013, 26, 185–230. [Google Scholar] [CrossRef] [Green Version]
- Negm, W.; Abo El-Seoud, K.; Kabbash, A.; El-Aasr, M. Investigation of the Biological Activity Some Gymnosperm Plants Belong to Cycadales Order. J. Adv. Med Pharm. Res. 2020, 1, 9–13. [Google Scholar]
- Negm, W.A.; Abo El-Seoud, K.A.; Kabbash, A.; Kassab, A.A.; El-Aasr, M. Hepatoprotective, cytotoxic, antimicrobial and antioxidant activities of Dioon spinulosum leaves Dyer Ex Eichler and its isolated secondary metabolites. Nat. Prod. Res. 2020, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sabra, S.; Al-Masoudi, L. The effect of using frankincense (Boswellia sacra) chewing gum on the microbial contents of buccal/oral cavity, taif, KSA. J. Dent. Med. Sci. 2014, 13, 77–82. [Google Scholar]
- Al-Yasiry, A.R.M.; Kiczorowska, B. Frankincense-therapeutic properties. Adv. Hyg. Exp. Med. Postepy Hig. I Med. Dosw. 2016, 4, 70. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.M.; Aluru, S.; Sambasivarao, K.; Matcha, B. Antimicrobial activity of frankincense of Boswellia serrata. Int. J. Curr. Microbiol. App. Sci 2014, 3, 1095–1101. [Google Scholar]
- Hosain, N.A.; Ghosh, R.; Bryant, D.L.; Arivett, B.A.; Farone, A.L.; Kline, P.C. Isolation, structure elucidation, and immunostimulatory activity of polysaccharide fractions from Boswellia carterii frankincense resin. Int. J. Biol. Macromol. 2019, 133, 76–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shahlari, M. Frankincense (Rǔ Xiāng; Boswellia species): From the selection of traditional applications to the novel phytotherapy for the prevention and treatment of serious diseases. J. Tradit. Complement. Med. 2013, 3, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Al-Harrasi, A.; Hussain, H.; Csuk, R.; Khan, H.Y. Taxonomy of Boswellia tree, Traditional Medicinal Uses of Frankincense and Historical Aspects of Boswellic Acids. In Chemistry and Bioactivity of Boswellic Acids and Other Terpenoids of the Genus Boswellia; Elsevier: Amsterdam, The Netherlands, 2018; p. 1. [Google Scholar]
- Lemenith, M.; Teketay, D. Frankincense and myrrh resources of Ethiopia: II. Medicinal and industrial uses. SINET: Ethiop. J. Sci. 2003, 26, 161–172. [Google Scholar] [CrossRef]
- Ammon, H. Boswellic acids in chronic inflammatory diseases. Planta Med. 2006, 72, 1100–1116. [Google Scholar] [CrossRef] [Green Version]
- Al-Hamdoni, S.A.S.; Al-Rawi, A.M.M. Assessment the Effect of Some Reagents on the Planktonic Cells and Biofilms of Red Complex Periodontal Pathogens. Int. J. Sci. Basic Appl. Res. 2020, 51, 1–13. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. JNCI J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Ribeiro-Vidal, H.; Bartolomé, B.; Figuero, E.; Moreno-Arribas, M.; Sanz, M.; Herrera, D. New Evidences of Antibacterial Effects of Cranberry Against Periodontal Pathogens. Foods 2020, 9, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm activity of plant polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef] [PubMed]
- Krist, S.; Banovac, D.; Tabanca, N.; Wedge, D.E.; Gochev, V.K.; Wanner, J.; Schmidt, E.; Jirovetz, L. Antimicrobial activity of nerolidol and its derivatives against airborne microbes and further biological activities. Nat. Prod. Commun. 2015, 10. [Google Scholar] [CrossRef]
- Ambrosio, S.R.; Furtado, N.A.; de Oliveira, D.C.; da Costa, F.B.; Martins, C.H.; de Carvalho, T.C.; Porto, T.S.; Veneziani, R.C. Antimicrobial activity of kaurane diterpenes against oral pathogens. Z. Für Nat. C 2008, 63, 326–330. [Google Scholar] [CrossRef]
- Sasidharan, I.; Menon, A.N. Comparative chemical composition and antimicrobial activity fresh & dry ginger oils (Zingiber officinale Roscoe). Int. J. Curr. Pharm. Res. 2010, 2, 40–43. [Google Scholar]
- Stoyanova, A.; Konakchiev, A.; Damyanova, S.; Stoilova, I.; Suu, P.T. Composition and antimicrobial activity of ginger essential oil from Vietnam. J. Essent. Oil Bear. Plants 2006, 9, 93–98. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [Green Version]
- Abers, M.; Schroeder, S.; Goelz, L.; Sulser, A.; Rose, T.S.; Puchalski, K.; Langland, J. Antimicrobial activity of the volatile substances from essential oils. BMC Complement. Med. Ther. 2021, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, V.; Schillaci, D.; Cusimano, M.G.; Rishan, M.; Rashan, L. In vitro antimicrobial activity of frankincense oils from Boswellia sacra grown in different locations of the Dhofar region (Oman). Antibiotics 2020, 9, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macia, M.; Rojo-Molinero, E.; Oliver, A. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin. Microbiol. Infect. 2014, 20, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial biofilms: Development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, X.; Chen, V.; Xu, X. Novel approaches to the control of oral microbial biofilms. BioMed Res. Int. 2018, 2018, 1–52. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Melo, M.A.; Weir, M.D.; Xie, X.; Reynolds, M.A.; Xu, H.H. Novel bioactive nanocomposite for Class-V restorations to inhibit periodontitis-related pathogens. Dent. Mater. 2016, 32, e351–e361. [Google Scholar] [CrossRef]
- Vu, B.; Chen, M.; Crawford, R.J.; Ivanova, E.P. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 2009, 14, 2535–2554. [Google Scholar] [CrossRef]
- Cao, W.; Liu, X.; Bai, T.; Fan, H.; Hong, K.; Song, H.; Han, Y.; Lin, L.; Ruan, L.; Li, T. High-Dose Intravenous Immunoglobulin as a Therapeutic Option for Deteriorating Patients with Coronavirus Disease 2019; Open Forum Infectious Diseases, 2020; Oxford University Press: Oxford, UK, 2020; p. ofaa102. [Google Scholar]
- Cozens, D.; Read, R.C. Anti-adhesion methods as novel therapeutics for bacterial infections. Expert Rev. Anti-Infect. Ther. 2012, 10, 1457–1468. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, L.; Xu, L.; Meng, H.; Lu, R.; Chen, Z.; Shi, D.; Wang, X. Detection of eight periodontal microorganisms and distribution of Porphyromonas gingivalis fimA genotypes in Chinese patients with aggressive periodontitis. J. Periodontol. 2014, 85, 150–159. [Google Scholar] [CrossRef]
- Karin Kristoffersen, A.; Solli, S.J.; Duy Nguyen, T.; Enersen, M. Association of the rgpB gingipain genotype to the major fimbriae (fimA) genotype in clinical isolates of the periodontal pathogen Porphyromonas gingivalis. J. Oral Microbiol. 2015, 7, 29124. [Google Scholar] [CrossRef] [PubMed]
- Negm, W.; Ibrahim, A.; Aboelsauod, K.; Ragab, A.; Attia, G. GC-MS Analysis of Petroleum Ether Extract and Volatiles of Cycas revoluta Thunb Growing in Egypt. Inventi Rapid Planta Act 2016, 2016, 1–5. [Google Scholar]
- Condorelli, F.; Scalia, G.; Calì, G.; Rossetti, B.; Nicoletti, G.; Bue, A.M.L. Isolation of Porphyromonas gingivalisand Detection of Immunoglobulin A Specific to Fimbrial Antigen in Gingival Crevicular Fluid. J. Clin. Microbiol. 1998, 36, 2322–2325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wayne, A.; Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twentieth Informational Supplement; CLSI: Annapolis Junction, MD, USA, 2010. [Google Scholar]
- Su, P.-W.; Yang, C.-H.; Yang, J.-F.; Su, P.-Y.; Chuang, L.-Y. Antibacterial activities and antibacterial mechanism of Polygonum cuspidatum extracts against nosocomial drug-resistant pathogens. Molecules 2015, 20, 11119–11130. [Google Scholar] [CrossRef] [Green Version]
- Njeru, S.N.; Obonyo, M.A.; Nyambati, S.O.; Ngari, S.M. Antimicrobial and cytotoxicity properties of the crude extracts and fractions of Premna resinosa (Hochst.) Schauer (Compositae): Kenyan traditional medicinal plant. BMC Complement. Altern. Med. 2015, 15, 1–9. [Google Scholar] [CrossRef] [Green Version]
- El-Banna, T.; Abd El-Aziz, A.; Sonbol, F.; El-Ekhnawy, E. Adaptation of Pseudomonas aeruginosa clinical isolates to benzalkonium chloride retards its growth and enhances biofilm production. Mol. Biol. Rep. 2019, 46, 3437–3443. [Google Scholar] [CrossRef]
- Sonbol, F.I.; El-Banna, T.; Abd El-Aziz, A.A.; El-Ekhnawy, E. Impact of triclosan adaptation on membrane properties, efflux and antimicrobial resistance of Escherichia coli clinical isolates. J. Appl. Microbiol. 2019, 126, 730–739. [Google Scholar] [CrossRef]
- Qi, H.; Li, B.; Wang, H.; Cai, Q.; Quan, X.; Cui, Y.; Meng, W. Effects of d-valine on periodontal or peri-implant pathogens: Porphyromonas gingivalis biofilm. J. Periodontol. 2018, 89, 303–314. [Google Scholar] [CrossRef]
- Elekhnawy, E.; Sonbol, F.; Abdelaziz, A.; Elbanna, T. An investigation of the impact of triclosan adaptation on Proteus mirabilis clinical isolates from an Egyptian university hospital. Braz. J. Microbiol. 2021, 1–11. [Google Scholar]
- Zhang, Y.; Wu, Y.-T.; Zheng, W.; Han, X.-X.; Jiang, Y.-H.; Hu, P.-L.; Tang, Z.-X.; Shi, L.-E. The antibacterial activity and antibacterial mechanism of a polysaccharide from Cordyceps cicadae. J. Funct. Foods 2017, 38, 273–279. [Google Scholar] [CrossRef]
- Blando, F.; Russo, R.; Negro, C.; De Bellis, L.; Frassinetti, S. Antimicrobial and antibiofilm activity against Staphylococcus aureus of Opuntia ficus-indica (L.) Mill. cladode polyphenolic extracts. Antioxidants 2019, 8, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Tian, F.; Cui, S.; Song, J.; Zhao, S.; Brown, E.W.; Meng, J. Differential gene expression by RamA in ciprofloxacin-resistant Salmonella Typhimurium. PLoS ONE 2011, 6, e22161. [Google Scholar] [CrossRef] [PubMed]
- Allam, R.M.; Al-Abd, A.M.; Khedr, A.; Sharaf, O.A.; Nofal, S.M.; Khalifa, A.E.; Mosli, H.A.; Abdel-Naim, A.B. Fingolimod interrupts the cross talk between estrogen metabolism and sphingolipid metabolism within prostate cancer cells. Toxicol. Lett. 2018, 291, 77–85. [Google Scholar] [CrossRef]








| Peak No. | Rt (min.) | Name | Peak Area% |
|---|---|---|---|
| 1 | 11.106 | Acetic acid, octyl ester | 2.073 |
| 2 | 17.443 | Dodecanoic acid | 0.273 |
| 3 | 22.090 | 1,3,6,10-Cyclotetradecatetraene,3,7,11-trimethyl-14-(1-methylethyl)-, [S-(E,Z,E,E)] | 0.424 |
| 4 | 22.310 | Bicyclo [9.3.1]pentadeca-3,7-dien-12-ol,4,8,12,15,15-pentamethyl-, [1R-(1R,3E,7E,11R 12R)]- | 0.256 |
| 5 | 22.605 | Cyclohexane, 1-ethenyl-1-methyl-2,4-bis(1-methylethenyl)- | 1.831 |
| 6 | 22.770 | n-Hexadecanoic acid | 0.177 |
| 7 | 22.920 | 1,6,10,14-Hexadecatetraen-3-ol,3,7,11,15-tetramethyl-, (E,E) | 1.337 |
| 8 | 23.346 | ç-Elemene | 0.658 |
| 9 | 23.651 | Kaur-16-ene | 7.459 |
| 10 | 23.786 | Cycloheptane, 4-methylene-1-methyl-2-(2-methyl-1-propen-1-yl)-1-vinyl- | 0.584 |
| 11 | 24.066 | Thunbergol | 2.380 |
| 12 | 24.351 | Aromadendrene oxide-(2) | 0.456 |
| 13 | 25.011 | 1-Heptatriacotanol | 1.488 |
| 14 | 25.581 | 2,6,10,14-Hexadecatetraen-1-ol, 3,7,11,15-tetramethyl-, acetate, (E,E,E)- | 1.601 |
| 15 | 25.932 | Trans-Nerolidyl formate | 19.880 |
| 16 | 26.137 | Cis-Z-α-Bisabolene epoxide | 9.410 |
| 17 | 26.222 | Androstan-17-one, 3-ethyl-3-hydroxy-,(5à)- | 0.175 |
| 18 | 26.417 | Butyl 4,7,10,13,16,19-docosahexaenoate | 0.165 |
| 19 | 26.827 | Vitamin A aldehyde | 0.187 |
| 20 | 26.997 | i-Propyl 5,8,11,14,17-eicosapentaenoate | 0.125 |
| 21 | 27.122 | 1-Naphthalenepropanol, à-ethenyldecahydro-2-hydroxy-à,2,5,5,8a-pentamethyl-, [1R-[1à(R *),2á,4aá,8aà]]- | 0.280 |
| 22 | 27.732 | Cholestan-3-ol,2-methylene-,(3á,5à)- | 0.497 |
| 23 | 27.782 | 2-[4-methyl-6-(2,6,6-trimethylcyclohex-1-enyl) hexa-1,3,5-trienyl] cyclohex-1-en-1-carboxaldehyde | 0.482 |
| 24 | 28.057 | Docosahexaenoic acid | 0.136 |
| 25 | 28.132 | Isoaromadendrene epoxide | 0.153 |
| 26 | 28.613 | Retinol, acetate | 0.397 |
| 27 | 29.313 | Card-20(22)-enolide, 3,5,14,19-tetrahydroxy-,(3á,5á)- | 0.159 |
| 28 | 29.853 | 9,10-Secocholesta-5,7,10(19)-triene-3,25,26-triol, (3á,5Z,7E)- | 0.175 |
| 29 | 31.289 | 3-Oxatricyclo [20.8.0.0(7,16)] triaconta-1(22),7(16), 9,13,23,29-hexaene | 0.236 |
| 30 | 31.494 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 0.269 |
| 31 | 31.859 | Methyl 2-hydroxy-octadeca-9,12,15-trienoate | 0.360 |
| 32 | 32.274 | Butyl 6,9,12,15-octadecatetraenoate | 0.174 |
| 33 | 37.656 | 2H-Cyclopenta[a]phenanthrene-3,17-dione, 16-(1,3-dimethyl-1H-pyrazol-4-ylmethylene)-10,13-dimethyl-1,6,7,8,9,10,11,12,13,14,15,16-dodecahydro- | 0.438 |
| 34 | 38.477 | Retinol | 4.236 |
| 35 | 38.732 | 2(1H)Naphthalenone, 3,5,6,7,8,8a-hexahydro-4,8a-dimethyl-6-(1-methylethenyl)- | 6.204 |
| 36 | 38.977 | Prasterone | 0.199 |
| 37 | 39.442 | Urs-12-en-24-oic acid, 3-oxo-, methyl ester, (+)-7 | 12.420 |
| 38 | 39.997 | 9,19-Cycloergost-24(28)-en-3-ol,4,14-dimethyl-, acetate, (3á,4à) | 0.157 |
| 39 | 42.283 | Oleana-11,13(18)-diene | 0.341 |
| 40 | 42.558 | Betulin | 0.196 |
| 41 | 42.973 | Urs-12-ene | 1.064 |
| 42 | 43.673 | Lanosterol | 0.149 |
| 43 | 44.224 | 9,19-Cyclolanost-24-en-3-ol, acetate, (3a)-(cycloartenol acetate) | 13.560 |
| 44 | 44.659 | 4,4,6a,6b,8a,11,11,14b-Octamethyl-1,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-octadecahydro-2H-picen-3-one | 0.170 |
| 45 | 45.019 | Acetic acid, 3-hydroxy-7-isopropenyl-1,4a-dimethyl-2,3,4,4a,5,6,7,8-octahydronaphthalen-2-yl ester | 0.280 |
| 46 | 45.264 | α-Amyrin | 0.472 |
| 47 | 45.624 | Stigmasterol | 0.144 |
| 48 | 45.844 | 2-Oleanen-3-yl acetate, (3à)- | 0.822 |
| 49 | 47.630 | Lupeol | 0.206 |
| Isolate Code | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC value (µg/mL) | 500 | 1000 | 1000 | 1000 | 500 | 500 | 500 | 1000 | 1000 | 500 | 500 | 500 |
| Isolate Code | Relative Gene Expression * | ||||
|---|---|---|---|---|---|
| fimA | hagA | hagB | rgpA | Kgp | |
| P1 | 0.1 ± 0.3 | 0.6 ± 0.1 | 1.1 ± 0.3 | 1.1 ± 0.1 | 1.2 ± 0.2 |
| P2 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.2 | 0.8 ± 0.2 | 1.0 ± 0.3 |
| P3 | 1.4 ± 0.0 | 0.1 ± 0.1 | 0.3 ± 0.1 | 1.2 ± 0.3 | 1.2 ± 0.1 |
| P4 | 1.1 ± 0.3 | 0.5 ± 0.2 | 0.4 ± 0.1 | 1.0 ± 0.2 | 1.4 ± 0.2 |
| P5 | 0.5 ± 0.1 | 0.1 ± 0.2 | 0.4 ± 0.2 | 0.8 ± 0.2 | 1.1 ± 0.2 |
| P6 | 0.3 ± 0.2 | 1.2 ± 0.1 | 0.2 ± 0.09 | 0.8 ± 0.1 | 1.4 ± 0.1 |
| P7 | 1.2 ± 0.2 | 1.4 ± 0.1 | 0.6 ± 0.1 | 1.2 ± 0.2 | 0.9 ± 0.3 |
| P8 | 1.4 ± 0.0 | 1.2 ± 0.4 | 1.2 ± 0.1 | 0.8 ± 0.1 | 1.4 ± 0.0 |
| P9 | 1.3 ± 0.2 | 1.0 ± 0.2 | 1.3 ± 0.2 | 1.0 ± 0.2 | 0.8 ± 0.2 |
| P10 | 0.2 ± 0.2 | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.1 ± 0.3 | 1.2 ± 0.0 |
| P11 | 1.2 ± 0.0 | 1.2 ± 0.0 | 1.2 ± 0.0 | 1.2 ± 0.1 | 0.9 ± 0.3 |
| P12 | 0.4 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.1 | 1.1 ± 0.3 | 1.2 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attallah, N.G.M.; Negm, W.A.; Elekhnawy, E.; Altwaijry, N.; Elmongy, E.I.; El-Masry, T.A.; Alturki, E.A.; Yousef, D.A.; Y. Shoukheba, M. Antibacterial Activity of Boswellia sacra Flueck. Oleoresin Extract against Porphyromonas gingivalis Periodontal Pathogen. Antibiotics 2021, 10, 859. https://doi.org/10.3390/antibiotics10070859
Attallah NGM, Negm WA, Elekhnawy E, Altwaijry N, Elmongy EI, El-Masry TA, Alturki EA, Yousef DA, Y. Shoukheba M. Antibacterial Activity of Boswellia sacra Flueck. Oleoresin Extract against Porphyromonas gingivalis Periodontal Pathogen. Antibiotics. 2021; 10(7):859. https://doi.org/10.3390/antibiotics10070859
Chicago/Turabian StyleAttallah, Nashwah G. M., Walaa A. Negm, Engy Elekhnawy, Najla Altwaijry, Elshaymaa I. Elmongy, Thanaa A. El-Masry, Eman A. Alturki, Doaa A. Yousef, and Malak Y. Shoukheba. 2021. "Antibacterial Activity of Boswellia sacra Flueck. Oleoresin Extract against Porphyromonas gingivalis Periodontal Pathogen" Antibiotics 10, no. 7: 859. https://doi.org/10.3390/antibiotics10070859
APA StyleAttallah, N. G. M., Negm, W. A., Elekhnawy, E., Altwaijry, N., Elmongy, E. I., El-Masry, T. A., Alturki, E. A., Yousef, D. A., & Y. Shoukheba, M. (2021). Antibacterial Activity of Boswellia sacra Flueck. Oleoresin Extract against Porphyromonas gingivalis Periodontal Pathogen. Antibiotics, 10(7), 859. https://doi.org/10.3390/antibiotics10070859








