Abstract
Endodontic biomaterials have significantly improved dental treatment techniques in several aspects now that they can be used for vital pulp treatments, as temporary intracanal medication, in definitive fillings, in apical surgeries, and for regenerative procedures. Calcium silicate-based cement is a class of dental material that is used in endodontics in direct contact with the dental structures, connective tissue, and bone. Because the material interacts with biological tissues and stimulates biomineralization processes, its properties are of major importance. The main challenge in endodontic treatments is the elimination of biofilms that are present in the root canal system anatomical complexities, as it remains even after chemical-mechanical preparation and disinfection procedures. Thus, an additional challenge for these biomaterials is to exert antimicrobial activity while maintaining their biological properties in parallel. This article reviews the literature for studies considering the antimicrobial properties of calcium silicate-based dental biomaterials used in endodontic practice. Considering the reviewed studies, it can be affirmed that the reduced antimicrobial effect exhibited by calcium silicate-based endodontic materials clearly emphasizes that all clinical procedures prior to their use must be carefully performed. Future studies for the evaluation of these materials, and especially newly proposed materials, under poly-microbial biofilms associated with endodontic diseases will be necessary.
1. Introduction
Endodontics in dentistry concerns the study of the morphology, physiology, and pathology of human dental pulp and apical tissues. This includes the normal biology, etiology of alterations, methods of diagnosis, preventive procedures, and clinical approaches for these treatments [1]. The growing demand for continuous improvements in the techniques and materials used in endodontics has been remarkable. In this sense, biomaterials have become a promising field of research and are now being developed to interact with complex biological systems and are mainly used in endodontic techniques involving dental perforation accidents, apexification treatments, and root canal filling after chemical-mechanical preparation [2]. The main reason for filling the root canal system is to seal the majority of its spaces, thus preventing the survival of microorganisms that interfere with promoting the forthcoming repair of the apical tissues [3], and eliminating any potential residual infection [4].
Among the endodontic materials indicated for root canal filling are tricalcium silicate-based materials as the main compound. The main advantages of these materials are related to their physicochemical and biological properties [5,6]. These materials have an alkaline pH after immersion, a high calcium ion release, and adequate flowability for endodontic use. Additionally, they can be considered as bioactive materials once they have a certain ability to induce the formation of hard tissue in both dental pulp tissue and bone; this encourages new treatment approaches for dentinal remineralization, vital pulp therapy, and bone regeneration [7] through the stimulation of cell proliferation and gene expression related to stem cell differentiation [8]. The potential antimicrobial properties of endodontic cements were previously attributed to their alkalinity and release of calcium ions [4].
Although considerable microbial reduction can be achieved after chemical-mechanical preparation, irrigation, and intracanal medication, the presence of bacteria in dentinal tubules and cementum after treatment still occurs, mainly due to the anatomical features of the root canal [9,10]. For this reason—especially when there is pulp necrosis and apical periodontitis—choosing a material with a certain level of antimicrobial activity can potentially help to reduce or prevent the growth of remaining microorganisms [11,12].
Primary infections in root canals contain microorganisms able to access and colonize the pulp tissue, impairing its function and leading to its necrosis [13]. Their microbial profile consists of several bacterial species that may lead to apical periodontitis once they reach the apical region. The most prevalent are Fusobacterium, Porphyromonas, Prevotella, Parvimonas, Tannerella, Treponema, Dialister, Filifactor, Actinomyces, Olsenella, and Pseudoramibacter. In addition, root canals with persistent/secondary infection are usually associated with post-treatment apical periodontitis, in which the first endodontic treatment has failed. The microbiota in these cases are composed of a group of species involving a predominance of facultative and Gram-positive anaerobic bacteria, such as Streptococcus mutans, Streptococcus anginosus, Enteroccocus faecalis, and Staphylococcus aureus. Therefore, the prevalence of biofilms is high, and clinically, one of their main characteristics is their greater resistance to antimicrobials [9,10,14,15,16,17].
Several in vitro studies have investigated the antimicrobial activity of endodontic materials through methods such as the agar diffusion test and the direct contact test [18,19,20,21]. Endodontic cements may have different inhibitory effects depending on their composition, as well as the evaluation method and selected test times. The direct contact test has been widely used to assess the antimicrobial effect of endodontic cements and root filling materials. The test is quantitative and is indicated for the analysis of insoluble materials and in standardized configurations [22].
This literature review aimed to investigate the published information regarding the antimicrobial activity of materials for root canal filling and reparative procedures with a calcium silicate-based composition used in endodontics.
2. Search Strategy
The literature search was performed on PubMed without language or year restrictions, according to the following search strategy:
(anti-infective agents OR antimicrobial AND biofilms AND bioactive materials OR calcium silicate-based dental materials OR biocompatible materials OR biomaterials AND endodontics AND root canal filling OR sealer OR repair material OR reparative endodontic materials OR hydraulic endodontic materials).
Duplicates were removed manually with help from a reference manager (Mendeley Desktop, software version 1.19.8). After the article screening, a manual search was conducted for the download of the complete texts. Other articles were then added by hand searching of grey literature (OpenGrey and Google Scholar). The details of the main outcomes classified by material type are shown in Table 1.

Table 1.
Main outcomes reviewed, grouped in chronological order by material type.
2.1. Inclusion Criteria
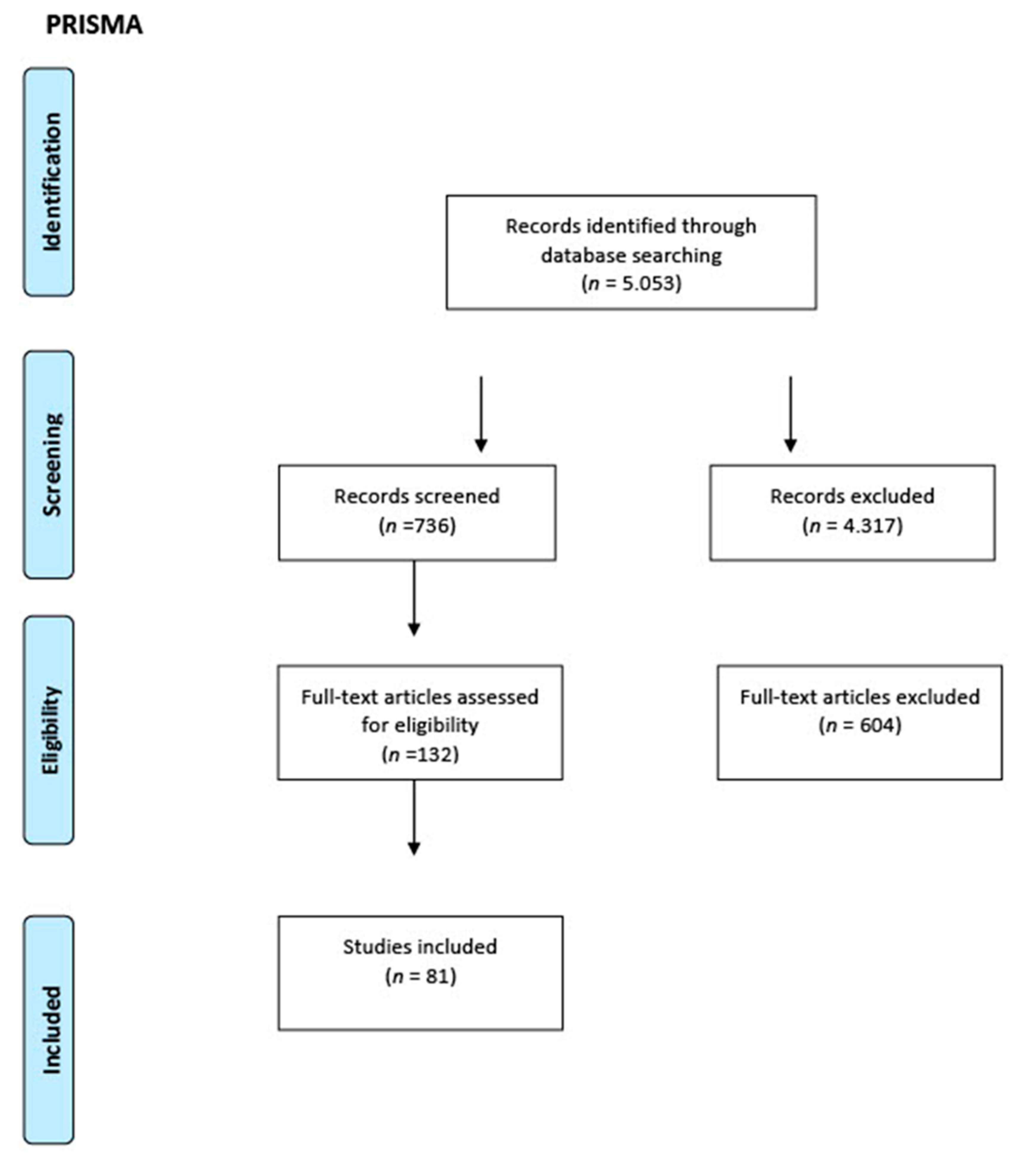
The PRISMA checklist was followed for this category of review; its workflow is shown in Figure 1. Studies of any design that analyzed the antimicrobial properties of hydraulic calcium silicate-based endodontic sealers and reparative materials, in vivo studies on both humans and animals, and in vitro studies conducted on any type of laboratory model were considered for inclusion in this review.
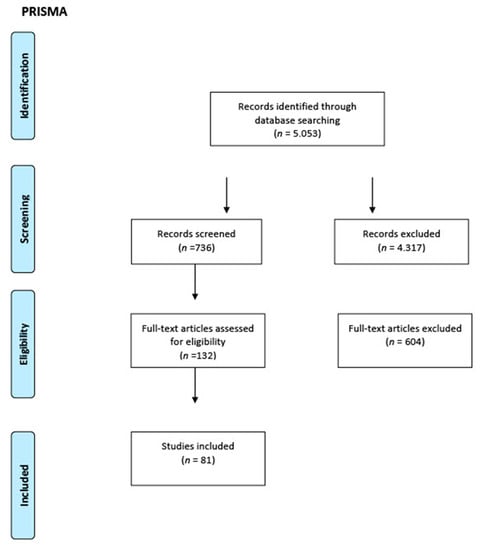
Figure 1.
PRISMA workflow.
2.2. Exclusion Criteria
The exclusion criteria were as follows: articles in languages other than English, narrative reviews, experts’ opinions, and guideline reports. Studies were excluded if they evaluated the antimicrobial properties of other types of materials.
3. Root Canal Filling Materials (Sealers)
Antimicrobial activity is an expected feature of an endodontic sealer because these materials are used in contaminated clinical sites [34,35,36]. Gram-negative bacteria secrete virulence factors such as lipopolysaccharides, an endotoxin that potentially stimulates bone resorption by acting in the synthesis and release of cytokines, which in turn activate osteoclasts, thus being directly related to the occurrence of periapical lesions. Enterococcus faecalis is a facultative Gram-positive bacterium that has been associated with several oral diseases, including endodontic infections, apical periodontitis, peri-implantitis, and endodontic-periodontal diseases. For this reason, this microorganism is used in several in vitro studies to test the antimicrobial properties of dental materials and their effectiveness in endodontic procedures [16,37].
The antimicrobial properties inherent to endodontic cements, in some cases, are transient and rarely extend beyond 7 days, not being sufficient against persistent infections [28,38]. The use of a material with long-term antimicrobial capacity could be decisive for the success of an endodontic treatment, as it would help to reduce the residual microbial load and prevent the formation of new biofilms. Furthermore, the use of antimicrobial additives in cements can be advantageous with the addition of quaternary ammonium compounds such as benzalkonic chloride and cetylpyridinium chloride and may increase the antimicrobial effect of the materials [39,40].
The use of nanomaterials—due to their small particle size—also potentially offers a large surface area/mass ratio and an increase in chemical reactivity when compared to its correspondent original material [41]. The addition of nanoparticles to endodontic cements has increased the antimicrobial activity inside dentinal tubules and the consistency of the materials [42,43]. Chlorhexidine is an example of a substance used in its nanoparticulated form in endodontic cements, offering broad-spectrum antimicrobial action, which makes it effective against Gram-positive bacteria, Gram-negative bacteria, and fungi [29].
Endodontic sealers such as EndoSequence BC Sealer (Brasseler, Savannah, GA, USA, EUA), BioRoot RCS (Septodont, Saint-Maur-des-Fossés, France) and Endoseal MTA (Maruchi, Wonju-si, Korea) have been widely studied. These sealers contain an amount of oxide compounds known to potentially have antimicrobial activity, such as Al2O3, Fe2O3, MgO, Na2O, NiO, and SO3 [23]. Additionally, the potential antimicrobial activity of the endodontic sealers may be associated with their alkaline pH, release of Ca2+ ions, and formation of hydroxyapatite with the interaction of dentin, significantly reducing biofilm formation and viability [6,44]. These cements after hydration undergo a reaction forming calcium hydroxide [45], which is responsible for their biological properties [24,46].
Microbial recontamination in root canal fillings is directly related to the presence of voids between the filling material and the gutta-percha within the walls of root canals [47]. The amount of sealer between the root canal walls and the gutta-percha points is critical, as it should be as thin as possible, aiming to reduce the infiltration of microorganisms, especially after single-cone techniques associated with the use of endodontic sealers [48]. Especially in round-shaped root canals, this technique has shown favorable results in relation to filling capacity; however, in canals with a large buccolingual extension, the single-cone technique requires a greater amount of cement, potentially resulting in a greater presence of voids [23,49].
Another important aspect to be evaluated is the physicochemical properties of root canal sealers that can influence the bacterial contamination of root canals. A number of studies have shown that biomaterials that have high solubility require more time for their complete setting [27,50,51,52]. Thus, this high solubility can influence the quality of root canal treatment and increase microbial infiltration over time [33]. Calcium silicate-based endodontic sealers are currently commercially presented in ready-to-use or powder/liquid formulas. Pre-mixed cements depend on ambient humidity to initiate the setting reaction, while for powder/liquid cements, water is present in the formulation itself [53].
The most used antimicrobial test for endodontic cements is the direct contact test [18,22,31]. The use of the agar diffusion test for antimicrobial analysis of endodontic cements has been discouraged, as it reflects only the diffusion capacity of the tested material, and not its antimicrobial potential [34]. Therefore, it was replaced by the direct contact test, which gives more reliable results. However, direct contact tests do not take into account the presence of dentin and the potential effect as part of the complex nature of root canal anatomy or for biofilm formation [26]. However, recent modifications were introduced to assess the antimicrobial effect of materials under conditions that most resemble the clinical condition, those found in endodontic infections using viability staining and confocal laser scanning microscopy inside root canals [25,54].
Based on these results, it seems fair to affirm that preventing bacterial recontamination by sealing is an important feature that the sealer must provide. For this reason, the long-term dimensional stability must be considered when developing this type of materials, and its stability in clinical use should be evaluated in a variety of methodologies. The available sealer compositions per se do not possess a robust and significant antibacterial effect; however, the root canal chemical-mechanical preparation is undoubtedly still the most crucial step of endodontic therapy regarding the reduction of levels of bacteria and their sub-products.
4. Hydraulic Calcium Silicate-Based Reparative Materials
Reparative endodontic materials have been widely used since their development in the 1990s, with the first generation of mineral trioxide aggregate (MTA), mainly composed of calcium and silicate elements [55,56,57]. This patent described the origin of this material, which had a gray color, as being based on type I Portland cement partially replaced with bismuth oxide serving as a radiopacifying agent. After this patent, the first commercially available material emerged: ProRoot MTA (Dentsply, Tulsa, OK, USA). However, from a biological point of view, there were no studies at that time demonstrating the full potential that this material would present, which ended up being an inversion of the material development process, where the industry indicated the material emphasizing its sealing properties for clinical use.
Considering the aesthetic aspect of using this material, a white cement was proposed in a new patent on 25 July 2002 [58]. The reduction of the iron oxide concentration from the composition of ProRoot MTA—which resulted in a gray material—gave space to start the production of ProRoot MTA white; however, the radiopacifying agent based on bismuth oxide remained unchanged even in this new white composition [59,60]. A similar alteration was made with to Gray MTA Angelus (Angelus, Londrina, Brazil) which was renamed to white MTA Angelus, also reducing the concentration of iron oxide in its powder [61] but keeping the bismuth oxide as the radiopacifier agent. A second formula alteration around 2017 in MTA Angelus altered its radiopacifier from bismuth oxide to calcium tungstate.
However, later studies indicated that the interaction of bismuth oxide with the collagen present in dental structures, together with the irrigating solution used during endodontic treatment of root canal therapy, were the main reasons for tooth pigmentation [62,63,64]. These studies resulted in the replacement of bismuth oxide with other substances such as calcium tungstate, zirconium oxide, and tantalum oxide serving as alternative radiopacifiers in compositions such as Biodentine (Septodont, Saint-Maur-des-Fossés, France), EndoSequence BC RRM Putty (Brasseler, Savannah, GA, USA), MTA Repair HP (Angelus, Londrina, Brazil), and White-MTAFlow (Ultradent Products Inc., South Jordan, UT, USA) [32,65,66,67,68].
Currently, hydraulic calcium silicate-based endodontic materials have gained significant prominence due to their potential antimicrobial properties, alkaline pH, and bioactivity [4]. These materials have the ability to release calcium and hydroxyl ions in the surrounding tissue where they are applied, thus favoring the creation of a favorable environment for cell differentiation both in dentinal tissues and bone [36]. Currently, these materials are widely used in dental clinics—not only in endodontics—such as in the processes of pulp revascularization, repair of accidental or carious perforations, treatment of internal/external root resorption, pulp capping, and retro-filling in endodontic surgery [69,70,71,72,73,74].
The antimicrobial potential of reparative endodontic materials is directly related to their surface of contact, potential alkaline pH, and hydroxyl release [75], as these factors are directly responsible for damage to lipids, proteins, and DNA in the cell membranes of microorganisms [76]. Another antimicrobial mechanism of these materials is the presence of calcium in their composition, which reduces the presence of carbon dioxide in tissues, a molecule which is used by anaerobic bacteria, in addition to their alkaline pH, caused by the hydroxyl ions, which potentially also favors tissue repair [77].
The chemical compositions, as well as the crystalline phases, are of fundamental importance for the understanding of the physicochemical and antimicrobial properties of reparative endodontic biomaterials. In addition, the powder particle size before the hydration process varies widely depending on the materials, and the smaller the particle, the potentially easier it will be to mix and handle the material. The presence of particles with a diameter smaller than the dentinal tubules could potentially play an important role in the perforation sealing capacity, assuming that the smear layer and debris of the application site have been previously removed [35,78,79,80,81,82].
It is known that long-term antimicrobial challenges constantly occur after restorative procedures, and these clinical conditions may cause treatment failures [67]. An attempt to add an additional antimicrobial mechanism was the addition of a nano-hydroxyapatite capable of eliciting antibacterial activity on Streptococcus and Enterococcus faecalis [83]. The use of different species and methods of cultivation (aerobic and anaerobic conditions) when testing materials is crucial when prediction of its clinical behavior is intended.
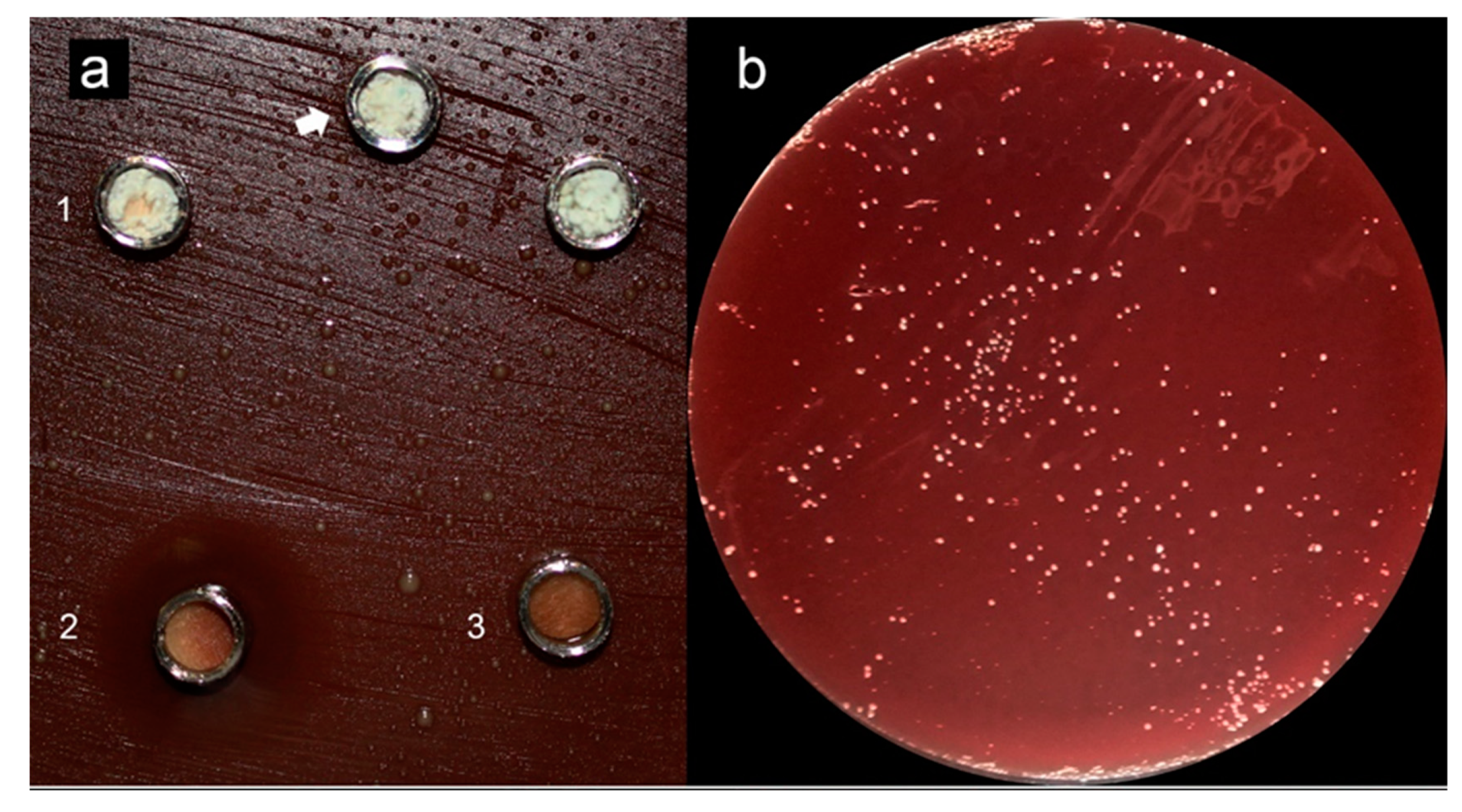
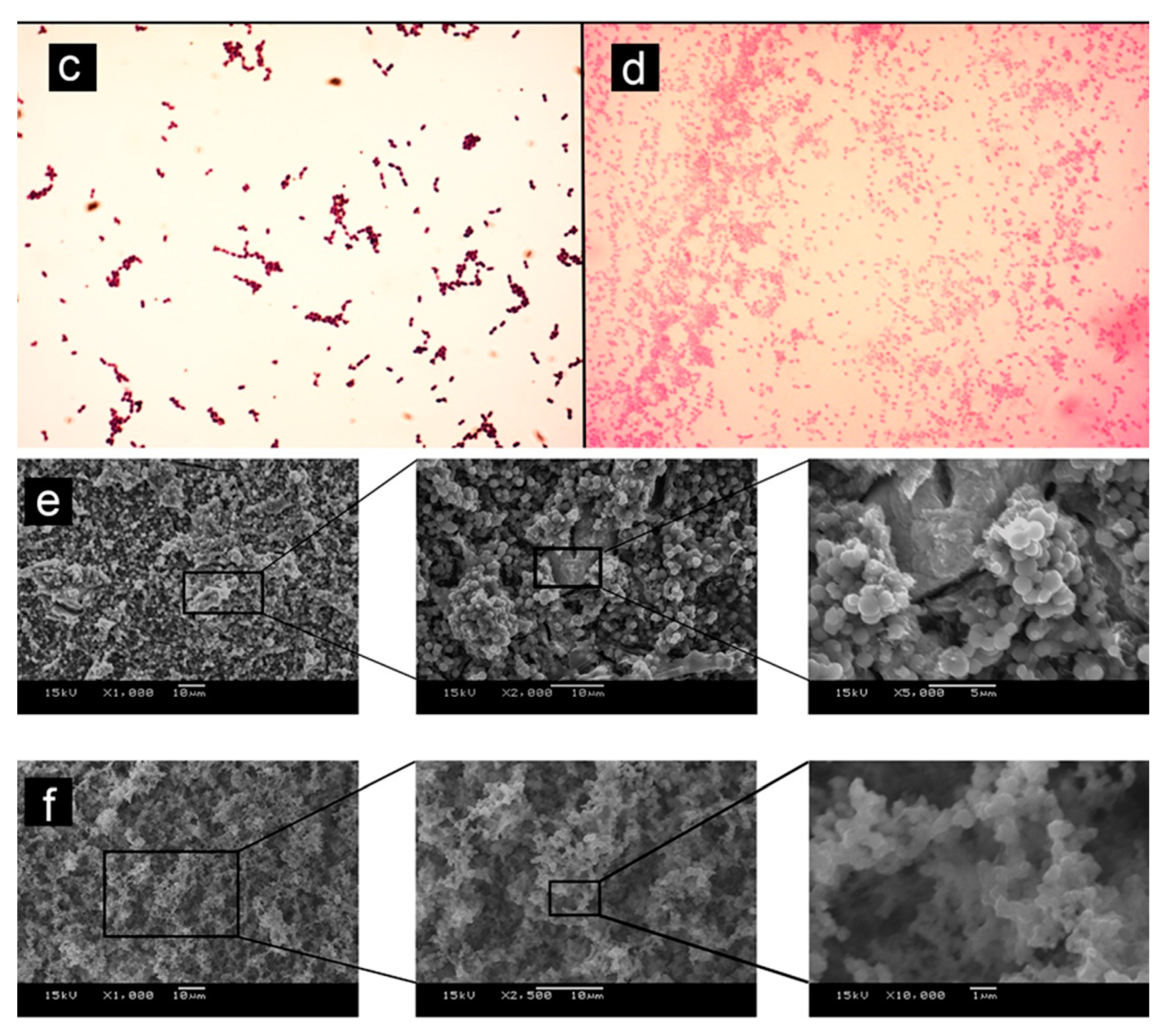
Testing soluble materials—such as reparative endodontic materials—over agar plates seems inappropriate once solubility halos are observed, indicating possible misleading interpretations [34]. Previous studies reported similar observations when testing hydraulic endodontic materials in contact with agar [67,68,75], reporting a limited antimicrobial effect of the reparative endodontic tested materials (Figure 2—Adapted from cited reference [74]); other studies using other methods such as confocal microscopy in contact with dentin and a mature biofilm [84] also concluded that previous disinfection of the site to be treated with these materials is crucial and mandatory in order to expect a positive clinical outcome. Different storage methods for antimicrobial testing can also obtain varying results; in water, for example, ProRoot MTA showed higher antimicrobial activity than when aged in blood and exhibited significant antimicrobial activity reduction after 7 days [35].
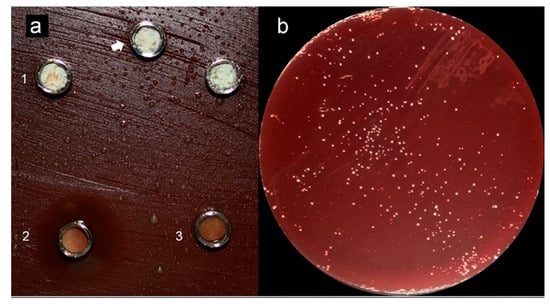
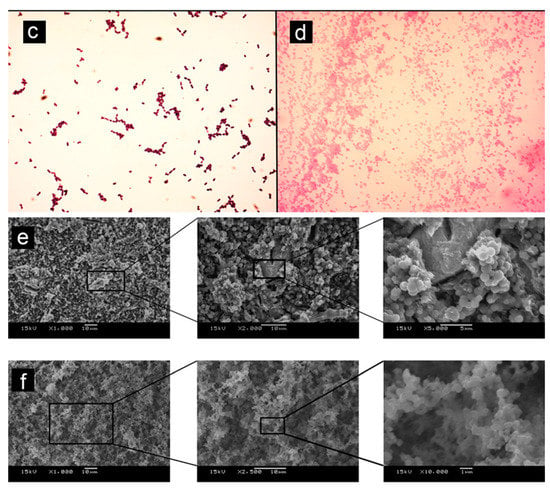
Figure 2.
Adapted from [75]. (a) (1) Grey-MTAFlow cement without inhibition halos in BHI medium containing inoculated E. faecalis; (2) Chlorhexidine gel used as a control for antimicrobial activity presenting inhibition halo; (3) Additional metallic disc containing only silicon-based gel without inhibition halo as well; the arrow indicates the collection area for smear and viability tests. (b) Viability test for E. faecalis after 7 days showing viable bacteria adjacent to the disc. (c) The smear of E. faecalis after 7 days in contact with fresh Grey-MTAFlow cement. (d) The smear of P. gingivalis after 5 days in contact with fresh Grey-MTAFlow cement. (e) Representative SEM images of the surface of Grey-MTAFlow in contact with E. faecalis at 1000×, 2000×, and 5000× magnifications. (f) Representative SEM images of the surface of Grey-MTAFlow in contact with P. gingivalis at 1000×, 2500×, and 10,000× magnifications.
Standardization of antimicrobial testing is crucial for the evolution of material research on different bacterial strains. A minimum of at least three specimens for each material/group should be tested, and three test replicates should be performed; additionally, they should be run by the same operator under the same laboratory conditions [85]. Other aspects such as the nature of the material, its chemical characterization, adequate sample size, the sample dimensions, and the sterilization method should also be broadly considered prior to the antimicrobial tests [30,86,87,88]. To date, no specific ISO standard is yet available for testing hydraulic endodontic calcium silicate-based reparative materials.
Regarding the results observed for the antimicrobial effect of reparative endodontic materials, it could be inferred that during the clinical use of these materials, the application sites must be thoroughly disinfected in advance, as the materials—similarly to the case observed for sealers—do not possess strong antimicrobial efficacy. Additionally, further studies with reproducible and standardized methods are necessary for further assumptions. Clinical long-term controlled studies considering both the success rates and analyzing the cause of failures regarding the use of these materials are utterly necessary to understand and improve endodontic materials.
The main limitation of this review is the fact that it did not provide details on the overall research strategy and several materials in both categories: sealers and reparative. However, a manual search of papers that discussed the standardization of these materials was included. Additionally, the lack of standardization (i.e., ISO) for calcium silicate-based materials is a crucial concern when developing, testing, and providing clearance for clinical use of these materials.
5. Conclusions
Long-term antimicrobial challenges can occur after endodontic and restorative procedures and can cause failures in dental treatment. The reduced antimicrobial effect exhibited by calcium silicate-based endodontic materials per se clearly emphasizes that all clinical procedures prior to their use must be carefully performed, aiming for exhaustive disinfection of the dental tissues. It cannot be expected that these materials will achieve bacterial reductions attributable to their properties (i.e., alkaline pH) once they are constantly challenged by infection and body fluid interactions that might cause failure of their antimicrobial or sealing properties in the long run. Therefore, it is necessary that future in vitro studies use greater methodological standardization for antimicrobial analysis of endodontic cements. Preferably, new studies are indicated to evaluate polymicrobial biofilms associated with endodontic diseases, as well as the addition of new compounds and formulations to optimize the antimicrobial effect of calcium silicate-based materials.
Author Contributions
A.C.P.J. performed the literature review and original drafting of the manuscript. G.F.B., M.A.M. and L.E.P. participated in manuscript conceptualization, writing, reviewing, and editing. M.A.M. directed the development of all aspects of the manuscript and participated in manuscript conceptualization, writing, reviewing, and editing. All authors approved the final manuscript for publication and agree to be accountable for all aspects of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sao Paulo Research Foundation, Sao Paulo, Brazil—2019/22098-9.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- American Association of Endodontists. Glossary of endodontic terms 2016. Gloss Endod. Terms 2015, 9, 43. [Google Scholar]
- Patel, E.; Pradeep, P.; Kumar, P.; Choonara, Y.E.; Pillay, V. Oroactive dental biomaterials and their use in endodontic therapy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 201–212. [Google Scholar] [CrossRef]
- Schilder, H. Filling root canals in three dimensions. J. Endod. 2006, 32, 281–290. [Google Scholar] [CrossRef]
- Al-Haddad, A.; Ab Aziz, Z.A.C. Bioceramic-based root canal sealers: A review. Int. J. Biomater. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Muedra, P.; Forner, L.; Lozano, A.; Sanz, J.; Rodríguez-Lozano, F.; Guerrero-Gironés, J.; Riccitiello, F.; Spagnuolo, G.; Llena, C. Could the calcium silicate-based sealer presentation form influence dentinal sealing? An in vitro confocal laser study on tubular penetration. Materials (Basel) 2021, 14, 659. [Google Scholar] [CrossRef]
- de Miranda Candeiro, G.T.; Correia, F.C.; Duarte, M.A.H.; Ribeiro-Siqueira, D.C.; Gavini, G. Evaluation of radiopacity, pH, release of calcium ions, and flow of a bioceramic root canal sealer. J. Endod. 2012, 38, 842–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prati, C.; Gandolfi, M.G. Calcium silicate bioactive cements: Biological perspectives and clinical applications. Dent. Mater. 2015, 31, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wei, L.; Liu, X.; Li, J.; Li, B.; Wang, G.; Meng, F. Influences of ionic dissolution products of dicalcium silicate coating on osteoblastic proliferation, differentiation and gene expression. Acta Biomater. 2009, 5, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.R.; Herrera, D.R.; Francisco, P.A.; Pereira, A.C.; Lemos, J.; Abranches, J.; Gomes, B.P. Detection of Streptococcus mutans in symptomatic and asymptomatic infected root canals. Clin. Oral Investig. 2021, 25, 3535–3542. [Google Scholar] [CrossRef]
- Gabrielli, E.S.; Lima, A.R.; Francisco, P.A.; Herrera, D.R.; De-Jesus-Soares, A.; Ferraz, C.C.R.; Almeida, J.F.A.; Marciano, M.A.; Gomes, B.P.F.A. Comparative analysis of bacterial content, levels of lipopolysaccharides and lipoteichoic acid in symptomatic and asymptomatic endodontic infections at different stages of endodontic treatment. Clin. Oral Investig. 2021, 1–16. [Google Scholar] [CrossRef]
- Ozcan, E.; Eldeniz, A.U.; Ari, H. Bacterial killing by several root filling materials and methods in an ex vivo infected root canal model. Int. Endod. J. 2011, 44, 1102–1109. [Google Scholar] [CrossRef]
- Zhou, H.-M.; Shen, Y.; Zheng, W.; Li, L.; Zheng, Y.-F.; Haapasalo, M. Physical Properties of 5 Root Canal Sealers. J. Endod. 2013, 39, 1281–1286. [Google Scholar] [CrossRef]
- Narayanan, L.L.; Vaishnavi, C. Endodontic microbiology. J. Conserv. Dent. 2010, 13, 233. [Google Scholar] [CrossRef]
- Munson, M.A.; Pitt-Ford, T.; Chong, B.; Weightman, A.; Wade, W.G. Molecular and cultural analysis of the microflora associated with endodontic infections. J. Dent. Res. 2002, 81, 761–766. [Google Scholar] [CrossRef]
- Siqueira, J.; Rôças, I. Diversity of endodontic microbiota revisited. J. Dent. Res. 2009, 88, 969–981. [Google Scholar] [CrossRef]
- Gomes, B.P.; Berber, V.B.; Kokaras, A.S.; Chen, T.; Paster, B.J. Microbiomes of endodontic-periodontal lesions before and after chemome-chanical preparation. J. Endod. 2015, 41, 1975–1984. [Google Scholar] [CrossRef]
- Gomes, B.P.F.D.A.; Herrera, D.R. Etiologic role of root canal infection in apical periodontitis and its relationship with clinical symptomatology. Braz. Oral Res. 2018, 32, 82–110. [Google Scholar] [CrossRef] [Green Version]
- Weiss, E.I.; Shalhav, M.; Fuss, Z. Assessment of antibacterial activity of endodontic sealers by a direct contact test. Dent. Traumatol. 1996, 12, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Candeiro, G.T.M.; Moura-Netto, C.; D’Almeida-Couto, R.S.; Azambuja-Júnior, N.; Marques, M.M.; Cai, S.; Gavini, G. Cytotoxicity, genotoxicity and antibacterial effectiveness of a bioceramic endodontic sealer. Int. Endod. J. 2016, 49, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Rezende, G.C.; Massunari, L.; Queiroz, I.O.D.A.; Gomes, J.E.; Jacinto, R.C.; Lodi, C.S.; Dezan, E. Antimicrobial action of calcium hydroxide-based endodontic sealers after setting, against E. faecalis biofilm. Braz. Oral Res. 2016, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, G.; Gupta, I.; Elshamy, F.M.; Boreak, N.; Homeida, H.E. In vitro comparison of antibacterial properties of bioceramic-based sealer, res-in-based sealer and zinc oxide eugenol based sealer and two mineral trioxide aggregates. Eur. J. Dent. 2016, 10, 366–369. [Google Scholar]
- Zhang, H.; Shen, Y.; Ruse, N.D.; Haapasalo, M. Antibacterial activity of endodontic sealers by modified direct contact test against Enter-ococcus faecalis. J. Endod. 2009, 35, 1051–1055. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, Y.; Haapasalo, M. Dentin extends the antibacterial effect of endodontic sealers against enterococcus faecalis biofilms. J. Endod. 2014, 40, 505–508. [Google Scholar] [CrossRef]
- Arias-Moliz, M.T.; Camilleri, J. The effect of the final irrigant on the antimicrobial activity of root canal sealers. J. Dent. 2016, 52, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Alsubait, S.; Albader, S.; Alajlan, N.; Alkhunaini, N.; Niazy, A.; Almahdy, A. Comparison of the antibacterial activity of calcium silicate- and epoxy resin-based endodontic sealers against Enterococcus faecalis biofilms: A confocal laser-scanning microscopy analysis. Odontology 2019, 107, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.; Karabucak, B. The antimicrobial effect of bioceramic sealer on an 8-week matured enterococcus faecalis biofilm attached to root canal dentinal surface. J. Endod. 2019, 45, 1047–1052. [Google Scholar] [CrossRef]
- Zordan-Bronzel, C.L.; Tanomaru-Filho, M.; Chávez-Andrade, G.M.; Torres, F.F.E.; Abi-Rached, G.P.C.; Guerreiro-tanomaru, j.m. calcium silicate-based experimental sealers: Physicochemical properties evaluation. Mater. Res. 2021, 24. [Google Scholar] [CrossRef]
- Barbosa, A.F.A.; Silva, E.J.N.L.; Coelho, B.P.; Ferreira, C.M.A.; Lima, C.O.; Sassone, L.M. The influence of endodontic access cavity de-sign on the efficacy of canal instrumentation, microbial reduction, root canal filling and fracture resistance in mandibular molars. Int. Endod. J. 2020, 53, 1666–1679. [Google Scholar] [CrossRef]
- Carvalho, N.K.; Barbosa, A.F.A.; Coelho, B.D.P.; Gonçalves, L.D.S.; Sassone, L.M.; Silva, E.J.N.L. Antibacterial, biological, and physicochemical properties of root canal sealers containing chlorhexidine-hexametaphosphate nanoparticles. Dent. Mater. 2021, 37, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Bhavana, V.; Chaitanya, K.P.; Gandi, P.; Patil, J.; Dola, B.; Reddy, R.B. Evaluation of antibacterial and antifungal activity of new calcium-based cement (Biodentine) compared to MTA and glass ionomer cement. J. Conserv. Dent. 2015, 18, 44–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ElReash, A.A.; Hamama, H.; Eldars, W.; Lingwei, G.; El-Din, A.Z.; Xiaoli, X. Antimicrobial activity and pH measurement of calcium silicate cements versus new bioactive resin composite restorative material. BMC Oral Health 2019, 19, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Duarte, M.A.H.; D’arc de Oliveira El, G.; Vivan, R.R.; Tanomaru, J.M.G.; Tanomaru Filho, M.; de Moraes, I.G. Radiopacity of portland cement associated with different radiopacifying agents. J. Endod. 2009, 35, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.B.; Torres, F.F.E.; Rodrigues, E.M.; Viola, K.S.; Bosso-Martelo, R.; Chavez-Andrade, G.M.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M. Physicochemical, biological, and antibacterial evaluation of tricalcium sili-cate-based reparative cements with different radiopacifiers. Dent. Mater. 2021, 37, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, C.; Haider, J.; Camilleri, L.; Camilleri, J. Clinical relevance of antimicrobial testing results for dental restorative materials. J. Appl. Biomater. Funct. Mater. 2017, 15, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, C.; Baca, P.; Camilleri, J.; Arias-Moliz, M.T. Antimicrobial activity of proroot MTA in contact with blood. Sci. Rep. 2017, 7, 41359. [Google Scholar] [CrossRef]
- Arias-Moliz, M.T.; Farrugia, C.; Lung, C.Y.; Schembri-Wismayer, P.; Camilleri, J. Antimicrobial and biological activity of leachate from light curable pulp capping materials. J. Dent. 2017, 64, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.G.; Ferreira, N.S.; Martinho, F.; Nascimento, G.G.; Manhães, L.R.; Rocco, M.A.; Carvalho, C.T.; Valera, M.C. Correlation between volume of apical periodontitis determined by cone-beam computed tomography analysis and endotoxin levels found in primary root canal infection. J. Endod. 2015, 41, 1015–1019. [Google Scholar] [CrossRef]
- Wong, J.; Zou, T.; Lee, A.H.C.; Zhang, C. The potential translational applications of nanoparticles in endodontics. Int. J. Nanomed. 2021, 16, 2087–2106. [Google Scholar] [CrossRef]
- Gjorgievska, E.S.; Nicholson, J.W.; Coleman, N.; Booth, S.; Dimkov, A.; Hurt, A. Component release and mechanical properties of endodontic sealers following incorporation of antimicrobial agents. BioMed Res. Int. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Arias-Moliz, M.; Ruiz-Linares, M.; Cassar, G.; Ferrer-Luque, C.; Baca, P.; Ordinola-Zapata, R.; Camilleri, J. The effect of benzalkonium chloride additions to AH plus sealer. Antimicrobial, physical and chemical properties. J. Dent. 2015, 43, 846–854. [Google Scholar] [CrossRef]
- Curtis, A.; Wilkinson, C. Nantotechniques and approaches in biotechnology. Trends Biotechnol. 2001, 19, 97–101. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Antibacterial nanoparticles in endodontics: A review. J. Endod. 2016, 42, 1417–1426. [Google Scholar] [CrossRef]
- Loyola-Rodríguez, J.P.; Torres-Méndez, F.; Espinosa-Cristobal, L.F.; García-Cortes, J.O.; Loyola-Leyva, A.; González, F.J.; Soto-Barreras, U.; Nieto-Aguilar, R.; Contreras-Palma, G. Antimicrobial activity of endodontic sealers and med-ications containing chitosan and silver nanoparticles against Enterococcus faecalis. J. Appl. Biomater. Funct. Mater. 2019, 17. [Google Scholar] [CrossRef] [Green Version]
- Borges, R.P.; Sousa-Neto, M.D.; Versiani, M.A.; Rached-Júnior, F.A.; De-Deus, G.; Miranda CE, S.; Pécora, J.D. Changes in the surface of four calcium silicate-containing endodontic mate-rials and an epoxy resin-based sealer after a solubility test. Int. Endod. J. 2012, 45, 419–428. [Google Scholar] [CrossRef]
- Camilleri, J. Characterization of hydration products of mineral trioxide aggregate. Int. Endod. J. 2008, 41, 408–417. [Google Scholar] [CrossRef]
- Koutroulis, A.; Kuehne, S.A.; Cooper, P.R.; Camilleri, J. The role of calcium ion release on biocompatibility and antimicrobial proper-ties of hydraulic cements. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sönmez, I.S.; Oba, A.A.; Sönmez, D.; Almaz, M.E. In vitro evaluation of apical microleakage of a new MTA-based sealer. Eur. Arch. Paediatr. Dent. 2012, 13, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Heran, J.; Khalid, S.; Albaaj, F.; Tomson, P.L.; Camilleri, J. The single cone obturation technique with a modified warm filler. J. Dent. 2019, 89. [Google Scholar] [CrossRef] [PubMed]
- Orhan, K.; Jacobs, R.; Celikten, B.; Huang, Y.; Vasconcelos, K.D.F.; Nicolielo, L.F.P.; Buyuksungur, A.; Van Dessel, J. Evaluation of Threshold Values for Root Canal Filling Voids in Micro-CT and Nano-CT Images. Scanning 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Almeida LH, S.; Moraes, R.R.; Morgental, R.D.; Pappen, F.G. Are premixed calcium silicate–based endodontic sealers comparable to conventional materials? a systematic review of in vitro studies. J. Endod. 2017, 43, 527–535. [Google Scholar] [CrossRef]
- Tanomaru-Filho, M.; Andrade, A.S.; Rodrigues, E.; Viola, K.S.; Faria, G.; Camilleri, J.; Guerreiro-Tanomaru, J.M. Biocompatibility and mineralized nodule formation of Neo MTA Plus and an experimental tricalcium silicate cement containing tantalum oxide. Int. Endod. J. 2017, 50, e31–e39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, E.J.; Cardoso, M.L.; Rodrigues, J.P.; De-Deus, G.; Fidalgo, T.K.D.S. Solubility of bioceramic- and epoxy resin-based root canal sealers: A system-atic review and meta-analysis. Aust. Endod. J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Antunes, T.B.M.; Janini, A.C.P.; Pelepenko, L.E.; Abuna, G.F.; Paiva, E.M.; Sinhoreti, M.A.C.; Raimundo, I.M.; Gomes, B.P.F.A.; De-Jesus-Soares, A.; Marciano, M.A.; et al. Heating stability, physical and chemical analysis of calcium silicate-based endodontic sealers. Int. Endod. J. 2021, 13496. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Z.; Shen, Y.; Haapasalo, M. A new noninvasive model to study the effectiveness of dentin disinfection by using confocal laser scanning microscopy. J. Endod. 2011, 37, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Monsef, M.; Torabinejad, M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J. Endod. 1993, 19, 541–544. [Google Scholar] [CrossRef]
- Torabinejad, M.; Hong, C.; Ford, T.P.; Kettering, J. Antibacterial effects of some root end filling materials. J. Endod. 1995, 21, 403–406. [Google Scholar] [CrossRef]
- Cehreli, Z.C.; Sara, S.; Uysal, S.; Turgut, M.D. MTA apical plugs in the treatment of traumatized immature teeth with large periapical lesions. Dent. Traumatol. 2010, 27, 59–62. [Google Scholar] [CrossRef]
- Primus, C.M. A White, Substantially Non-Iron Containing Dental Material Formed from Portland Cement; World Intellect Prop Organ: Geneva, Switzerland, 2002; pp. 1–15. [Google Scholar]
- Cutajar, A.; Mallia, B.; Abela, S.; Camilleri, J. Replacement of radiopacifier in mineral trioxide aggregate; Characterization and deter-mination of physical properties. Dent. Mater. 2011, 27, 879–891. [Google Scholar] [CrossRef]
- Dammaschke, T.; Gerth, H.U.; Züchner, H.; Schäfer, E. Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent. Mater. 2005, 21, 731–738. [Google Scholar] [CrossRef]
- Marciano, M.A.; Camilleri, J.; Costa, R.M.; Matsumoto, M.A.; Guimarães, B.M.; Duarte, M.A.H. Zinc oxide inhibits dental discoloration caused by white mineral trioxide aggregate angelus. J. Endod. 2017, 43, 1001–1007. [Google Scholar] [CrossRef] [Green Version]
- Camilleri, J. Tricalcium silicate cements with resins and alternative radiopacifiers. J. Endod. 2014, 40, 2030–2035. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J. Color stability of white mineral trioxide aggregate in contact with hypochlorite solution. J. Endod. 2014, 40, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Marciano, M.A.; Costa, R.M.; Camilleri, J.; Mondelli, R.F.L.; Guimarães, B.M.; Duarte, M.A.H. Assessment of color stability of white mineral trioxide aggregate angelus and bismuth oxide in contact with tooth structure. J. Endod. 2014, 40, 1235–1240. [Google Scholar] [CrossRef]
- Camilleri, J. Hydration characteristics of Biodentine and Theracal used as pulp capping materials. Dent. Mater. 2014, 30, 709–715. [Google Scholar] [CrossRef]
- Moinzadeh, A.T.; Portoles, C.A.; Schembri-Wismayer, P.; Camilleri, J. Bioactivity potential of EndoSequence BC RRM putty. J. Endod. 2016, 42, 615–621. [Google Scholar] [CrossRef]
- Pelepenko, L.E.; Saavedra, F.; Antunes, T.B.M.; Bombarda, G.F.; Gomes, B.P.F.D.A.; Zaia, A.A.; Marciano, M.A. Investigation of a modified hydraulic calcium silicate-based material-Bio-C Pulpo. Braz. Oral Res. 2021, 35, e077. [Google Scholar] [CrossRef]
- Pelepenko, L.E.; Saavedra, F.; Antunes, T.B.M.; Bombarda, G.F.; Gomes, B.P.F.A.; Zaia, A.A.; Camilleri, J.; Marciano, M.A. Physicochemical, antimicrobial, and biological properties of White-MTAFlow. Clin. Oral Investig. 2021, 25, 663–672. [Google Scholar] [CrossRef]
- Duarte, M.A.H.; Marciano, M.A.; Vivan, R.R.; Filho, M.T.; Tanomaru, J.M.G.; Camilleri, J. Tricalcium silicate-based cements: Properties and modifications. Braz. Oral Res. 2018, 32, e70. [Google Scholar] [CrossRef] [Green Version]
- Banchs, F.; Trope, M. Revascularization of immature permanent teeth with apical periodontitis: New treatment protocol? J. Endod. 2004, 30, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Zamparini, F.; Siboni, F.; Prati, C.; Taddei, P.; Gandolfi, M.G. Properties of calcium silicate-monobasic calcium phosphate materials for endodontics containing tantalum pentoxide and zirconium oxide. Clin. Oral Investig. 2019, 23, 445–457. [Google Scholar] [CrossRef]
- Pace, R.; Giuliani, V.; Pagavino, G. Mineral trioxide aggregate as repair material for furcal perforation: Case series. J. Endod. 2008, 34, 1130–1133. [Google Scholar] [CrossRef]
- Bjørndal, L.; Simon, S.; Tomson, P.L.; Duncan, H.F. Management of deep caries and the exposed pulp. Int. Endod. J. 2019, 52, 949–973. [Google Scholar] [CrossRef]
- Borges, Á.H.; Bandeca, M.C.; Estrela, C.R.D.A.; Amezcua, O.; Gonzalez, Á.C.; Estrela, C. Sealing Ability of Root-end Filling Materials. J. Contemp. Dent. Pract. 2015, 16, 210–214. [Google Scholar] [CrossRef]
- Pelepenko, L.E.; Saavedra, F.; Bombarda, G.F.; Gomes, B.P.F.D.A.; de-Jesus-Soares, A.; Zaia, A.A.; Duarte, M.A.H.; Tanomaru-Filho, M.; Marciano, M.A. Dental discoloration caused by Grey-MTAFlow cement: Analysis of its phys-icochemical, biological and antimicrobial properties. J. Appl. Oral Sci. 2020, 28. [Google Scholar] [CrossRef]
- Samanta, A.; Podder, S.; Ghosh, C.K.; Bhattacharya, M.; Ghosh, J.; Mallik, A.K.; Dey, A.; Mukhopadhyay, A.K. ROS mediated high anti-bacterial efficacy of strain tolerant layered phase pure nano-calcium hydroxide. J. Mech. Behav. Biomed. Mater. 2017, 72, 110–128. [Google Scholar] [CrossRef]
- Bueno, C.R.E.; Valentim, D.; Marques, V.A.S.; Gomes-Filho, J.E.; Cintra, L.T.A.; Jacinto, R.C.; Dezan-Junior, E. Biocompatibility and biomineralization assessment of bioceramic-, epoxy-, and calcium hydroxide-based sealers. Braz. Oral Res. 2016, 30. [Google Scholar] [CrossRef] [PubMed]
- Belío-Reyes, I.A.; Bucio, L.; Cruz-Chavez, E. Phase composition of ProRoot mineral trioxide aggregate by X-ray powder dif-fraction. J. Endod. 2009, 35, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J. Hydration mechanisms of mineral trioxide aggregate. Int. Endod. J. 2007, 40, 462–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camilleri, J. Evaluation of the physical properties of an endodontic Portland cement incorporating alternative radiopacifiers used as root-end filling material. Int. Endod. J. 2010, 43, 231–240. [Google Scholar] [CrossRef]
- Camilleri, J. Scanning electron microscopic evaluation of the material interface of adjacent layers of dental materials. Dent. Mater. 2011, 27, 870–878. [Google Scholar] [CrossRef]
- Ha, W.N.; Kahler, B.; Walsh, L.J. Particle size changes in unsealed mineral trioxide aggregate powder. J. Endod. 2014, 40, 423–426. [Google Scholar] [CrossRef]
- Guerreiro-Tanomaru, J.M.; Vazquez-Garcia, F.A.; Bosso-Martelo, R.; Bernardi, M.I.B.; Faria, G.; Tanomaru, M. Effect of addition of nano-hydroxyapatite on physi-co-chemical and antibiofilm properties of calcium silicate cements. J. Appl. Oral Sci. 2016, 24, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Jardine, A.P.; Montagner, F.; Quintana, R.M.; Zaccara, I.M.; Kopper, P.M.P. Antimicrobial effect of bioceramic cements on multispecies microcosm bio-film: A confocal laser microscopy study. Clin. Oral Investig. 2019, 23, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Moliz, T.A.; Bettencourt, A.; Costa, J.; Martins, F.; Rabadijeva, D.; Rodriguez, D.; Visai, L.; Combes, C.; Farrugia, C.; et al. Standardization of antimicrobial testing of dental devices. Dent. Mater. 2020, 36, e59–e73. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, C.; Cassar, G.; Valdramidis, V.; Camilleri, J. Effect of sterilization techniques prior to antimicrobial testing on physical properties of dental restorative materials. J. Dent. 2015, 43, 703–714. [Google Scholar] [CrossRef]
- Meschi, N.; Li, X.; Van Gorp, G.; Camilleri, J.; Van Meerbeek, B.; Lambrechts, P. Bioactivity potential of Portland cement in regenerative endodontic procedures: From clinic to lab. Dent. Mater. 2019, 35, 1342–1350. [Google Scholar] [CrossRef]
- Rohanová, D.; Horkavcová, D.; Helebrant, A.; Boccaccini, A.R. Assessment of in vitro testing approaches for bioactive inorganic materials. J. Non-Cryst. Solids 2016, 432, 53–59. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).